Abstract
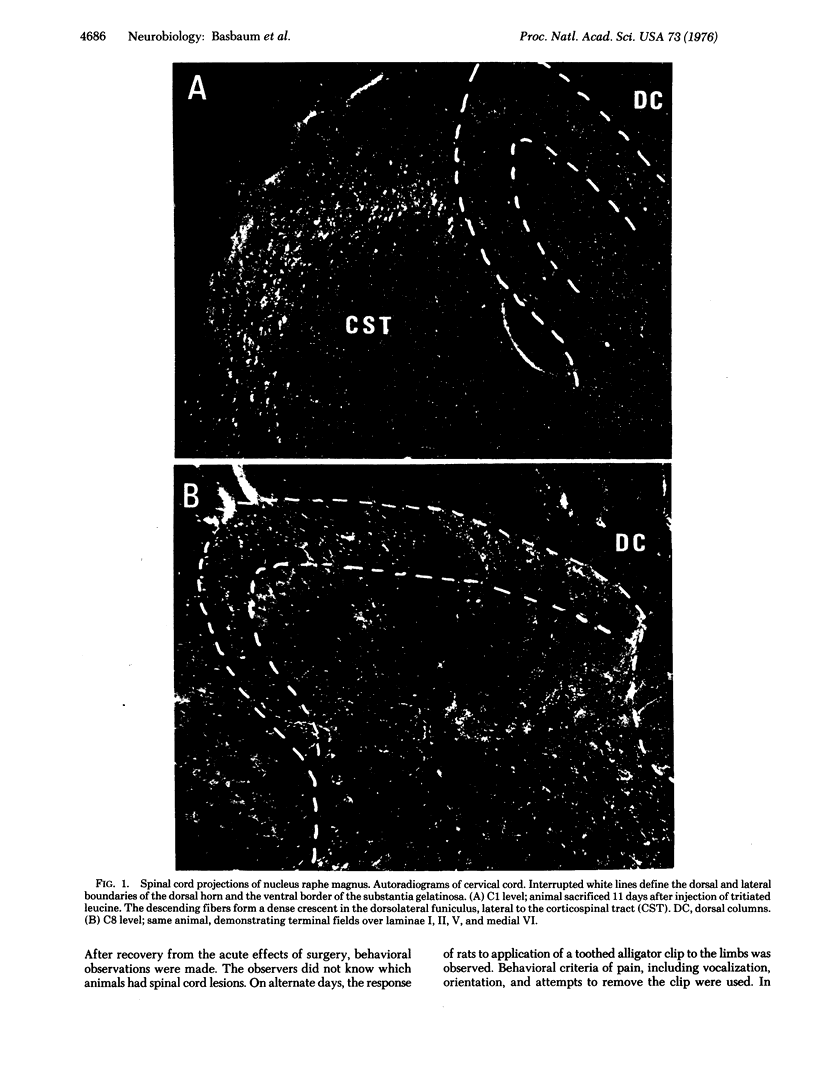
Neurons in ventromedial medulla, including the nucleus raphe magnus, project to trigeminal nucleus caudalis and, via the dorsolateral funiculus, to spinal dorsal horn. The terminals of this descending system are in loci containing cells responsive to noxious stimuli. Electrical stimulation of nucleus raphe magnus selectively inhibits spinal dorsal horn neurons that respond to noxious stimuli. These neurons are located near the anatomically demonstrated terminals of this descending system. Dorsolateral funiculus lesions block this descending inhibition of spinal neurons as well as the analgesic action of morphine. This evidence supports the hypothesis that this neuron population mediates the analgesia produced by opiates and electrical stimulation of certain diencephalic and brainstem sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. E. Naloxone reversal of analgesia produced by brain stimulation in the human. Pain. 1976 Jun;2(2):161–166. [PubMed] [Google Scholar]
- Akil H., Mayer D. J. Antagonism of stimulation-produced analgesia by p-CPA, a serotonin synthesis inhibitor. Brain Res. 1972 Sep 29;44(2):692–697. doi: 10.1016/0006-8993(72)90338-1. [DOI] [PubMed] [Google Scholar]
- Akil H., Mayer D. J., Liebeskind J. C. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976 Mar 5;191(4230):961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- Bars D. L., Menetrey D., Conseiller C., Besson J. M. Comparaison chez le chat spinal et le chat décérébré, des effets de la morphine sur les activités des interneurones de type V de la corne dorsale de la moelle. C R Acad Sci Hebd Seances Acad Sci D. 1974 Oct 14;279(16):1369–1371. [PubMed] [Google Scholar]
- Basbaum A. I. Conduction of the effects of noxious stimulation by short-fiber multisynaptic systems of the spinal cord in the rat. Exp Neurol. 1973 Sep;40(3):699–716. doi: 10.1016/0014-4886(73)90105-2. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A., Ryall R. W. Is the tonic decerebrate inhibition of reflex paths mediated by monoaminergic pathways? Acta Physiol Scand. 1968 Jan-Feb;72(1):123–133. doi: 10.1111/j.1748-1716.1968.tb03834.x. [DOI] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Fields H. L., Wagner G. M., Anderson S. D. Some properties of spinal neurons projecting to the medial brain-stem reticular formation. Exp Neurol. 1975 Apr;47(1):118–134. doi: 10.1016/0014-4886(75)90241-1. [DOI] [PubMed] [Google Scholar]
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Res. 1975 May 2;88(2):295–308. doi: 10.1016/0006-8993(75)90391-1. [DOI] [PubMed] [Google Scholar]
- Jacquet Y. F., Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976 Feb 27;103(3):501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- KENNARD M. A. The course of ascending fibers in the spinal cord of the cat essential to the recognition of painful stimuli. J Comp Neurol. 1954 Jun;100(3):511–524. doi: 10.1002/cne.901000304. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Liebeskind J. C. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 1974 Mar 15;68(1):73–93. doi: 10.1016/0006-8993(74)90534-4. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Wolfle T. L., Akil H., Carder B., Liebeskind J. C. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971 Dec 24;174(4016):1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- NYBERG-HANSEN R. SITES AND MODE OF TERMINATION OF RETICULO-SPINAL FIBERS IN THE CAT. AN EXPERIMENTAL STUDY WITH SILVER IMPREGNATION METHODS. J Comp Neurol. 1965 Feb;124:71–99. doi: 10.1002/cne.901240107. [DOI] [PubMed] [Google Scholar]
- Oliveras J. L., Besson J. M., Guilbaud G., Liebeskind J. C. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974 Apr 30;20(1):32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- Oliveras J. L., Redjemi F., Guilbaud G., Besson J. M. Analgesia induced by electrical stimulation of the inferior centralis nucleus of the raphe in the cat. Pain. 1975 Jun;1(2):139–145. doi: 10.1016/0304-3959(75)90098-6. [DOI] [PubMed] [Google Scholar]
- Pert A., Yaksh T. Sites of morphine induced analgesia in the primate brain: relation to pain pathways. Brain Res. 1974 Nov 8;80(1):135–140. doi: 10.1016/0006-8993(74)90731-8. [DOI] [PubMed] [Google Scholar]
- Proudfit H. K., Anderson E. G. Morphine analgesia: blockade by raphe magnus lesions. Brain Res. 1975 Nov 21;98(3):612–618. doi: 10.1016/0006-8993(75)90380-7. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Reynolds D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969 Apr 25;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Sato M., Takagi H. Enhancement by morphine of the central descending inhibitory influence on spinal sensory transmission. Eur J Pharmacol. 1971;14(1):60–65. doi: 10.1016/0014-2999(71)90122-1. [DOI] [PubMed] [Google Scholar]
- Simantov R., Kuhar M. J., Pasternak G. W., Snyder S. H. The regional distribution of a morphine-like factors enkephalin in monkey brain. Brain Res. 1976 Apr 16;106(1):189–197. doi: 10.1016/0006-8993(76)90086-x. [DOI] [PubMed] [Google Scholar]
- TABER E., BRODAL A., WALBERG F. The raphe nuclei of the brain stem in the cat. I. Normal topography and cytoarchitecture and general discussion. J Comp Neurol. 1960 Apr;114:161–187. doi: 10.1002/cne.901140205. [DOI] [PubMed] [Google Scholar]
- Vogt M. The effect of lowering the 5-hydroxytryptamine content of the rat spinal cord on analgesia produced by morphine. J Physiol. 1974 Jan;236(2):483–498. doi: 10.1113/jphysiol.1974.sp010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L., DuChateau J. C., Rudy T. A. Antagonism by methysergide and cinanserin of the antinociceptive action of morphine administered into the periaqueductal gray. Brain Res. 1976 Mar 12;104(2):367–372. doi: 10.1016/0006-8993(76)90634-x. [DOI] [PubMed] [Google Scholar]