Abstract
AIM: Peroxynitrite (ONOO-) is a powerful oxidant shown to damage membranes. In the present study, the effect of taurine on changes of liver plasma membrane Na+, K+-ATPase induced by ONOO- was investigated.
METHODS: Liver plasma membrane was exposed to ONOO- with or without taurine. Na+, K+-ATPase activity and lipid peroxidation as thiobarbituric acid reactive substances (TBARS) levels were measured.
RESULTS: Different concentrations of ONOO- (100, 200, 500, and 1000 μmol/L) were found to decrease liver plasma membrane Na+, K+-ATPase activity significantly. The depletion of enzyme activity was not concentration dependent. Effects of different concentrations of taurine on liver plasma membrane Na+, K+-ATPase activity were also measured. Taurine did not cause any increase in enzyme activity. When plasma membranes were treated with 200 μmol/L ONOO- with different concentrations of taurine, a restoring effect of taurine on enzyme activity was observed. TBARS levels were also measured and taurine was found to decrease the elevated values.
CONCLUSION: Taurine is observed to act as an antioxidant of ONOO- to decrease lipid peroxidation and thus affect liver plasma membrane Na+, K+-ATPase by restoring its activity.
Keywords: Na+, K+-ATPase; Taurine; Peroxynitrite
INTRODUCTION
Taurine (2-aminoethanesulfonic acid) is present as a free amino acid in mammalian tissues like liver, heart, brain, and leukocytes[1]. Although it does not take part in protein structure, it has a pivotal role in maintenance of various cellular functions like osmoregulator, neuromodulator, and membrane stabilizer[2]. Besides its hypolipidemic and antiatherosclerotic effects, it is proved to be an effective antioxidant[3-6].
ONOO- is a powerful oxidant that can be formed by the reaction of superoxide anion and nitric oxide in significant amount in vivo. It has been shown to be associated with both beneficial and harmful effects. Its contribution to the host-defense response to bacterial invasion is known to be due to its production by neutrophils and macrophages. On the other hand, the role of ONOO- in ischemia-reperfusion has been implicated to cause myocardial dysfunction and infarction[7]. ONOO- can react with DNA leading to mutations and strand breaks. It can cause oxidation of thiol groups, proteins and low molecular antioxidants and induce lipid peroxidation[8]. It is also known to nitrate macromolecules to contribute to various pathophysiological conditions.
Na+, K+-ATPase is a plasma membrane-bound enzyme that provides the necessary electrochemical gradients of Na+ and K+ to maintain the cell volume and thus it plays a crucial role in homeostasis[9]. It functions by exporting intracellular Na+ and importing extracellular K+ across the plasma membrane to provide energy for membrane transport of various metabolites taking part in special cell functions. Therefore, dysfunction of the enzyme may lead to severe consequences.
Na+, K+-ATPase has been known to be a good target of free radical induced membrane damage[10]. Lipid peroxidation induced by free radicals has been shown to inactivate the enzyme by particularly modifying the active site for binding of the substrates[11]. ONOO- has also been known to disrupt membrane fluidity and decrease Na+, K+-ATPase activity by oxidation of its thiol groups and probably partially due to nitration of its aromatic amino acids[12,13].
In this study we investigated the effects of taurine on changes of liver plasma membrane Na+, K+-ATPase induced by ONOO-.
MATERIALS AND METHODS
Materials
Bovine liver obtained from slaughterhouse from freshly slaughtered animal was rapidly rinsed with ice-cold physiological saline and frozen in liquid nitrogen. It was stored at -80 °C. All reagents used were analytically pure.
Liver plasma membrane preparation
Frozen liver portions were homogenized in ice-cold buffer containing 8% saccharose, 0.1 mmol/L phenylmethane sulfonyl fluoride, 1 mmol/L EDTA and 30 mmol/L imidazole-HCl, pH 7.4[14]. The homogenate was centrifuged at 120 r/min for 5 min, 6800 r/min for 15 min and 48000 g for 30 min using Sorvall centrifuge with AH-650 rotor. The obtained pellet was resuspended in 8% saccharose and 30 mmol/L imidazole-HCl, pH 7.4 and stored at -80 °C until use.
ONOO- preparation
Five milliliters of 0.6 mol/L NaNO2 and 5 mL 0.6 mol/L H2O2 in 0.7 mol/L HCl were filled in two syringes separately[15]. They were immersed in ice for about 30 min. A beaker containing 5 mL 1.2 mol/L NaOH solution with a magnetic stirrer was also cooled on ice. The syringes, after being cooled, were held with a T-piece above the NaOH solution that was in ice. Both plungers were rapidly pressed down at the same time. Excess H2O2 was removed by using granular MnO2 (2 g) at 4 °C. Concentration of ONOO- was determined by measuring the absorbance at 302 nm using the extinction coefficient of 1670/M×cm. ONOO- solution was kept at -80 °C.
Preparation of decomposed ONOO-
Samples of the ONOO- solution were allowed to decompose overnight in imidazole-HCl buffer to control the effect of decomposition products, nitrite and nitrate, and H2O2.
Treatment of liver plasma membrane with ONOO- and taurine
One hundred microliters of plasma membrane samples (30 μg protein) were incubated with 5 μL of 100, 200, 500, and 1000 μmol/L ONOO- solutions at room temperature. The incubations were done with decomposed ONOO- as well. Following incubations, membrane Na+,K+-ATPase activity and thiobarbituric acid reactive substances (TBARS) levels were assayed.
One hundred microliters of plasma membrane samples (30 μg protein) were incubated with taurine (1, 2, and 5 mmol/L) and 200 μmol/L ONOO- (5 μL) plus 10 μL of taurine (1, 2, and 5 mmol/L). Following incubations, membrane Na+, K+-ATPase activity and TBARS levels were measured.
Assay of Na+, K+-ATPase activity
Enzymatic activity was measured in triplicate by the inorganic phosphate (Pi) released from ATP in the presence or absence of 1 mmol/L ouabain[12]. Membrane preparations (20 μg) were added to the medium containing 150 mmol/L NaCl, 5 mmol/L KCl, 2.5 mmol/L MgCl2 and 20 mmol/L imidazole-HCl buffer, pH 7.4. After 8 min of preincubation at 37 °C, 2.5 mmol/L ATPNa2 was added to make the final volume of 0.5 mL and to start the reaction. The samples were incubated at 37 °C for 30 min. The reaction was stopped by the addition of 100 μL of 35% ice-cold trichloroacetic acid. The amount of liberated Pi was measured in the supernatant by using FeSO4-ammonium molybdate solution. The mixtures were kept for 5 min in the dark and the absorbances were measured at 700 nm.
Determination of lipid peroxidation
The level of lipid peroxidation was assessed by the determination of TBARS[16]. Following incubation with ONOO-, membrane samples were reacted with TBA to yield a pink colored product. Absorbances were measured at 532 nm and the amount of TBARS was calculated by using the extinction coefficient of 1.56×105/M×cm.
Protein determinations were done by the method of Lowry et al[17], using bovine serum albumin as a standard.
Statistical analysis
Ten experiments were performed separately. All results were expressed as mean±SD. Statistically significant differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test (THS test).
RESULTS
Effect of ONOO- on liver plasma membrane Na+, K+-ATPase
When plasma membrane was treated with 100, 200, 500, and 1000 μmol/L ONOO- solutions, significant depletion of enzyme activity was observed with all ONOO- concentrations (all P<0.05). However, ONOO- with a concentration above 200 μmol/L produced no additional decreasing effect (Figure 1). Plasma membrane samples were also incubated with decomposed ONOO-. This incubation was made to eliminate the effect of decayed products and contaminants. Decomposed ONOO- solutions were also found to cause significant decreases in enzyme activity (P<0.05).
Figure 1.
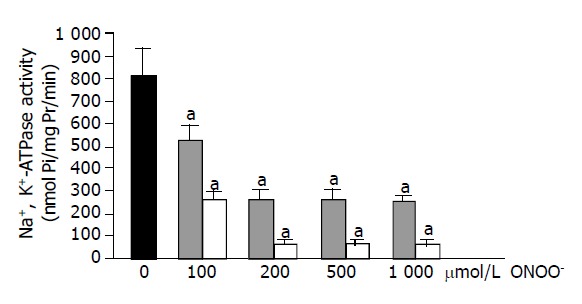
Effects of decomposed ONOO- and ONOO- on liver plasma membrane Na+, K+-ATPase activity. Values are mean±SD, n = 10. Values not sharing a common superscript letter are significantly different by ANOVA (THS test), aP<0.05 vs controls. Control (black), decomposed ONOO- (gray lines), ONOO- (white lines).
Effect of taurine on liver plasma membrane Na+, K+-ATPase
The effect of taurine on Na+, K+-ATPase is shown in Table 1. After incubation of liver plasma membrane with 1, 2, and 5 mmol/L taurine, Na+, K+-ATPase activities were unchanged.
Table 1.
Effect of taurine on liver plasma membrane Na+, K+-ATPase activity (values are mean±SD, n = 10).
| Groups | Na+, K+-ATPase activity (nmol Pi/mg Pr/min) |
| Control | 815.64±116.4 |
| Taurine (1 mmol/L) | 798.60±31.52 |
| Taurine (2 mmol/L) | 806.20±61.39 |
| Taurine (5 mmol/L) | 781.06±77.62 |
Effect of taurine on ONOO- induced inhibition of liver plasma membrane Na+, K+-ATPase
Since ONOO- at concentration >200 μmol/L has not caused additional depletion in enzyme activity, 200 μmol/L was chosen to observe the effect of different concentrations of taurine on the change in enzyme activity (Table 2). With all chosen taurine concentrations, significant activity increases from the depleted values by decomposed ONOO- and ONOO- were observed (all P<0.05).
Table 2.
Effects of 200 μmol/L decomposed ONOO- and ONOO- with and without taurine on liver plasma membrane Na+, K+-ATPase activity (values are mean±SD, n = 10).
| Groups | Na+,K+-ATPase activity (nmol Pi/mg Pr/min) |
| Decomposed ONOO- (200 mmol/L) | 261.86±38.32a |
| Decomposed ONOO- (200 mmol/L) +taurine (1 mmol/L) | 460.24±51.28a |
| Decomposed ONOO- (200 mmol/L) +taurine (2 mmol/L) | 452.07±50.92a |
| Decomposed ONOO- (200 mmol/L) +taurine (5 mmol/L) | 435.61±71.44a |
| ONOO- (200 mmol/L) | 62.36±18.77a |
| ONOO- (200 mmol/L)+taurine (1 mmol/L) | 115.37±27.19a |
| ONOO- (200 mmol/L)+taurine (2 mmol/L) | 114.34±27.34a |
| ONOO- (200 mmol/L)+taurine (5 mmol/L) | 113.86±16.32a |
Values not sharing a common superscript letter are significantly different by ANOVA (THS test),
P<0.05 vs others.
Effect of taurine on liver plasma membrane lipid peroxidation
TBARS levels were measured when liver plasma membrane was incubated with 200 μmol/L decomposed ONOO- and 200 μmol/L ONOO- with or without taurine (1, 2, and 5 mmol/L) (Table 3). The elevation of lipid peroxide levels was decreased with taurine in a concentration-dependent manner (P<0.05).
Table 3.
Effects of taurine, 200 μmol/L decomposed ONOO- and ONOO- with and without taurine on liver plasma membrane. TBARS levels (values are mean±SD, n = 10).
| Groups | TBARS (nmol MDA/mg Pr) |
| Control | 4.84±0.45a |
| Taurine (1 mmol/L) | 4.80±0.43a |
| Taurine (2 mmol/L) | 4.73±0.39a |
| Taurine (5 mmol/L) | 4.31±0.41a |
| Decomposed ONOO- 200 mmol/L | 6.10±0.80a |
| ONOO- (200 mmol/L) | 25.62±1.08a |
| ONOO- (200 mmol/L)+taurine (1 mmol/L) | 19.80±0.78a |
| ONOO- (200 mmol/L)+taurine (2 mmol/L) | 17.52±0.73a |
| ONOO- (200 mmol/L)+taurine (5 mmol/L) | 14.80±0.85a |
Values not sharing a common superscript letter are significantly different by ANOVA (THS test),
P<0.05 vs others.
DISCUSSION
In this study, ONOO- at 200 μmol/L was found to significantly decrease Na+, K+-ATPase activity, when liver plasma membrane was treated with different concentrations of ONOO-. Increasing ONOO- concentration did not appear to cause an additional decreasing effect. In our previous works, human sperm Na+, K+-ATPase located in plasma membrane was also found to be significantly decreased when treated with 100 μmol/L ONOO-[13], with increasing lipid peroxide levels and decreasing total sulfhydryl content[18]. Indeed, ONOO- releasing agents like 3-morpholinosy-dnonimine (SIN-1) have been found to inhibit Na+,K+-ATPase activity by interacting with a sulfhydryl group at the active site[19]. Incubation of liver plasma membrane with SIN-1 reduced Na+, K+-ATPase activity and it was suggested that ONOO- caused inhibition both by oxidizing thiol groups and in part by decreasing membrane fluidity[12].
Rat erythrocyte membrane Na+, K+-ATPase was also found to be inhibited by ONOO-, which was considered to be compatible with oxidation of thiol groups either directly by being involved in ATP binding or of those located outside the active site being important for enzyme activity[20].
Taurine has been found to restore depletion of membrane Na+, K+-ATPase activity due to ozone exposure or cholesterol enrichment, thus considered both as an antioxidant to prevent lipid peroxidation and as a membrane stabilizer to maintain the environment for Na+, K+-ATPase to function properly[21]. Glucose-induced lipid peroxidation and protein glycosylation have been lowered and erythrocyte Na+, K+-ATPase and Ca2+-ATPase activities preserved by taurine treatment, implicating the inhibition of development of diabetic complications[22]. Chronic taurine supplementation has also been proved to decrease lipid peroxidation and preserve retinal Na+, K+-ATPase activity in diabetic rats[23].
The scavenging activity of taurine against superoxide anion and peroxides is under debate[24-27]. There are studies suggesting the absence of a direct reaction of taurine and oxygen-derived radicals, as well as taurine protection of hepatocytes against H2O2-stimulated damage[24]. While protection provided by taurine against reactive oxygen species has been assumed as an indirect action, its reaction with hypochlorous acid is clearly shown[28]. In the study of Redmond et al[29], however, treatment of cultured hepatocytes with 4 mmol/L taurine has been found to reduce apoptosis and necrosis by inhibiting nitric oxide and oxy radicals due to suppression of nitric oxide synthase mRNA. Likewise in another study, it has been demonstrated that 1 mmol/L taurine could not reduce ONOO- formation by SIN-1 treatment of cerebellar granular cells[30]. Indeed in the study of Mehta and Dawson, taurine at concentrations above 30 mmol/L has been shown to be only a modest scavenger of ONOO- produced from SIN-1[31].
Our study suggests that in vitro taurine treatment can protect liver plasma membrane against oxidative damage caused by ONOO-, by acting as an antioxidant and thus contribute to restoring of Na+, K+-ATPase activity.
Footnotes
Supported by the Istanbul University Research Foundation, No. BYP-247/20082003
References
- 1.Schuller-Levis GB, Park E. Taurine: new implications for an old amino acid. FEMS Microbiol Lett. 2003;226:195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 2.Hagar HH. The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett. 2004;151:335–343. doi: 10.1016/j.toxlet.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Balkan J, Kanbağli O, Hatipoğlu A, Kücük M, Cevikbaş U, Aykaç-Toker G, Uysal M. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci Biotechnol Biochem. 2002;66:1755–1758. doi: 10.1271/bbb.66.1755. [DOI] [PubMed] [Google Scholar]
- 4.Balkan J, Oztezcan S, Aykaç-Toker G, Uysal M. Effects of added dietary taurine on erythrocyte lipids and oxidative stress in rabbits fed a high cholesterol diet. Biosci Biotechnol Biochem. 2002;66:2701–2705. doi: 10.1271/bbb.66.2701. [DOI] [PubMed] [Google Scholar]
- 5.Balkan J, Doğru-Abbasoğlu S, Kanbağli O, Cevikbaş U, Aykaç-Toker G, Uysal M. Taurine has a protective effect against thioacetamide-induced liver cirrhosis by decreasing oxidative stress. Hum Exp Toxicol. 2001;20:251–254. doi: 10.1191/096032701678227758. [DOI] [PubMed] [Google Scholar]
- 6.Balkan J, Kanbağli O, Aykaç-Toker G, Uysal M. Taurine treatment reduces hepatic lipids and oxidative stress in chronically ethanol-treated rats. Biol Pharm Bull. 2002;25:1231–1233. doi: 10.1248/bpb.25.1231. [DOI] [PubMed] [Google Scholar]
- 7.Vinten-Johansen J. Physiological effects of peroxynitrite: potential products of the environment. Circ Res. 2000;87:170–172. doi: 10.1161/01.res.87.3.170. [DOI] [PubMed] [Google Scholar]
- 8.Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 9.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelièvre L, Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- 10.Mense M, Stark G, Apell HJ. Effects of free radicals on partial reactions of the Na,K-ATPase. J Membr Biol. 1997;156:63–71. doi: 10.1007/s002329900188. [DOI] [PubMed] [Google Scholar]
- 11.Mishra OP, Delivoria-Papadopoulos M, Cahillane G, Wagerle LC. Lipid peroxidation as the mechanism of modification of the affinity of the Na+, K+-ATPase active sites for ATP, K+, Na+, and strophanthidin in vitro. Neurochem Res. 1989;14:845–851. doi: 10.1007/BF00964813. [DOI] [PubMed] [Google Scholar]
- 12.Muriel P, Sandoval G. Nitric oxide and peroxynitrite anion modulate liver plasma membrane fluidity and Na(+)/K(+)-ATPase activity. Nitric Oxide. 2000;4:333–342. doi: 10.1006/niox.2000.0285. [DOI] [PubMed] [Google Scholar]
- 13.Koçak-Toker N, Aktan G, Aykaç-Toker G. The role of Na,K-ATPase in human sperm motility. Int J Androl. 2002;25:180–185. doi: 10.1046/j.1365-2605.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 14.Sennoune S, Gerbi A, Duran MJ, Benkoël L, Pierre S, Lambert R, Dodero F, Chamlian A, Vague P, Maixent JM. A quantitative immunocytochemical study of Na+,K+-ATPase in rat hepatocytes after STZ-induced diabetes and dietary fish oil supplementation. J Histochem Cytochem. 1999;47:809–816. doi: 10.1177/002215549904700610. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Oztezcan S, Türkoğlu UM, Kervancioğlu E, Koçak T, Koçak-Toker N, Aykaç-Toker G. In vitro effects of peroxynitrite on human spermatozoa. Andrologia. 1999;31:195–198. doi: 10.1046/j.1439-0272.1999.00279.x. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Kamata Y, Irifune M, Nishikawa T. Inhibition of purified (Na+,K+)-ATPase activity from porcine cerebral cortex by NO generating drugs. Brain Res. 1995;704:117–120. doi: 10.1016/0006-8993(95)01165-x. [DOI] [PubMed] [Google Scholar]
- 20.Muriel P, Castañeda G, Ortega M, Noël F. Insights into the mechanism of erythrocyte Na+/K+-ATPase inhibition by nitric oxide and peroxynitrite anion. J Appl Toxicol. 2003;23:275–278. doi: 10.1002/jat.922. [DOI] [PubMed] [Google Scholar]
- 21.Qi B, Yamagami T, Naruse Y, Sokejima S, Kagamimori S. Effects of taurine on depletion of erythrocyte membrane Na-K ATPase activity due to ozone exposure or cholesterol enrichment. J Nutr Sci Vitaminol (Tokyo) 1995;41:627–634. doi: 10.3177/jnsv.41.627. [DOI] [PubMed] [Google Scholar]
- 22.Nandhini TA, Anuradha CV. Inhibition of lipid peroxidation, protein glycation and elevation of membrane ion pump activity by taurine in RBC exposed to high glucose. Clin Chim Acta. 2003;336:129–135. doi: 10.1016/s0009-8981(03)00337-1. [DOI] [PubMed] [Google Scholar]
- 23.Di Leo MA, Santini SA, Cercone S, Lepore D, Gentiloni Silveri N, Caputo S, Greco AV, Giardina B, Franconi F, Ghirlanda G. Chronic taurine supplementation ameliorates oxidative stress and Na+ K+ ATPase impairment in the retina of diabetic rats. Amino Acids. 2002;23:401–406. doi: 10.1007/s00726-002-0202-2. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is taurine cytoprotective? Adv Exp Med Biol. 2003;526:307–321. doi: 10.1007/978-1-4615-0077-3_39. [DOI] [PubMed] [Google Scholar]
- 25.Pokhrel PK, Lau-Cam CA. Protection by taurine and structurally related sulfur-containing compounds against erythrocyte membrane damage by hydrogen peroxide. Adv Exp Med Biol. 2000;483:411–429. doi: 10.1007/0-306-46838-7_47. [DOI] [PubMed] [Google Scholar]
- 26.Koyama I, Nakamura T, Ogasawara M, Nemoto M, Yoshida T. The protective effect of taurine on the biomembrane against damage produced by the oxygen radical. Adv Exp Med Biol. 1992;315:355–359. doi: 10.1007/978-1-4615-3436-5_41. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Ogasawara M, Koyama I, Nemoto M, Yoshida T. The protective effect of taurine on the biomembrane against damage produced by oxygen radicals. Biol Pharm Bull. 1993;16:970–972. doi: 10.1248/bpb.16.970. [DOI] [PubMed] [Google Scholar]
- 28.Nakamori K, Koyama I, Nakamura T, Yoshida T, Umeda M, Inoue K. Effectiveness of taurine in protecting biomembrane against oxidant. Chem Pharm Bull (Tokyo) 1990;38:3116–3119. doi: 10.1248/cpb.38.3116. [DOI] [PubMed] [Google Scholar]
- 29.Redmond HP, Wang JH, Bouchier-Hayes D. Taurine attenuates nitric oxide- and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg. 1996;131:1280–1287; discussion 1287-1288. doi: 10.1001/archsurg.1996.01430240034004. [DOI] [PubMed] [Google Scholar]
- 30.Boldyrev AA, Johnson P, Wei Y, Tan Y, Carpenter DO. Carnosine and taurine protect rat cerebellar granular cells from free radical damage. Neurosci Lett. 1999;263:169–172. doi: 10.1016/s0304-3940(99)00150-0. [DOI] [PubMed] [Google Scholar]
- 31.Mehta TR, Dawson R. Taurine is a weak scavenger of peroxynitrite and does not attenuate sodium nitroprusside toxicity to cells in culture. Amino Acids. 2001;20:419–433. doi: 10.1007/s007260170038. [DOI] [PubMed] [Google Scholar]


