Abstract
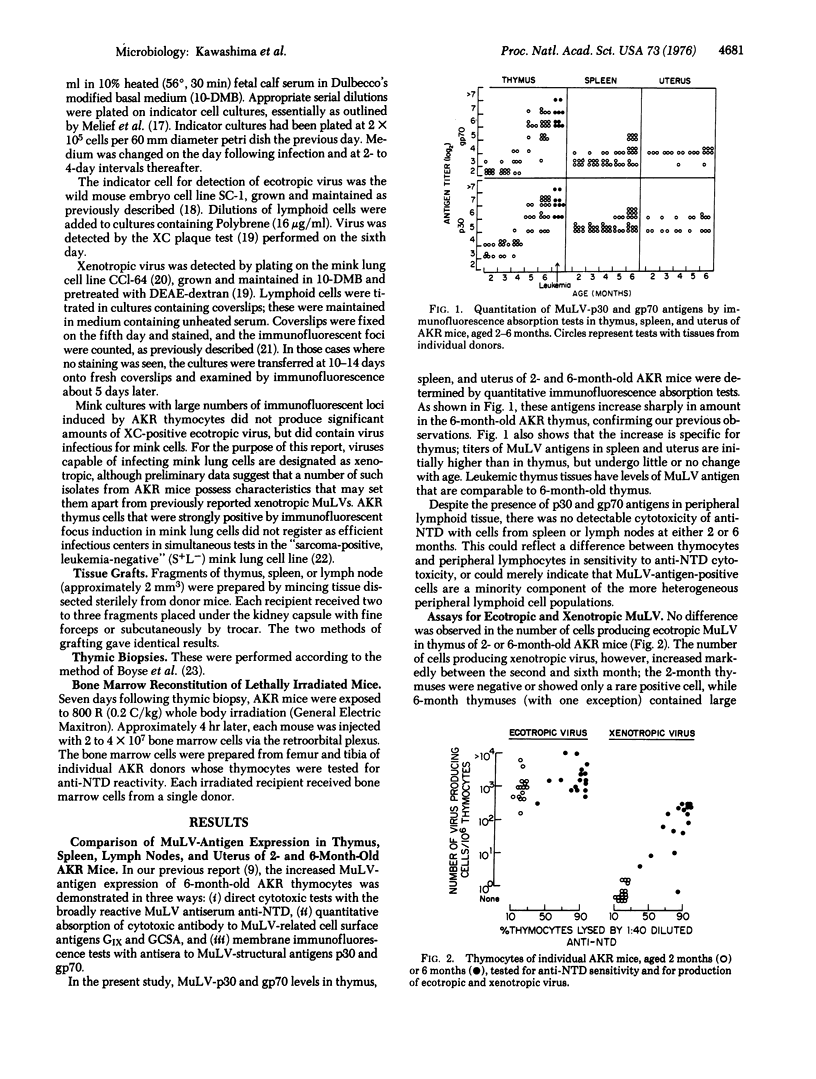
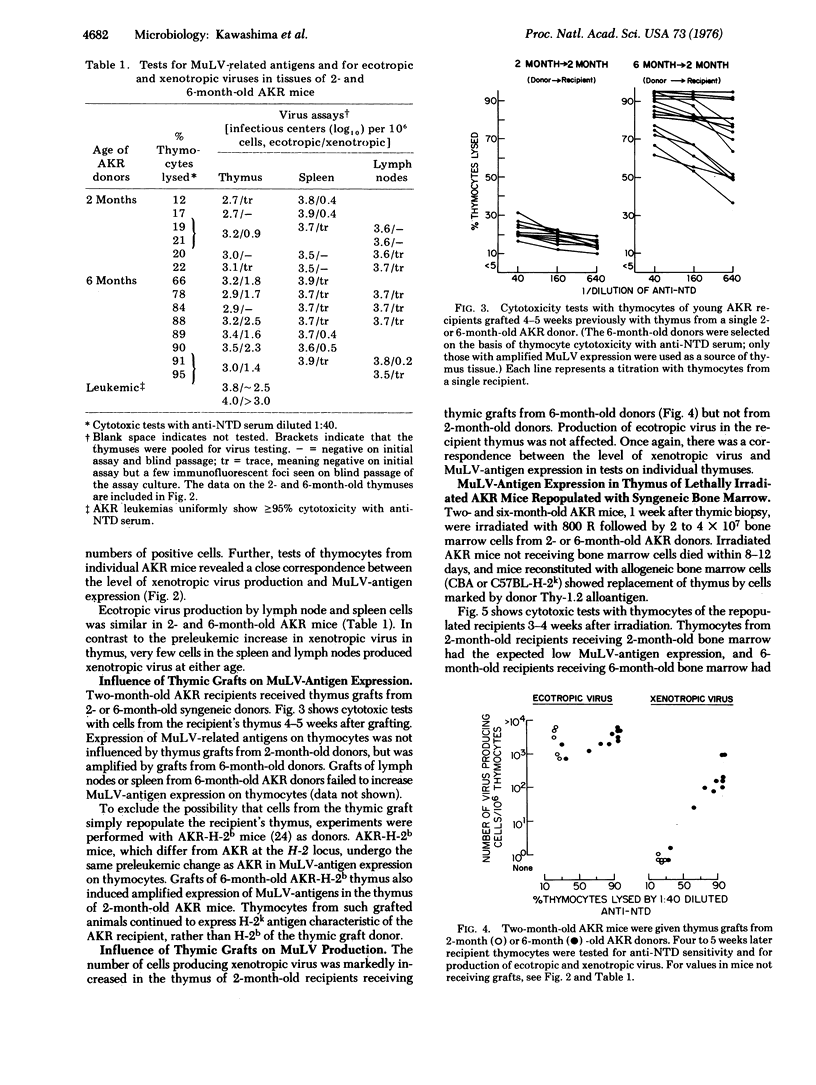
We recently reported that thymocytes from 6-month-old preleukemic AKR mice express higher levels of murine leukemia virus (MuLV)-related antigens that thymocytes from 2-month-old mice. We have now found that the level of xenotropic MuLV (defined operationally as MuLV able to infect mink cell cultures) is also markedly increased in thymus of 6-month-old AKR mice and that this increase in virus correlates closely with increased MuLV-antigen expression. There is no increase of MuLV antigen or xenotropic virus in spleen or lymph nodes. Production of ecotropic MuLV remains unchanged with age in thymus, lymph nodes, and spleen. Thymic grafts from 6-month-old AKR mice, but not from 2-month-old mice, induce both amplified MuLV-antigen expression and xenotropic virus production in the thymus of young AKR recipients. Experiments with lethally irradiated AKR mice reconstituted with syngeneic bone marrow cells indicate that age-related changes in the thymus rather than in bone marrow precursor cells are responsible for MuLV-antigen amplification.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyse E. A., Old L. J., Iritani C. A. The technique of obtaining thymocytes from living mice. Transplantation. 1971 Jul;12(1):93–95. doi: 10.1097/00007890-197107000-00019. [DOI] [PubMed] [Google Scholar]
- Chazan R., Haran-Ghera N. The role of thymus subpopulations in "T" leukemia development. Cell Immunol. 1976 May;23(2):356–375. doi: 10.1016/0008-8749(76)90200-8. [DOI] [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Ihle J. N., Yurconic M., Jr, Hanna M. G., Jr Autogenous immunity to endogenous RNA tumor virus. Radioimmune precipitation assay of mouse serum antibody levels. J Exp Med. 1973 Jul 1;138(1):194–208. doi: 10.1084/jem.138.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Hardy W., Jr, Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. V. Identification of a new murine viral protein, p15(E). J Virol. 1975 Jul;16(1):53–61. doi: 10.1128/jvi.16.1.53-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Kassel R. L., Old L. J., Carswell E. A., Fiore N. C., Hardy W. D., Jr Serum-mediated leukemia cell destruction in AKR mice. J Exp Med. 1973 Oct 1;138(4):925–938. doi: 10.1084/jem.138.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K., Ikeda H., Stockert E., Takahashi T., Old L. J. Age-related changes in cell surface antigens of preleukemic AKR thymocytes. J Exp Med. 1976 Jul 1;144(1):193–208. doi: 10.1084/jem.144.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp R. G., Duquesnoy R. J. Thymus adenylate cyclase activity during murine leukemogenesis. Science. 1974 Jan 18;183(4121):218–219. doi: 10.1126/science.183.4121.218. [DOI] [PubMed] [Google Scholar]
- Melief C. J., Datta S. K., Louie S., Johnson S., Melief M., Schwartz R. S. Splenocyte plaque assay for the detection of murine leukemia virus. Proc Soc Exp Biol Med. 1975 Sep;149(4):1015–1018. doi: 10.3181/00379727-149-38946. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Ford C. E., Evans E. P., Gray J. Interrelationships of myeloid and lymphoid cells: studies with chromosome-marked cells transfused into lethally irradiated mice. Proc R Soc Lond B Biol Sci. 1966 Jul 19;165(998):78–102. doi: 10.1098/rspb.1966.0059. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Nakakuki K. Acceleration of leukemogenesis in AKR mice by grafts, cell suspensions, and cell-free centrifugates of thymuses from preleukemic AKR donors. Int J Cancer. 1968 Mar 15;3(2):203–210. doi: 10.1002/ijc.2910030205. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Kaehler S. L. Antibody to leukemia virus: widespread occurrence in inbred mice. Science. 1974 Sep 6;185(4154):869–871. doi: 10.1126/science.185.4154.869. [DOI] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A. Current enigmas in cancer research. Harvey Lect. 1973;67:273–315. [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Stockert E. The G (Gross) leukemia antigen. Cancer Res. 1965 Jul;25(6):813–819. [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Del Villano B. C., Dixon F. J. Autologous immune responses to the major oncornavirus polypeptides in unmanipulated AKR/J mice. J Virol. 1976 Apr;18(1):176–181. doi: 10.1128/jvi.18.1.176-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- RUDALI G., DUPLAN J. F., LATARJET R. Latence des leucoses chez des souris Ak injectées avec un extrait leucémique a-cellulaire Ak. C R Hebd Seances Acad Sci. 1956 Feb 6;242(6):837–839. [PubMed] [Google Scholar]
- Rowe W. P. Genetic factors in the natural history of murine leukemia virus infection: G. H. A. Clowes Memorial Lecture. Cancer Res. 1973 Dec;33(12):3061–3068. [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Stockert E., Old L. J., Boyse E. A. The G-IX system. A cell surface allo-antigen associated with murine leukemia virus; implications regarding chromosomal integration of the viral genome. J Exp Med. 1971 Jun 1;133(6):1334–1355. doi: 10.1084/jem.133.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]


