Abstract
AIM: To elucidate the frequency and characteristics of pancreatic disorders in the course of chronic viral hepatitis.
METHODS: We prospectively assessed the serum pancreatic enzyme levels and imaging findings in patients with chronic viral hepatitis and healthy control subjects.
RESULTS: Serum amylase (t-Amy), salivary amylase (s-Amy), pancreatic amylase (p-Amy) and serum lipase levels were higher in hepatitis patients in comparison to control subjects. However, in asymptomatic viral carriers, only the serum t-Amy levels were higher than those of the controls. The levels of each enzyme rose with the progression of liver disease in patients with hepatitis B or C; whereas the levels of each enzyme within the same clinical stage of the disease did not differ between patients diagnosed with either hepatitis B or hepatitis C virus. Imaging findings demonstrated chronic pancreatitis in only 1 out of 202 patients (0.5%).
CONCLUSION: Our data suggest that serum levels of pancreatic enzymes increase with the progression of liver disease in patients diagnosed with viral hepatitis. Pancreatic disease, asymptomatic in most cases, may represent an extrahepatic manifestation of chronic viral hepatitis.
Keywords: Hepatitis C, Hepatitis B, Pancreatic disorder, Amylase, Lipase
INTRODUCTION
Anatomically, the pancreas and the liver are in close proximity; many of the blood vessels and ducts associated with these organs anatomies with each other. Also, pancreatic diseases which effect bile flow may result in concomitant liver damage. However, whether or not diseases affect pancreatic functions has not been clearly resolved. A number of studies reported that patients diagnosed with acute or fulminant hepatitis also suffer from acute pancreatitis[1-14]. Hepatitis A[3-6,8,9,13,14], hepatitis B[7], hepatitis C[11] and hepatitis E[9,10,12] have been reported to cause acute pancreatitis in patients with acute viral hepatitis. Furthermore, a case with acute exacerbation of chronic hepatitis (CH) B complicated by acute pancreatitis was reported[15], which suggests that pancreatitis may be an extrahepatic manifestation of CH.
It was reported that exocrine pancreatic function is damaged in chronic liver disease (CLD)[16]. This report has shown that pancreatic amylase output increases in the patients with nonalcoholic, non-cirrhotic CLD, which suggests that pancreatic enzymes may elevate in chronic non-cirrhotic viral diseases. On the contrary, a study from Italy has shown that elevation of pancreatic enzymes is observed only in cirrhotic patients[17]. Therefore, whether or not the pancreas is damaged in patients with CLD is not elucidated.
The aim of this study was to determine whether or not the pancreas is damaged in patients with chronic viral hepatitis.
MATERIALS AND METHODS
Materials
Patients seen in the outpatient clinic of our hospital between April 2002 and March 2003 were included in the study. The patient pool, defined as the CLD group, included 202 subjects, 111 males and 91 females, with a mean age of 51.9±14.9 years. CLD group consisted of asymptomatic carriers (AsC), patients with CH and those with liver cirrhosis (LC). AsC was defined by normal serum ALT levels measured repeatedly over a period of more than 1 year. CH and LC were diagnosed by liver biopsy and/or imaging studies. Patients with a history of alcohol intake exceeding 75 g/d were excluded from the study.
Eighty patients were positive for HBsAg, but negative for anti-HCV and were defined as the B-CLD group, which consisted of 34 AsC, 38 diagnosed with CH and 8 diagnosed with LC. The remaining 122 patients were negative for HBsAg, but positive for anti-HCV and were defined as the C-CLD group, which consisted of 14 AsC, 68 diagnosed with CH and 40 diagnosed with LC (Table 1). Three hundred and fifty-five healthy employees of St. Marianna University who underwent annual health checks constituted the control group.
Table 1.
Number of studied patients.
| HBsAg positive | Anti-HCV positive | Total | |
| AsC | 34 (28) | 14 (12) | 48 (40) |
| CH | 38 (35) | 68 (49) | 106 (84) |
| LC | 8 (5) | 40 (7) | 48 (12) |
| Total | 80 (68) | 122 (68) | 202 (136) |
Number of patients with the age between 30 and 60 years.
Methods
The levels of serum amylase (t-Amy) and lipase were determined using enzymatic assay (Pureauto sAmy-G2, Daiichi Pure Chemicals Co., Tokyo, Japan). The levels of pancreatic (p-Amy) and salivary amylase (s-Amy) were determined by isoenzyme pattern determined by cellulose acetate membrane electrophoresis method and total amylase levels. Fisher’s exact test, the unpaired t-test and Spearman’s rank correlation test were used for statistical analysis; P values less than 0.05 were considered significant.
All of the patients in the CLD group underwent abdominal ultrasonography and/or computed tomography within 3 mo of their blood chemistries. Informed consent was obtained from each patient and healthy volunteer enrolled in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as well as those implemented in our institutions.
RESULTS
Pancreatic enzymes in patients with chronic liver disease
The mean age of the patient group was higher than that in the control group. Therefore, 79 male and 59 female patients between 30 and 60 years of age (mean age 44±8.8 years) were selected and compared with age and sex-matched control cohorts.
The levels of t-Amy, s-Amy, p-Amy and lipase in the patient group were 20%, 18%, 25%, and 32% higher than those in their respective control cohorts (Figure 1). Table 2 demonstrates the frequency of abnormal elevation in the patient and control groups. Macroamylase was not detected in any patient.
Figure 1.

Levels of serum or tissue enzymes in patients with CLD compared to those measured in age and sex-matched control subjects. bP<0.01 vs controls.
Table 2.
Frequency of hyperamylasemia and hyperlipasemia.
| Enzymes | CLD (total) (n = 202, %) | CLD (30-60 yr old) (n = 136, %) | Controls (n = 355, %) |
| t-Amy | 27 (13.4) | 12 (8.8)b | 7 (2.0) |
| s-Amy | 8 (4.0) | 6 (4.4) | 18 (5.1) |
| p-Amy | 9 (4.5) | 7 (5.1) | 7 (2.0) |
| Lipase | 13 (6.4) | 6 (4.4)a | 3 (0.8) |
Statistical significance in comparison with differences between patients with CLD (30-60 years old) and age, sex-matched controls:
P<0.05,
P<0.01 vs controls. CLD: chronic liver disease.
Subsequently, we compared the enzyme levels in each stage of chronic viral hepatitis with those in healthy control subjects. Figure 2 shows the results from patients diagnosed with chronic HBV infection. The asymptomatic HBV carriers exhibited higher p-Amy and lipase levels than did their respective control cohorts. Furthermore, patients diagnosed with CH and LC exhibited higher levels of both t-Amy and s-Amy, in addition to p-Amy and lipase. Figure 3 shows the levels of pancreatic enzyme obtained from patients diagnosed with chronic HCV infection. In contrast to HBV patients, the levels of all four enzymes were not significantly different between the asymptomatic HCV carriers and the control subjects. The patients with CH and cirrhosis exhibited higher levels of these four enzymes than did their controls as seen in the patients diagnosed with chronic HBV.
Figure 2.
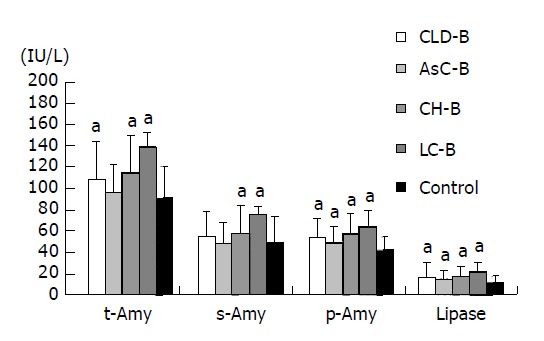
Levels of serum or tissue enzymes at each clinical stage of CH B. aP<0.05 vs controls.
Figure 3.

Levels of serum or tissue enzymes at several stages of CH C. aP<0.05 vs controls.
Figures 4 and 5 illustrate the distribution of enzyme levels at each clinical stage of CH. The levels of t-Amy, s-Amy, p-Amy and lipase increased concomitantly with the progression of liver disease in patients with HBV and HCV infection.
Figure 4.

Distribution of serum enzyme levels at several stages of CH B. Box plots are given with horizontal lines for the medians, upper and lower edges indicating the 25th and 75th percentiles, respectively, and bars represent the extremes without including outliers. _ indicates outliers and ● indicates extremities. P values indicate the correlation between the enzyme levels and the progression of liver disease (A-D). aP<0.05, bP<0.01 vs others.
Figure 5.

Distribution of serum enzyme levels in several stages of CH C. Box plots are given with horizontal lines for the medians, upper and lower edges indicating the 25th and 75th percentiles, respectively, and bars represent the extremes without including outliers. _ indicates outliers and ● indicates extremities. P values indicate the correlation between the enzyme levels and the progression of liver disease (A-D). aP<0.05, bP<0.01 vs others.
Pancreatic enzyme levels in HBV and HCV carriers
The enzyme levels at several stages of CH were compared between HBV and HCV carriers. Figure 6 clearly demonstrates that the enzyme levels are not significantly different between the HBV and HCV carriers.
Figure 6.
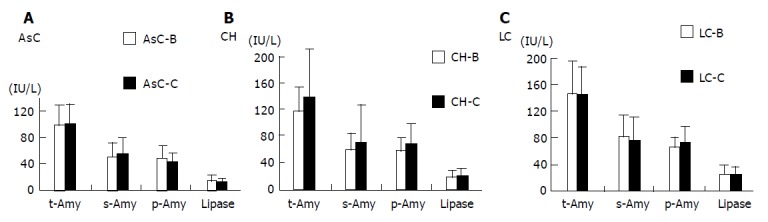
Comparison of serum and tissue enzyme levels between HBV and HCV carriers (A-C).
Pancreatic disease in patients with chronic liver disease
Only 1 of the 202 patients (0.5%) diagnosed with CLD and who underwent imaging for pancreatic disease exhibited demonstrable pancreatic disease. This patient suffered from LC caused by HCV and he had no history of alcohol abuse or biliary disease. His pancreatic enzyme levels were as follows: t-Amy, 223 IU/L; s-Amy, 105 IU/L; p-Amy, 118 IU/L; and lipase, 26 IU/L. The levels of CEA, CA19-9 and DUPAN-2 were as follows: 2.1 ng/mL, 48 U/mL, and 66 U/mL, respectively. An abdominal ultrasonography disclosed dilation in the main pancreatic duct; whereas magnetic resonance cholangiopancreatography revealed an irregularity in the pancreatic ducts. The patient was diagnosed as having chronic pancreatitis.
DISCUSSION
Patients diagnosed with CLD, particularly those with HCV infection, often present with extrahepatic diseases, such as mixed cryoglobulinemia[18-22], glomerulonephritis[23-25], porphyria cutanea tarda[26,27] and oral lichen planus[28-31]. However, there is little information pertaining to associated pancreatic complications. The present study revealed that pancreatic enzymes are elevated in some patients with CLD.
The frequency of elevated serum levels of t-Amy in patients with chronic viral hepatitis is not well known. Tsianos et al[32], reported that hyperamylasemia was found in 6 of 30 (20%) patients with CH. Pezzilli et al[17], showed that 27 of 78 (35%) patients with chronic viral liver diseases were complicated by hyperamylasemia. Our present study has shown that 27 of 202 (13.4%) patients with CLD are complicated by hyperamylasemia. One reason for the discrepancy among studies is the background liver disease. Tsianos’s and Pezzilli’s paper did not include asymptomatic patients. After excluding asymptomatic patients, the frequency of hyperamylasemia in our present study is 16.9%, which is comparable to Tsianos’ results.
The tissue source of the elevated serum t-Amy is not conclusive. Skrha et al[33], suggested that elevated amylase in chronic active hepatitis may be originated from salivary gland. Tsianos et al[32], reported that the origin of elevated amylase was pancreas. Pezzilli et al[17], reported that elevated amylase originated from pancreas in patients with cirrhotic changes. Our results have clearly shown that elevated amylase among patients with CLD originated from both pancreas and extrapancreatic organs (mainly salivary gland). Furthermore, elevated amylase in HBV AsC originated from pancreas, which is discussed below.
Contrary to serum t-Amy levels, there has been only two reports that measured lipase levels in patients with CLD. Pezzilli et al[17], reported that 21% of CLD patients exhibited elevated levels of serum lipase. Yoffe et al[34], reported that 25% of patients with CH C exhibited elevated levels of serum lipase; whereas our data demonstrate a 6.4% frequency of hyperlipasemia. This discrepancy may partly result from the background of the patients. As stated above, our study includes asymptomatic patients. After excluding asymptomatic patients, the frequency of hyperlipasemia in our study was 7.8%. Another reason for the discrepancy may be differences inherent in the quantitative methods, e.g., Pezzilli et al, and Yoffe et al, used a comparative turbidity assay which measures not only pancreatic lipase, but also hepatic lipase, lipoprotein lipase, as well as elastase. Our enzymatic assay, however, is specific for pancreatic lipase. Therefore, we suggest that our data correctly reflect the frequency of hyperlipasemia in CLD patients.
The levels of p-Amy and lipase were higher in patients diagnosed with CLD-B at all stages of liver disease in comparison to those measured in the healthy control subjects suggesting that HBV infection affects pancreatic enzyme secretion. Several studies have reported the detection of HBV DNA in the pancreas of patients diagnosed with hepatitis B[35-37]. Nevertheless, these studies did not demonstrate that the infectivity of HBV into pancreas. Additional studies are necessary to demonstrate whether or not HBV infects the pancreas and, thus, affecting the pancreatic enzyme secretion.
In patients diagnosed with CLD-C, the levels of pancreatic enzymes in individuals with either CH or LC were higher in comparison to healthy control subjects. However, in AsC patients, the enzyme levels were comparable to those of the controls, suggesting that pancreatic enzyme secretion does not change in AsC. Yan et al, have shown by in situ hybridization and immunohistochemistry that HCV infects pancreas acinar cells and pancreatic duct epithelial cells. Furthermore, Yoffe et al, have recently shown that hyperlipasemia in patients with CH C improved during antiviral therapy, showing that hyperlipasemia in patients with chronic active HCV infection may be caused by direct effect of HCV[34]. These results suggest that HCV may infect pancreas. However, whether pancreatic enzymes’ elevation is caused by pancreatic HCV infection itself is uncertain. Our results, showing that frequency of elevated pancreatic enzymes increased with the progression of liver disease, suggest that the elevation in enzyme levels in patients with CLD may result from a delay in their hepatic metabolism[17,38].
Interestingly, the levels of s-Amy in patients diagnosed with CLD-C were significantly higher at all stages of CLD than those measured in control subjects. In contrast, the s-Amy levels in patients with CLD-B were higher than control levels only in patients diagnosed with CH and LC. The difference between CLD-C and CLD-B may be due to HCV infection of the salivary glands[39,40]. The fact that sialadenitis complicates CH C substantiates this hypothesis[41-44].
Imaging studies disclosed pancreatic disease in only 1 of 202 patients diagnosed with CLD in the present study. However, these data neither confirm nor deny that the frequency of pancreatic involvement in chronic viral hepatitis is low. Several studies have shown that pancreatic exocrine function is impaired in some patients with non-alcoholic CLD[16,45]. Furthermore, pancreatic fibrosis may be a contributing factor in patients with non-alcoholic LC[46]. These data suggest that a mild disturbance of pancreatic function may be a consequence of chronic viral hepatitis, despite the fact that imaging studies do not demonstrate significant changes. Subsequent studies on pancreatic exocrine function and histological observations focused on chronic viral liver diseases are required to confirm or deny this hypothesis.
In conclusion, pancreatic enzymes are elevated in a substantial percentage of patients diagnosed with chronic viral hepatitis. The elevation of pancreatic enzymes may reflect an extrahepatic manifestation of chronic viral hepatitis.
Footnotes
Co-first-authors: Yoshiki Katakura
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Parbhoo SP, Welch J, Sherlock S. Acute pancreatitis in patients with fulminant hepatic failure. Gut. 1973;14:428. [PubMed] [Google Scholar]
- 2.Gillespie WJ. Viral hepatitis and acute pancreatitis. J R Coll Surg Edinb. 1973;18:120–122. [PubMed] [Google Scholar]
- 3.Lopez Morante A, Rodriguez de Lope C, San Miguel G, Pons Romero F. Acute pancreatitis in hepatitis A infection. Postgrad Med J. 1986;62:407–408. doi: 10.1136/pgmj.62.727.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadranel JF, Guivarch P, Duvoux C, Desaint B, Florent C, Lévy VG. Acute pancreatitis in benign viral hepatitis A. Gastroenterol Clin Biol. 1987;11:344–345. [PubMed] [Google Scholar]
- 5.Davis TV, Keeffe EB. Acute pancreatitis associated with acute hepatitis A. Am J Gastroenterol. 1992;87:1648–1650. [PubMed] [Google Scholar]
- 6.Amarapurkar DN, Begani MM, Mirchandani K. Acute pancreatitis in hepatitis A infection. Trop Gastroenterol. 1996;17:30–31. [PubMed] [Google Scholar]
- 7.de Oliveira LC, Rezende PB, Ferreira AL, de Freitas AA, de Carvalho AM, Guedes CA, Costa WO. Concurrent acute hepatitis and pancreatitis associated with hepatitis B virus: case report. Pancreas. 1998;16:559–561. doi: 10.1097/00006676-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Sood A, Midha V. Hepatitis A and acute pancreatitis. J Assoc Physicians India. 1999;47:736–737. [PubMed] [Google Scholar]
- 9.Mishra A, Saigal S, Gupta R, Sarin SK. Acute pancreatitis associated with viral hepatitis: a report of six cases with review of literature. Am J Gastroenterol. 1999;94:2292–2295. doi: 10.1111/j.1572-0241.1999.01318.x. [DOI] [PubMed] [Google Scholar]
- 10.Majumder AK, Halder A, Talapatra DS, Bhaduri S. Hepatitis E associated with acute pancreatitis with pseudocyst. J Assoc Physicians India. 1999;47:1207–1208. [PubMed] [Google Scholar]
- 11.Alvares-Da-Silva MR, Francisconi CF, Waechter FL. Acute hepatitis C complicated by pancreatitis: another extrahepatic manifestation of hepatitis C virus? J Viral Hepat. 2000;7:84–86. doi: 10.1046/j.1365-2893.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 12.Maity SG, Ray G. Severe acute pancreatitis in acute hepatitis E. Indian J Gastroenterol. 2002;21:37–38. [PubMed] [Google Scholar]
- 13.Khanna S, Vij JC. Severe acute pancreatitis due to hepatitis A virus infection in a patient of acute viral hepatitis. Trop Gastroenterol. 2003;24:25–26. [PubMed] [Google Scholar]
- 14.Batra Y, Chakravarty S, Bhatt G. Severe acute pancreatitis associated with acute hepatitis A: a case report. Trop Gastroenterol. 2003;24:27–28. [PubMed] [Google Scholar]
- 15.Yuen MF, Chan TM, Hui CK, Chan AO, Ng IO, Lai CL. Acute pancreatitis complicating acute exacerbation of chronic hepatitis B infection carries a poor prognosis. J Viral Hepat. 2001;8:459–464. doi: 10.1046/j.1365-2893.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa T, Kondo T, Shibata T, Kitagawa M, Sakai Y, Sobajima H, Ishiguro H, Nakae Y, Kato K. Exocrine pancreatic function in chronic liver diseases. Am J Gastroenterol. 1991;86:201–204. [PubMed] [Google Scholar]
- 17.Pezzilli R, Andreone P, Morselli-Labate AM, Sama C, Billi P, Cursaro C, Barakat B, Gramenzi A, Fiocchi M, Miglio F, et al. Serum pancreatic enzyme concentrations in chronic viral liver diseases. Dig Dis Sci. 1999;44:350–355. doi: 10.1023/a:1026662719514. [DOI] [PubMed] [Google Scholar]
- 18.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 19.Ferri C, La Civita L, Longombardo G, Greco F, Bombardieri S. Hepatitis C virus and mixed cryoglobulinaemia. Eur J Clin Invest. 1993;23:399–405. doi: 10.1111/j.1365-2362.1993.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 20.Lunel F, Musset L. Mixed cryoglobulinemia and hepatitis C virus infection. Minerva Med. 2001;92:35–42. [PubMed] [Google Scholar]
- 21.Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002;36:978–985. doi: 10.1053/jhep.2002.35620. [DOI] [PubMed] [Google Scholar]
- 22.Okuse C, Yotsuyanagi H, Okazaki T, Yasuda K, Fujioka T, Tomoe M, Hashizume K, Hayashi T, Suzuki M, Iwabuchi S, et al. Detection, using a novel method, of a high prevalence of cryoglobulinemia in persistent hepatitis C virus infection. Hepatol Res. 2003;27:18–22. doi: 10.1016/s1386-6346(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 24.Schifferli JA, French LE, Tissot JD. Hepatitis C virus infection, cryoglobulinemia, and glomerulonephritis. Adv Nephrol Necker Hosp. 1995;24:107–129. [PubMed] [Google Scholar]
- 25.Fornasieri A, D'Amico G. Type II mixed cryoglobulinaemia, hepatitis C virus infection, and glomerulonephritis. Nephrol Dial Transplant. 1996;11 Suppl 4:25–30. doi: 10.1093/ndt/11.supp4.25. [DOI] [PubMed] [Google Scholar]
- 26.Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, Obando J, Di Bisceglie A, Tattrie C, Tortorelli K, LeClair P, Mercurio MG, Lambrecht RW. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27:1661–1669. doi: 10.1002/hep.510270627. [DOI] [PubMed] [Google Scholar]
- 27.Moran MJ, Fontanellas A, Brudieux E, Hombrados I, de Ledinghen V, Couzigou P, de Verneuil H, De Salamanca RE. Hepatic uroporphyrinogen decarboxylase activity in porphyria cutanea tarda patients: the influence of virus C infection. Hepatology. 1998;27:584–589. doi: 10.1002/hep.510270237. [DOI] [PubMed] [Google Scholar]
- 28.Figueiredo LC, Carrilho FJ, de Andrage HF, Migliari DA. Oral lichen planus and hepatitis C virus infection. Oral Dis. 2002;8:42–46. doi: 10.1034/j.1601-0825.2002.10763.x. [DOI] [PubMed] [Google Scholar]
- 29.Imhof M, Popal H, Lee JH, Zeuzem S, Milbradt R. Prevalence of hepatitis C virus antibodies and evaluation of hepatitis C virus genotypes in patients with lichen planus. Dermatology. 1997;195:1–5. doi: 10.1159/000245675. [DOI] [PubMed] [Google Scholar]
- 30.Mignogna MD, Fedele S, Lo Russo L, Ruoppo E, Adamo D, Lo Muzio L. Extrahepatic manifestations of Hepatitis C virus infection: the slowly unraveling picture of oral lichen planus. J Hepatol. 2002;37:412–413. doi: 10.1016/s0168-8278(02)00173-3. [DOI] [PubMed] [Google Scholar]
- 31.Pilli M, Penna A, Zerbini A, Vescovi P, Manfredi M, Negro F, Carrozzo M, Mori C, Giuberti T, Ferrari C, et al. Oral lichen planus pathogenesis: A role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446–1452. doi: 10.1053/jhep.2002.37199. [DOI] [PubMed] [Google Scholar]
- 32.Tsianos EB, Jalali MT, Gowenlock AH, Braganza JM. Serum isoamylases in liver diseases. Hepatogastroenterology. 1986;33:247–249. [PubMed] [Google Scholar]
- 33.Skrha J, Stĕpán J, Hazuka V, Pacovský V. Serum isoamylases in acute and chronic liver disease. Z Gastroenterol. 1984;22:255–258. [PubMed] [Google Scholar]
- 34.Yoffe B, Bagri AS, Tran T, Dural AT, Shtenberg KM, Khaoustov VI. Hyperlipasemia associated with hepatitis C virus. Dig Dis Sci. 2003;48:1648–1653. doi: 10.1023/a:1024744613671. [DOI] [PubMed] [Google Scholar]
- 35.Shimoda T, Shikata T, Karasawa T, Tsukagoshi S, Yoshimura M, Sakurai I. Light microscopic localization of hepatitis B virus antigens in the human pancreas. Possibility of multiplication of hepatitis B virus in the human pancreas. Gastroenterology. 1981;81:998–1005. [PubMed] [Google Scholar]
- 36.Yoshimura M, Sakurai I, Shimoda T, Abe K, Okano T, Shikata T. Detection of HBsAg in the pancreas. Acta Pathol Jpn. 1981;31:711–717. doi: 10.1111/j.1440-1827.1981.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoffe B, Burns DK, Bhatt HS, Combes B. Extrahepatic hepatitis B virus DNA sequences in patients with acute hepatitis B infection. Hepatology. 1990;12:187–192. doi: 10.1002/hep.1840120202. [DOI] [PubMed] [Google Scholar]
- 38.Møller-Peterson J, Dati F. Renal handling of pancreatic lipase. Clin Chem. 1984;30:343–344. [PubMed] [Google Scholar]
- 39.Arrieta JJ, Rodríguez-Iñigo E, Ortiz-Movilla N, Bartolomé J, Pardo M, Manzarbeitia F, Oliva H, Macías DM, Carreño V. In situ detection of hepatitis C virus RNA in salivary glands. Am J Pathol. 2001;158:259–264. doi: 10.1016/S0002-9440(10)63964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toussirot E, Le Huédé G, Mougin C, Balblanc JC, Bettinger D, Wendling D. Presence of hepatitis C virus RNA in the salivary glands of patients with Sjögren's syndrome and hepatitis C virus infection. J Rheumatol. 2002;29:2382–2385. [PubMed] [Google Scholar]
- 41.Haddad J, Deny P, Munz-Gotheil C, Ambrosini JC, Trinchet JC, Pateron D, Mal F, Callard P, Beaugrand M. Lymphocytic sialadenitis of Sjögren's syndrome associated with chronic hepatitis C virus liver disease. Lancet. 1992;339:321–323. doi: 10.1016/0140-6736(92)91645-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirisi M, Scott C, Fabris C, Ferraccioli G, Soardo G, Ricci R, Toniutto P, Avellini C, Vitulli D, Miotti AM. Mild sialoadenitis: a common finding in patients with hepatitis C virus infection. Scand J Gastroenterol. 1994;29:940–942. doi: 10.3109/00365529409094867. [DOI] [PubMed] [Google Scholar]
- 43.Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, Kurokawa K, Matsuura Y, Miyamura T. Sialadenitis histologically resembling Sjogren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci USA. 1997;94:233–236. doi: 10.1073/pnas.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott CA, Avellini C, Desinan L, Pirisi M, Ferraccioli GF, Bardus P, Fabris C, Casatta L, Bartoli E, Beltrami CA. Chronic lymphocytic sialoadenitis in HCV-related chronic liver disease: comparison of Sjögren's syndrome. Histopathology. 1997;30:41–48. doi: 10.1046/j.1365-2559.1997.d01-561.x. [DOI] [PubMed] [Google Scholar]
- 45.Sakai T. Pancreatic exocrine function in patients with chronic liver disease. Kurume Med J. 1998;45:181–185. doi: 10.2739/kurumemedj.45.181. [DOI] [PubMed] [Google Scholar]
- 46.Sobel HJ, Waye JD. Pancreatic Changes in Various Types of Cirrhosis in Alcoholics. Gastroenterology. 1963;45:341–344. [PubMed] [Google Scholar]


