Abstract
AIM: To determine if primary murine colonic epithelial cells (CEC) respond to commensal bacteria and discriminate between different types of bacteria.
METHODS: A novel CEC: bacteria co-culture system was used to compare the ability of the colonic commensal bacteria, Bacteroides ovatus, E. coli (SLF) and Lactobacillus rhamnosus (LGG) to modulate production of different cytokines (n = 15) by primary CEC. Antibody staining and flow cytometry were used to investigate Toll-like receptor (TLR) expression by CEC directly ex vivo and TLR responsiveness was determined by examining the ability of TLR ligands to influence CEC cytokine production.
RESULTS: Primary CEC constitutively expressed functional TLR2 and TLR4. Cultured in complete medium alone, CEC secreted IL-6, MCP-1 and IP-10 the levels of which were significantly increased upon addition of the TLR ligands peptidoglycan (PGN) and lipopolysaccharide (LPS). Exposure to the commensal bacteria induced or up-regulated different patterns of cytokine production and secretion. E. coli induced production of MIP-1α/β and β defensin3 whereas B. ovatus and L. rhamnosus exclusively induced MCP-1 and MIP-2α expression, respectively. TNFα, RANTES and MEC were induced or up-regulated in response to some but not all of the bacteria whereas ENA78 and IP-10 were up-regulated in response to all bacteria. Evidence of bacterial interference and suppression of cytokine production was obtained from mixed bacterial: CEC co-cultures. Probiotic LGG suppressed E. coli- and B. ovatus-induced cytokine mRNA accumulation and protein secretion.
CONCLUSION: These observations demonstrate the ability of primary CEC to respond to and discriminate between different strains of commensal bacteria and identify a mechanism by which probiotic bacteria (LGG) may exert anti-inflammatory effects in vivo.
Keywords: Epithelial cells, Colon, Commensal bacteria, Cytokines, Chemokines
INTRODUCTION
Intestinal mucosal surfaces are in continuous contact with heterogeneous populations of commensal microorganisms, which collectively make up the intestinal microbiota. Historically, the barrier function of intestinal epithelial cells (IEC) has been considered to be important in preventing the interaction of non-invasive microbes and protein antigens from making contact with, and activating the mucosal immune system and maintaining immune tolerance[1]. Indeed, breach of the epithelial barrier is a feature of chronic inflammatory disorders such as inflammatory bowel disease (IBD), and the virulence of enteric pathogens such as enterohemorrhagic E. coli, is in large part attributable to their ability to disrupt the epithelial barrier[2]. More recent studies suggest that IEC play a more active role in preventing or limiting host responses to harmless self-antigens. Studies using immortalized lines have shown that IEC selectively lack expression of pattern recognition receptors (PRRs) such as Toll-like receptors (TLR), or PRR-associated signal transduction complexes that mediate recognition of signature molecules of microorganisms (microbe-associated molecular patterns; MAMPs) including lipopolysaccharide (LPS) and peptidoglycan (PGN)[3]. The results of studies of TLR expression by IEC in vivo are however, ambiguous with some providing no evidence of TLR 2 or TLR4 expression in the normal intestinal mucosa whereas others have detected low levels of expression[4-6].
The host response to commensal bacteria in vivo has been investigated by profiling ileal tissue mRNA of germfree mice following conventionalization with commensal bacteria[7]. Although this study did not include an analysis of host immune response genes, it demonstrated that commensal bacteria could modulate expression of genes in ileal tissue and laser-capture microdissected epithelial cells that are involved in mucosal barrier integrity, xenobiotic metabolism, nutrient absorption, angiogenesis and postnatal intestinal maturation. Evidence that the host can distinguish between different commensal bacteria has been obtained by comparing the levels of mRNA encoding proteins involved in toxin metabolism (mdr1a), barrier function (sprr2a) and lipid metabolism (colipase), which showed quantitative differences in levels of ileal tissue mRNA in mice colonized by three different commensal bacteria. Since the bulk of these studies were carried out on intact ileal tissue samples, it was not possible to exclude non-epithelial cell contributions to the mRNA profile, and any indirect effects of other mucosal cells on epithelial cell responses to bacterial challenge in vivo. One way in which the molecular nature of IEC responses to commensal bacteria has been addressed is through the use of defined intestinal epithelial cell lines.
Studies using IEC lines have suggested that commensal bacteria may “programme” IEC to prevent or down-modulate pro-inflammatory responses to non-pathogenic bacteria by interfering with TLR expression[8] or NF-κB activation[9]. The involvement or requirement of NF-κB activation in IEC inflammatory responses is however questioned by in vivo studies, demonstrating that this transcription factor is primarily involved in IEC homeostasis and that NF-κB activation is associated with suppression of CEC proliferation[10]. Other studies using IEC lines have implied that there may be qualitative and/or quantitative differences in the response of IEC to harmless versus harmful microbes[9,11]. Thus, it is not clear if or how IEC normally respond to commensal bacteria. To address this issue we have used a novel CEC:bacteria co-culture system and three representative strains of commensal bacteria including a probiotic bacterium, to determine what effect non-pathogenic bacteria have on CEC cytokine production. Our results provide evidence for the ability of primary CEC to respond to and distinguish between different types of commensal bacteria.
MATERIALS AND METHODS
Animals
Specific pathogen free (SPF) C57BL/6 mice (Harlan, UK) were housed under SPF conditions at The University of Leeds and used between four and six weeks of age.
CEC isolation
CEC isolation, culture and characterization were extensively described previously[12,13]. CEC viability was routinely >95% and comprised >98% cytokeratin+ cells as determined by antibody staining and flow cytometry. CEC preparations with <90% viability, or >10% cytokeratin- cells were discarded. Cells were initially cultured for up to 72 h in complete medium MEM (Sigma, Poole, Dorset, UK), 20% heat-inactivated fetal bovine serum (Harlan, UK), 2% Luria broth and 2 mmol/L L-glutamine to obtain a semi-confluent monolayer of polarized CEC. The medium was changed after 24 h to remove dead and non-adherent cells. These culture conditions were not ideal for hematopoietic cell growth and the absence of contaminating hematopoietic (CD45+) cells was confirmed by antibody staining, flow cytometry, and RT-PCR using primers specific for CD45. Contamination by mesenchymal cells was evaluated by assaying the presence of vimentin mRNA by RT-PCR.
CEC:bacteria co-culture
CEC were cultured for 1-5 h at 37 °C in 50 mL/L CO2 in complete media alone or in media containing three representative members of the murine and human colonic microbiota[14]. A human colonic isolate of Bacteroides ovatus (V975) was provided by Dr. T Whitehead (Peoria, IL), a murine intestinal isolate of E. coli (slow lactose fermenting; SLF) was provided by Dr. J Cebra (Philadelphia, PA), and Lactobacillus rhamnosus (Lactobacillus GG; LGG) was originally isolated from human feces (ATCC, catalog number 53103). CEC and bacteria were cultured at a ratio of 10 bacteria: 1 CEC and the number of bacteria and CEC were determined at the beginning and end of the culture. In some experiments viable or non-viable (heat killed) L. rhamnosus was added to an equal number of E. coli or B. ovatus immediately prior to culture with CEC such that the total number of bacteria was the same as that in single bacteria:CEC co-culture. In additional experiments, CEC were cultured with 10 μg/mL LPS (Sigma, Poole, UK) or 1 μg/mL PGN (Sigma) for 8 h prior to analysis of TLR expression and levels of the active form of ERK kinase. Conditioned media from CEC:bacteria co-culture were harvested and stored at -80 °C until analyzed by ELISA and CEC were extensively washed prior to processing for RNA isolation.
RT-PCR analysis
Total cellular RNA was isolated from cultured CEC by lysis in 4 mol/L guanidinium isothiocyanate and CsCl density gradient centrifugation followed by acidic phenol extraction and ethanol precipitation. One to two micrograms of RNA was reverse transcribed into cDNA and amplified by capillary PCR (Idaho Technology, Idaho Falls, ID) using specific oligonucleotide primers (Table 1). Wherever possible primer pairs spanning an intron were used wherever possible. Optimal amplification conditions for each primer pair were determined empirically using cloned cDNAs or mRNA/cDNAs obtained from primary or established cell lines that expressed the gene of interest. Quantitative scanning densitometry of EtBr-stained gels was used to compare levels of PCR products obtained under different culture conditions.
Table 1.
PCR primer sequences.
| Gene | Forward 5'-3’ | Reverse 5'-3’ |
| Cytokines | ||
| IL-1b | GAGATTGAGCTGTCTGCTCA | AAGGAGAACCAAGCAACGAC |
| IL-6 | GATGCTACCAAACTGGATATAATC | GGTCCTTAGCCACTCCTTCTGTG |
| IL-18 | ACTGTACAACCGCAGTAATACGG | AGTGAACATTACAGATTTATCCC |
| TNFa | TGGGAGTAGACAAGGTACAACCC | CATCTTCTCAAAATTCGAGTGACAA |
| ENA78 (CXCL5) | CTTCCTCAGTCATAGCCGCAAC | ATCCGTGGGTGGAGAGAATCAG |
| IP-10 (CXCL10) | TTTCTGCCTCATCCTGCTGG | GGAGCCCTTTTAGACCTTTTTTGG |
| MIP-1a (CCL3) | GCCCTTGCTGTTCTTCTCTGT | GGCATTCAGTTCCAGGTCAGT |
| MIP-1b (CCL4) | ACACCATGAAGCTCTGCGT | CGCTGGAGCTGCTCAGTTC |
| MIP-2a (CXCL2) | CAAAGGCAAGGCTAACTG | TGTTCTACTCTCCTCGGT |
| MIP-3a (CCL-20) | GCAGAAGCAGCAAGCAACTACG | GAGGTTCACAGCCCTTTTCACC |
| MCP-1 (CCL2) | CTCACCTGCTGCTACTCATTC | GCTTGAGGTGGTTGTGGAAAA |
| MCP-3 (CCL7) | TGTGCCTGAACAGAAACCAACC | AAAAATGGGGAAAGGGGGAG |
| KC (GRO) | GTCCTTTGAACGTCTCTGTC | GCTGGCTTCTGACAACACTA |
| RANTES (CCL5) | CAAGGAGTATTTCTACACCAGCAGC | ATGCCGATTTTCCCAGGACC |
| MEC (CCL-28) | GCTGTGTGTGTGGCTTTTCAAAC | TTCTGTCCTTCCTGCTGGGTTG |
| TLR- signaling | ||
| MyD88 | ACCCCACTCGCAGTTTGTTGGATG | TGGTGATGCCTCCCAGTTCCTTTG |
| TIRAP | CAATCTACCTGGAATCGGCTGTC | GCATCTTCTTGGGCTTCTTCAAC |
| IRAK | TGCCGCTTCTACAAAGTGATGGACG | AATGGGTCTGGGAGCCTGGAAAAG |
| Caspase-1 | ATTACTGCTATGGACAAGGCACGG | CCCTCGGAGAAAGATGTTGAAACTC |
| TRAF-6 | AATGGAAGCACAGCAGTGTAACGGG | AAGGCAAGCAGTTCTGGTTTGGCG |
| IKKb | TGGAGCCTGGGAAATGAAAGAACG | TCTAAGAGCCGATGCGATGTCACTC |
| IKKa | AGCCATTCCTCACTTACCAGTCCG | CAGCAGTCTCTTCCACAGAAAACCC |
| Others | ||
| CD45 | CTTCAGAGCCTCGTACCAGC | TGTGTCCAGAAGGGCAAATC |
| b-actin | GCTTCTTTGCAGCTCCTTCGTTG | TTCTCCATGTCGTCCCAGTTGG |
| Vimentin | CTGTGTCCTCGTCCTCCTACC | GCAGTTCTACCTTCTCGTTGG |
| b-defensin3 | GCATTTGAGGAAAGGAACTCCACAAC | GTCTCCACCTGCAGCTTTTAGCAA |
| Angiogenin4 | CTCTGGCTCAGAATGTAAGGTACGA | GAAATCTTTAAAGGCTCGGTACCC |
| SAA-1 | GTAATTGGGGTCTTTGCC | TTCTGCTCCCTGCTCCTG |
Flow cytometric analysis of TLR expression
Highly purified (>98% cytokeratin+) preparations of freshly isolated CEC were stained with FITC-anti-TLR2 (clone 6C2, Hycult Biotechnology BV, Uden, The Netherlands) or TLR4 (Biocarta, Oxford, UK) antibodies followed by goat anti-rat-biotin (Caltag) and finally streptavidin-PE (Caltag). Samples were analyzed by flow cytometry. Appropriate isotype matched control antibodies were also used.
Cytokine ELISAs
Commercial preparations of paired antibodies for measurement of murine IL-1β, IL-6, TNFα, MCP-1, KC (Becton Dickinson Biosciences, Oxford, UK), MIP-1α, MIP-1β, and IP-10 (R&D Systems, Abingdon, UK) were used to develop ELISA according to the manufacturer’s instructions. Individual samples were tested in triplicate and the concentration of cytokines was determined using known amounts of recombinant protein. The amounts of cytokine detected in CEC:bacteria co-culture were normalized according to the number of CEC in each culture.
Statistical analysis
Cytokine concentrations were expressed as mean±SD and evaluated using the Student’s t test. P<0.05 was considered statistically significant.
RESULTS
Primary CEC expressed functional TLRs
To ascertain whether CEC had the potential to respond to commensal bacteria, we first examined CEC directly ex vivo for expression of TLR2 and TLR4 by antibody staining and flow cytometry. Examination of cellular levels of TLR2 and TLR4 protein showed that freshly isolated CEC expressed detectable levels of both TLR2 and TLR4 (Figure 1B). TLR2 and TLR4 expression by CEC were evaluated by determining the influence of TLR2 and four ligands PGN and LPS on cytokine production by CEC in vitro. For these experiments cultured CEC comprising crypts, enterocytes and goblet cells[12,13] were used. Contaminating hematopoietic (CD45+) cells were not detected in any of these cultures using a RT-PCR assay capable of detecting less than 2% contaminating CD45+ cells (Figure 1A). Vimentin antibody staining (data not shown) and RT-PCR analysis (Figure 1A) showed that vimentin+ cells including fibroblasts, were also absent from these cultures. CEC were cultured in vitro in the presence of PGN or LPS for 12 h, then the conditioned medium was assayed in the presence of the cytokines IL-6 and MCP-1, which are known to be produced by CEC both in vivo and in vitro[15]. As seen in Figure 1C, CEC constitutively secreted high levels of MCP-1 (>1000 pg/mL) and comparatively lower levels of IL-6 (<400 pg/mL). In response to LPS the levels of secretion of both cytokines were significantly increased (P<0.002 for MCP-1 and P<0.05 for IL-6). PGN also significantly increased the level of MCP-1 (P<0.01) and IL-6 (P<0.01) production when compared to cells cultured in medium alone. TLR4 expression by CEC was also confirmed by immunoblot analysis of the active and phosphorylated form of the MAP kinase, ERK 1/2, which showed that levels of the phosphorylated form of p42 and p44 isoforms of ERK were up-regulated in response to LPS and PGN (data not shown). Collectively, these data demonstrated that primary CEC could express functional TLR2 and TLR4.
Figure 1.
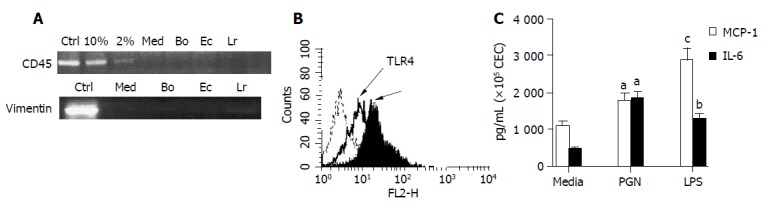
Evaluation of the purity and response of cultured murine CEC. A: CEC from 4-6 wk old C57BL/6 mice were cultured for 72 h in medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which CEC RNA was extracted, reverse transcribed and cDNA amplified by RT-PCR using primers specific for CD45 or vimentin. PCR products were separated by gel electrophoresis and EtBr-stained amplicons visualized and digitally recorded under UV illumination. The sensitivity of CD45 detection was determined by adding spleen cells to highly purified CECs so that they comprised 2% or 10% of the total cell population prior to RNA extraction and RT-PCR analysis. Control samples (Ctrl) were spleen cells (+Ctrl) and no cDNA (-Ctrl) for CD45 RT-PCR and fibroblasts for vimentin RT-PCR assay. The results are representative of more than 10 independent experiments; B: TLR2 and TLR4 expression by CEC. The dashed line on the histogram plots represents staining with control antibody, the bold line represents staining profile of anti-TLR4 and the filled in histogram plot represents anti-TLR2 antibody staining; C: Responsiveness of TLR expressed by CEC. Supernatants from 4 h cultures of CEC in medium alone (Med) or in medium containing LPS (10 μg/mL) or PGN (1 μg/mL) were assayed for the presence of IL-6 and MCP-1 by ELISA. The results shown were collated from three independent experiments. The error bars represent SEM. aP<0.05 LPS vs medium values, bP<0.01 PGN vs medium values, cP<0.002 LPS vs medium values.
Bacteria:CEC co-culture
CEC from 2-3 healthy adult C57BL/6 mice were pooled and cultured in vitro for up to 72 h to establish a semi-confluent monolayer of polarized epithelial cells prior to the addition of either B. ovatus, E. coli or LGG that were in logarithmic growth phase. The number of bacterial strains used was limited to three in order to facilitate screening of a large panel of genes (n = 18) encoding cytokines, acute phase proteins and antimicrobial proteins (Table 1). The choice of bacteria was based upon their prominence in the mouse and human colon[16,17], expression of antigens known to modulate immune (lymphocyte) cell activity[18-21], their availability and ability to sustain their normal growth activity under the conditions used to maintain CEC. L. rhamnosus (LGG) was also chosen as it is a widely used probiotic bacterium[22]. After co-culture supernatants were harvested for cytokine and chemokine analysis by ELISA and CEC were harvested for RNA isolation and RT-PCR analysis.
The duration of CEC:bacteria co-culture was restricted as short as possible in order to detect a response yet preventing bacterial overgrowth and any indirect effects on CEC as a result of changes in the culture media (e.g., pH, nutrient depletion). A ratio of 10 bacteria:1 CEC was found to be optimal for detecting changes in CEC gene expression without adversely affecting the composition of the culture medium and CEC viability (Lan and Carding, unpublished data). Approximately 2×105 CEC were cultured with different numbers of bacteria for 4-5 h, then the number of CEC and bacteria was determined. During a 4 h period the number of E. coli and L. rhamnosus bacteria increased approximately 4-fold (Figure 2A) whereas the number of viable CEC did not significantly change (Figure 2A). Although regarded as an anaerobe, B. ovatus is aerotolerant and over a 4 h period the viability and growth rate of B. ovatus were similar when cultured in CEC media under aerobic conditions (MEM 10% FCS, 100 mL/L CO2) or in bacterial culture media (RGM) under anaerobic conditions (Figure 2A). By seeding CEC cultures with a slightly higher density of B. ovatus, the total number of bacteria after 4 h of culture was equivalent to the number of E. coli and L. rhamnosus recovered from CEC co-culture after 4 h (Figure 2A). The optimal time for detecting changes in CEC gene expression was determined by co-culturing bacteria with CEC and harvesting CEC at hourly intervals for RT-PCR analysis of expression of representative cytokine genes. For MIP-1α and TNFα as well as the majority of genes analyzed (Table 1), the highest levels of mRNA were detected after 4 h (Figure 2B). A ratio of 10 bacteria:1 CEC and a 4 h co-culture period were therefore used for all subsequent experiments.
Figure 2.
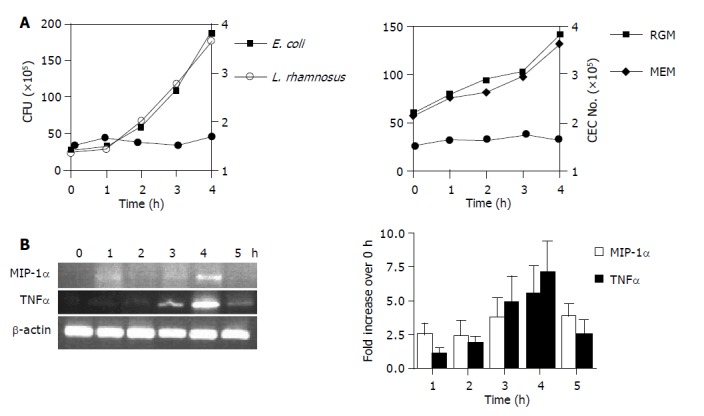
Bacterial growth (A) and kinetics of CEC cytokine gene expression (B) in CEC:bacteria co-cultures. A: Determination of bacterial (L. rhamnosus and E. coli) CFU by harvesting cells from CEC:bacteria co-cultures at hourly intervals up to 4 h by extensive washing of adherent CEC and plating serial dilutions onto agar plates and counting bacterial colonies 24 h later (left-hand panel). B. ovatus were cultured either alone under anaerobic conditions in RGM media or with CEC in 50 mL/L CO2 and complete MEM (right-hand panel). CEC numbers (solid circle on both graphs) were determined by counting the number of cells recovered from co-cultures at the indicated times using a counting chamber; B: CEC cultured in the presence of E. coli for up to 5 h. CECs were processed for RNA isolation and RT-PCR analysis using primers specific for the housekeeping gene β-actin, and MIP-1α and TNFα as described in Materials and Methods. Quantitative densitometry was carried out on EtBr-stained gels and the results from three independent experiments were compiled to produce the data shown. Error bars indicate 95% confidence limits.
Different regulation of cytokine gene expression by CEC in response to different bacteria
Activation of signal transduction pathways by TLRs led to the induction of various genes including inflammatory cytokines and chemokines that function in host defence. The ability of primary CEC to respond differently to different commensal bacteria at the level of cytokine mRNA expression was therefore assessed using semi-quantitative RT-PCR. Genes encoding 15 cytokines and three anti-microbial proteins (Tables 1 and 2) were chosen for this analysis on the basis of their constitutive or inducible production by cultured human or rodent IEC lines, or by IEC in healthy or inflamed colon of experimental animals or IBD patients[15,23-25].
Table 2.
Semi-quantitative assessment of bacteria-induced CEC gene expression.
| Gene | Medium | +B. ovatus | +E. coli | +L. rhamnosus |
| IL-1β | -1 | 12 | 1 | 3 |
| TNFα | - | 0 | 2 | 1 |
| IL-6 | + | 4 | 2 | 0 |
| IL-18 | 0 | 0 | 0 | 0 |
| ENA-78 (CXCL5) | 0 | 1 | 1 | 1 |
| IP-10 (CXCL10) | + | 1 | 2 | 1 |
| MIP-1α (CCL3) | - | 0 | 3 | 0 |
| MIP-1β (CCL4) | - | 0 | 4 | 0 |
| MIP-3α (CCL-20) | - | 1 | 3 | 1 |
| MCP-1 (CCL2) | + | 2 | 4 | 4 |
| MCP-3 (CCL5) | + | 1 | 2 | 2 |
| KC (GRO) | + | 1 | 4 | 2 |
| MIP-2α (CXCL2) | + | 0 | 0 | 2 |
| RANTES (CCL5) | - | 0 | 2 | 1 |
| MEC (CCL-28) | - | 0 | 2 | 1 |
| β-Defensin3 | - | 0 | 4 | 0 |
| SAA-1 | - | 0 | 0 | 0 |
| Angiogenin4 | - | 0 | 0 | 0 |
Absence (–) or presence (+) of EtBr-stained PCR amplicons in CECs cultured in media alone.
Relative intensity of amplicons expressed by CEC cultured in presence of bacteria compared to cultured in media alone as determined by scanning densitometry. 0 = No change, 1 = <2-fold higher, 2 = 2- to 3-fold higher, 3 = 3- to 4-fold higher, 4 = >4-fold higher.
Cultured CEC constitutively expressed detectable levels of mRNAs encoding IL-6, ENA78, MCP-1, MCP-3, IP-10, KC and MIP-2α (Figure 3 and Table 2). By contrast, it was not possible to detect constitutive expression of transcripts encoding the cytokines IL-18, IL-1β, TNFα (Figure 2B), MIP-1α, MIP-1β, MIP-3α and MEC, the acute phase protein serum amyloid A (SAA-1), or the anti-microbial proteins angiogenin 4 (Ang4) and β-defensin 3 (Figure 3 and Table 2).
Figure 3.
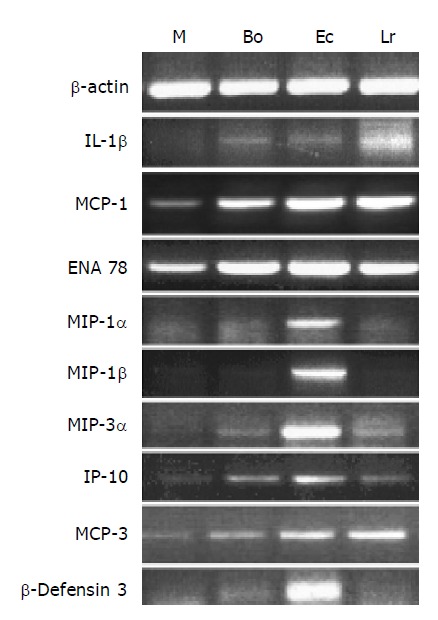
Changes in expression of cytokine genes in CEC in response to commensal bacteria. CECs were cultured for 4 h in complete medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which RNA was extracted from CECs and processed for RT-PCR analysis using primers for β-actin and genes encoding cytokines and the anti-microbial peptide, β-defensin3 as described in the Materials and methods section. The results shown are typical of those obtained from a total of six independent experiments.
The addition of bacteria resulted in different profiles of cytokine gene expression by CEC (Figure 3 and Table 2). The levels of expression of some cytokines (IL-18), acute phase (SAA-1) and anti-microbial proteins (Ang4) remained the same as that seen in CEC cultured in media alone. Changes in cytokine mRNA levels were seen in response to a single strain of bacteria. Induction of MIP-1α, MIP-1β and β-defensin 3 mRNA expression was only evident in response to E. coli, whereas induction of MIP-2α was only seen in response to L. rhamnosus. Some cytokine mRNAs (IL-6, TNFα RANTES and MEC) increased in response to 2/3 of the bacteria. ENA78, KC, MCP-1, MCP-3 and IP-10 mRNA levels were up-regulated to some extent in response to the three strains of bacteria. To substantiate these findings we determined if the changes seen in cytokine mRNA reflected changes in the level of secreted proteins.
Changes in cytokine secretion by CEC in response to different bacteria
A total of eight cytokines were used for this study, the choice of which was based upon those that were analyzed by RT-PCR, and necessarily restricted by the availability of suitable reagents (paired antibodies). Six of the eight cytokines (TNFα, IL-1β, IL-6, MCP-1, IP-10 and KC) analyzed were in good concordance with the profile of mRNA expression (Figure 3 and Table 2) and protein secretion (Figures 4 and 5) in CEC:bacteria co-culture. Consistent with the profile of constitutive expression of MCP-1, IP-10 and IL-6 mRNA by cultured CEC, it was also possible to demonstrate the constitutive secretion of these cytokines by CEC.
Figure 4.
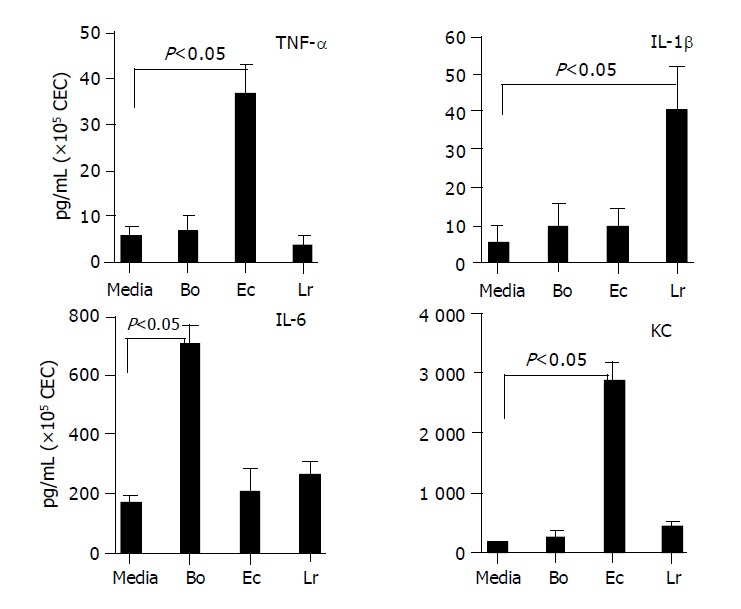
Profile of cytokines secreted by CEC in response to commensal bacteria. CECs were cultured for 4 h in complete medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which conditioned medium was assayed for the presence of IL-6, TNF-α, IL-1β and KC. The amount of cytokine present was determined by reference to a standard curve generated using known amounts of recombinant protein. The limit of detection of each assay was ~5 pg/mL. The results shown were obtained by combining the data sets from a minimum of three independent experiments. Error bars designate 95% confidence limits.
Figure 5.
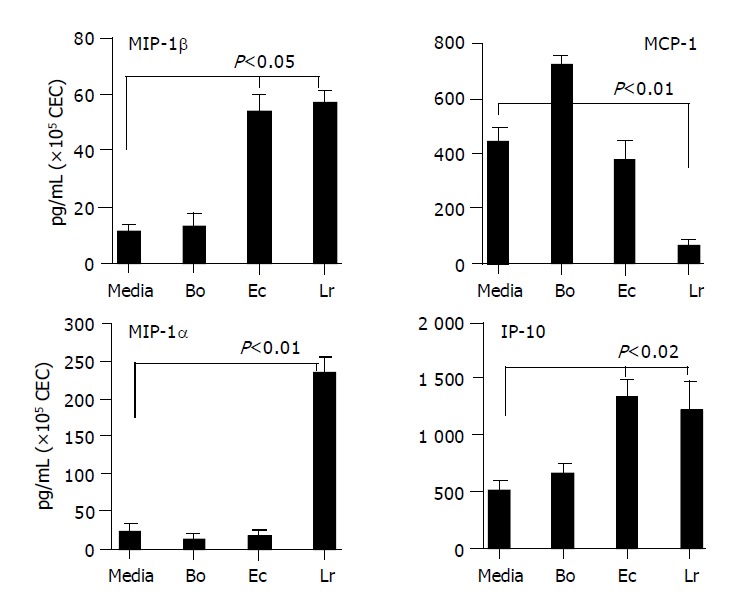
Chemokines secreted by CEC in response to commensal bacteria. CECs were cultured for 4 h in complete medium alone (M) or in medium containing Bacteroides ovatus (Bo), E. coli (Ec) or Lactobacillus rhamnosus (Lr) after which conditioned medium was assayed for the presence of MIP-1α, MIP-1β, IP-10 and MCP-1. The amount of chemokine present was determined by reference to a standard curve generated using known amounts of recombinant protein. The limit of detection of each assay was ~10 pg/mL. The results shown were obtained by combining the data sets from at least three independent experiments. Error bars designate 95% confidence limits.
The selectivity of CEC cytokine responses to different bacteria seen at the level of mRNA expression was also seen in TNFα, IL-1β and IP-10 secretion induced or up-regulated by one and/or two but not all three strains of bacteria. The differences in levels of individual cytokines secreted by CEC, which could be seen by comparing levels of TNF-α and IL-1β with KC and IP-10 secretion, varied by almost two orders of magnitude. Clearly, CEC produced some cytokines (e.g., KC and IP-10) at much higher levels than others. In general however, the amounts of cytokine produced by CEC in response to commensal bacteria were lower than those produced by hematopoietic cells. In cases where individual commensal bacteria induced or up-regulated cytokine production by CEC, the same bacteria induced higher levels of production of the same cytokine by splenic macrophages. For example, splenic macrophages produced 5-10-fold higher levels of MIP-1α and IL-6 and 50-80-fold higher levels of MIP-1β, TNF-α and IL-1β. Whereas for other cytokines such as IP-10 and MCP-1, splenic macrophages produced only 2-3-fold higher levels of cytokine (data not shown).
Discrepancies in CEC cytokine mRNA and protein profiles were evident between MIP-1α and MIP-1β. Most striking was MIP-1α for which a single yet different bacterium was identified inducing mRNA accumulation (E. coli) and protein secretion (L. rhamnosus). Interestingly, MCP-1 protein analysis indicated that the probiotic bacterium L. rhamnosus significantly (P<0.01) suppressed constitutive cytokine secretion by CEC for which there was no compelling case from the mRNA analysis (Table 2 and Figure 3). Although there was some overlap in MIP-1β mRNA and protein expression by CEC with E. coli up-regulating both, this was not the case for L. rhamnosus which up-regulated protein secretion in the absence of any influence on mRNA expression.
Probiotic bacterium, L. rhamnosus (LGG), interfered with E. coli- and B. ovatus-induced cytokine production by CEC
Although probiotic therapy has been associated with anti-inflammatory effects in specific groups of IBD patients, the mechanism of action of probiotic bacteria is not clear[26]. Since the probiotic bacterium L. rhamnosus (LGG) had a less potent effect on modulating cytokine production by CEC in comparison to E. coli and B. ovatus (Figure 4 and Table 2), we determined if L. rhamnosus could modulate or interfere with CEC cytokine production induced by other bacteria. CEC were therefore cultured with individual bacteria alone or with an equal number of two of the bacteria such that all co-cultures had the same number of bacteria. Combining two different bacteria species had no obvious effect on bacterial viability or growth during the 4 h culture period with the total number of viable bacteria recovered being indistinguishable from co-cultures containing the individual bacteria species alone (Lan and Carding, unpublished data). KC and IL-6 were the cytokines of choice for these experiments since they were produced at high levels by CEC in response to either E. coli (KC) or B. ovatus (IL-6). These cytokines are also major pro-inflammatory cytokines and they or their human homologs (murine KC and human IL-8) have been associated with inflammation[27,28].
The inclusion of LGG in E. coli:CEC cultures effectively reduced the level of KC secreted by CEC by more than 6-fold in response to E. coli to that seen in control cultures containing media or LGG alone (Figure 6A). This suppressive effect was dependent upon viable bacteria since non-viable and heat killed L. rhamnosus had no effect on E. coli-induced KC production (data not shown) and was mediated at the level of KC mRNA transcription or accumulation since the addition of LGG to E. coli:CEC co-cultures reduced the amount of KC mRNA expression to levels seen in cultures containing LGG alone (Figure 6A). This suppressive effect was not seen using B. ovatus which had no (negative) impact on the amount of KC produced by CEC, in response to E. coli in mixed bacteria:CEC co-cultures (Figure 6A). Since B. ovatus had little effect on KC production by CEC it was not possible to determine if the suppressive effect of LGG extended to B. ovatus. It was however possible to investigate this by analyzing IL-6 production by CEC since B. ovatus induced high levels of IL-6 production whereas E. coli and LGG had little or no effect on IL-6 production (Figure 4). The addition of LGG to B. ovatus:CEC co-cultures effectively neutralized the ability of B. ovatus to increase the level of IL-6 produced by CEC (Figure 6B). As seen for KC production, the effect of LGG on B. ovatus-induced IL-6 production appeared to be at the level of gene transcription and/or mRNA turnover. Whereas B. ovatus alone dramatically increased the levels of IL-6 mRNA expressed by CEC, in cultures containing both B. ovatus and LGG it was not possible to detect any IL-6 mRNA, which was also undetectable in cultures containing LGG alone (Figure 6B). This suppressive effect on IL-6 production was not seen with E. coli which was unable to exert a similar effect on B. ovatus-induced IL-6 mRNA or protein production (Figure 6B). These findings demonstrated that the probiotic bacteria LGG could influence and interfere with the interaction of other unrelated commensal bacteria with CEC.
Figure 6.
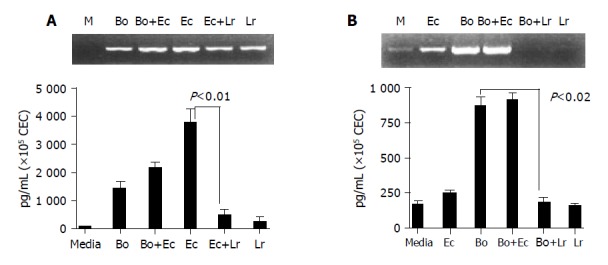
L. rhamnosus (LGG) interferes with E. coli- and B. ovatus-induced cytokine production by CEC. CEC were cultured for 4 h with individual bacteria alone or with a mixture of equal numbers of two different bacteria (B. ovatus+ E. coli or E. coli+L. rhamnosus) such that the total number of bacteria in each culture was the same. A: KC mRNA and protein expression by RT-PCE and ELISA; B: IL-6 mRNA and protein expression by RT-PCR and ELISA. ELISA data was obtained by combining the data sets from three independent experiments. Error bars designate 95% confidence limits.
DISCUSSION
This study represents one of the first attempts to investigate the interaction between isolated primary CEC and individual members of the commensal microbiota. A similar and complimentary in vivo study using conventionalized germfree mice has previously shown that commensal bacteria can modulate the expression of various (non-immune) genes in ileal IEC in vivo[7]. Our study although limited in the number and types of investigated commensal bacteria has focused on the ability of commensal bacteria to modulate expression of immune response-related genes and their products in isolated CEC. By studying CEC in isolation, any indirect effects of other mucosal cells that might occur in vivo have effectively been excluded. Our findings demonstrate that CEC have the potential to respond to commensal bacteria and that based upon differences in the cytokines they produce can at least discriminate between the three different bacteria used in this study. The ability of the probiotic bacterium LGG to interfere with the ability of other bacteria to modulate CEC cytokine production was unexpected and may have some relevance to the action of these probiotic bacteria in vivo.
Although the CEC:bacteria co-culture system described here provides a controlled in vitro accessible system for investigating host-microbe interactions, it is important to acknowledge its limitations. Similar to studies using immortalized IEC lines, our system cannot take into account the possibility that the behavior and response of CEC may normally be influenced directly or indirectly by other mucosal cell populations in vivo that are absent or underrepresented in our culture system. Our culture system by its design also cannot account for the complexity of bacterial populations that exist in the colon and as complex biofilms at mucosal epithelial surfaces, the constituents of which remain to be identified and may be uncultivable[14,16]. It should be noted however, that the species of bacteria we have used are major populations in the colon of laboratory rodents and collectively number 3-11×1010 organisms per gram of tissue[14,16]. Our culture system also cannot account for any interactions that might occur between different bacteria in vivo that might have an impact on the activity of individual bacteria and most likely the host response to them. As we have demonstrated here however, it is possible to compare IEC interactions with individual bacteria alone and in combination with other bacteria. While acknowledging these limitations, we believe our culture system represents an advance in the development of more physiologically relevant in vitro systems for investigating the nature of bacteria-IEC interactions. As complimenting existing methodologies can be used its in vitro accessibility is an advantage over more indirect and more expensive in vivo studies that use conventionalized gnotobiotic animals[29].
The significance of different cytokine responses by CEC to different commensal bacteria in vitro is not clear. In considering the limitations and somewhat artificial nature of our experimental system, the data should be interpreted cautiously. Since none of the bacteria used in this study is invasive, it is unlikely to be due to differences in the ability of the bacteria to “infect” CEC. It is possible that the cytokine-inducing activity of different bacteria may be dependent upon them reaching certain and perhaps different concentration thresholds. It may also be related to qualitative or quantitative differences in the MAMPs expressed by the different bacteria. For example, the E. coli strain used in this study produced a LPS that has been shown to elicit potent and persistent mucosal immune responses in germfree mice[18]. By contrast the MAMPs expressed by B. ovatus are poorly characterized, although the presence of antibodies to B. ovatus in the serum of IBD patients[19] suggests that they may not be entirely inert.
The biological significance of probiotic LGG-mediated modulation of cytokine production by CEC is not yet clear, although its ability to suppress MCP-1 production by CEC could have beneficial effects on limiting or preventing the recruitment to the colonic mucosa of inflammatory cells including granulocytes and activated T cells capable of responding to MCP-1. The ability of LGG to indirectly affect CEC immune responses by interfering with the ability of other commensal bacteria to induce or upregulate cytokine production by CEC identifies CEC as a cellular target for probiotoic bacteria in vivo. The ability of probiotic bacteria to down-modulate proinflammatory cytokine (TNF-α and IL-6) production has been demonstrated previously in vivo[30] and ex vivo in tissue explant:bacteria co-cultures[31]. However, the identity of cells affected by the bacteria and the cellular source(s) of cytokines were not established in these studies. Although the mechanisms of action of probiotics remain unclear[32], their ability to alter the composition of the colonic or fecal microbiota has been associated with beneficial effects in certain groups of IBD patients and infants[26]. In addition, Lactobacillus sp. can also prevent the development of intestinal inflammation in animal models of IBD[21,33]. Our findings suggest a mechanism by which non-pathogenic or probitoic bacteria might suppress or limit the ability of other “pathogenic” bacteria to promote or sustain inflammatory responses. How LGG interferes with the ability of E. coli or B. ovatus to induce cytokine production by CEC was not established in this study. It may include the production of antimicrobial peptides (bacteriocins), expression of MAMPs of higher density, affinity for LGG, induction of TLR anatagonistic signaling pathways or molecules such as phosphoinositide 3-kinase[34], suppressor of cytokine signaling-1[35,36], single immunoglobulin interleukin-1 receptor related molecule[37], or NOD2[38] by LGG in CECs. The CEC:bacteria co-culture system described here may be of value in investigating these potential mechanisms in more detail.
In summary, the results presented in this study provide evidence for the ability of primary CEC to respond to and discriminate between three different strains of commensal bacteria and new insights into the interaction of different commensal bacteria with the host.
ACKNOWLEDGMENTS
The authors thank Dr. John Cebra and Terry Whitehead for providing E. coli and B. ovatus bacteria.
Footnotes
Supported by the USA Public Health Service grants AI-41562 and PO1 RR12211 (SRC and PF), the Ann Gloag Fellowship of the Royal College of Surgeons Edinburgh and The Rays of Hope Charitable Trust (JS)
References
- 1.Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–694. doi: 10.1097/00075197-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hecht G. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1–G7. doi: 10.1152/ajpgi.2001.281.1.G1. [DOI] [PubMed] [Google Scholar]
- 3.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 4.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 8.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 9.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 10.Inan MS, Tolmacheva V, Wang QS, Rosenberg DW, Giardina C. Transcription factor NF-kappaB participates in regulation of epithelial cell turnover in the colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1282–G1291. doi: 10.1152/ajpgi.2000.279.6.G1282. [DOI] [PubMed] [Google Scholar]
- 11.Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgart DC, Olivier WA, Reya T, Peritt D, Rombeau JL, Carding SR. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell Immunol. 1998;187:52–66. doi: 10.1006/cimm.1998.1307. [DOI] [PubMed] [Google Scholar]
- 13.Telega GW, Baumgart DC, Carding SR. Uptake and presentation of antigen to T cells by primary colonic epithelial cells in normal and diseased states. Gastroenterology. 2000;119:1548–1559. doi: 10.1053/gast.2000.20168. [DOI] [PubMed] [Google Scholar]
- 14.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 15.McGee D. Inflammation and mucosal cytokine production. In: Ogra P, Mestecky J, lamm ME, Strober W, Bienenstock J, et al., editors. Mucosal Immunology. San Diego: Academic Press; 1999. pp. 559–573. [Google Scholar]
- 16.Savage DC. Gastrointestinal microflora of rodents. In: Ruitenberg EJ, Peters PWJ, eds , et al., editors. Handbook on Animal Production. VolC2. Amsterdam: Elsevier; 1985. pp. 85–117. [Google Scholar]
- 17.Ahrné S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85:88–94. doi: 10.1046/j.1365-2672.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- 18.Moreau MC, Ducluzeau R, Guy-Grand D, Muller MC. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–539. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitoh S, Noda S, Aiba Y, Takagi A, Sakamoto M, Benno Y, Koga Y. Bacteroides ovatus as the predominant commensal intestinal microbe causing a systemic antibody response in inflammatory bowel disease. Clin Diagn Lab Immunol. 2002;9:54–59. doi: 10.1128/CDLI.9.1.54-59.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cebra J, Cebra E, Shahin R. Perturbations in specific B cell subsets following deliberate contamination in germfree or natural colonization of mice by intestinal flora. In: Ryc C, Franek J, eds , et al., editors. Bacteria and the host. Prague: Avicenum; 1986. pp. 303–307. [Google Scholar]
- 21.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 22.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in't Veld JH. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 23.Ajuebor MN, Swain MG. Role of chemokines and chemokine receptors in the gastrointestinal tract. Immunology. 2002;105:137–143. doi: 10.1046/j.1365-2567.2002.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dwinell MB, Johanesen PA, Smith JM. Immunobiology of epithelial chemokines in the intestinal mucosa. Surgery. 2003;133:601–607. doi: 10.1067/msy.2003.143. [DOI] [PubMed] [Google Scholar]
- 25.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 26.Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin Exp Allergy. 2003;33:1634–1640. doi: 10.1111/j.1365-2222.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- 27.Bozic CR, Kolakowski LF, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 28.Boisvert WA, Curtiss LK, Terkeltaub RA. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol Res. 2000;21:129–137. doi: 10.1385/ir:21:2-3:129. [DOI] [PubMed] [Google Scholar]
- 29.Gordon HA, Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971;35:390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz M, Linde HJ, Lehn N, Zimmermann K, Grossmann J, Falk W, Schölmerich J. Immunomodulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. J Dairy Res. 2003;70:165–173. doi: 10.1017/s0022029903006034. [DOI] [PubMed] [Google Scholar]
- 31.Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–664. doi: 10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh S, van Heel D, Playford RJ. Probiotics in inflammatory bowel disease: is it all gut flora modulation? Gut. 2004;53:620–622. doi: 10.1136/gut.2003.034249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao Y, Nobaek S, Kasravi B, Adawi D, Stenram U, Molin G, Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996;111:334–344. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- 34.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 36.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 37.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]


