Abstract
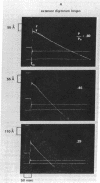
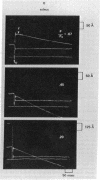
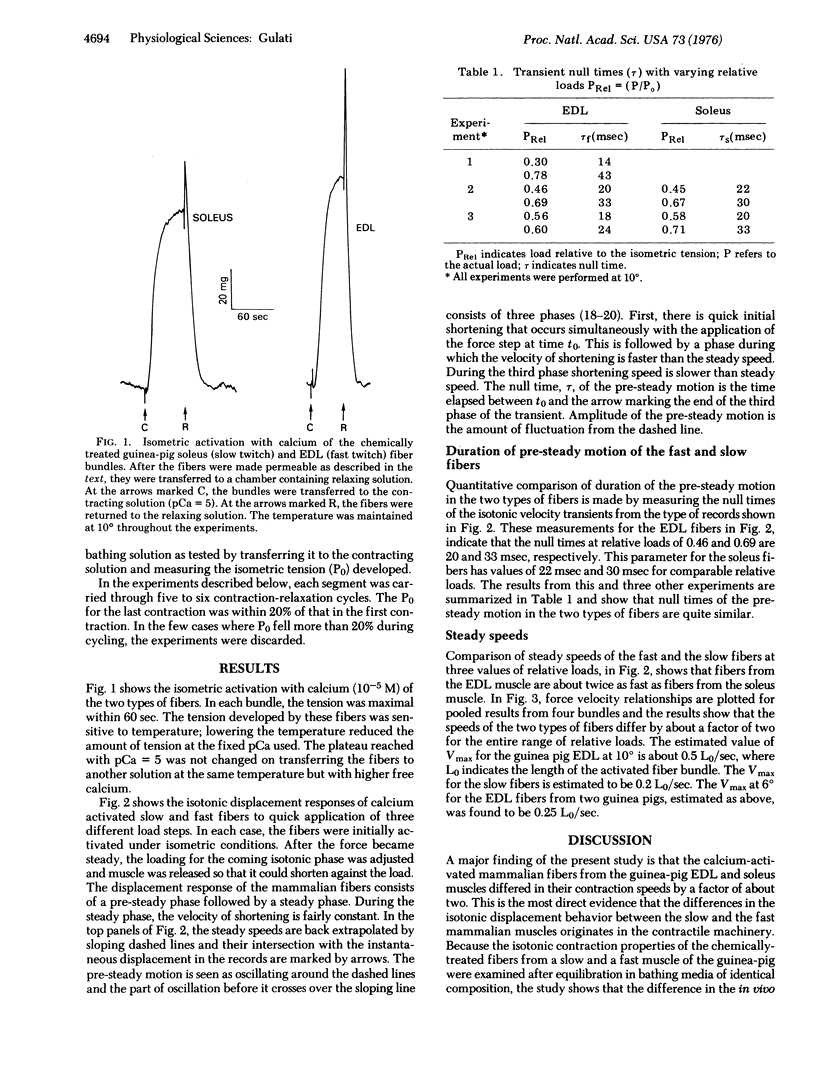
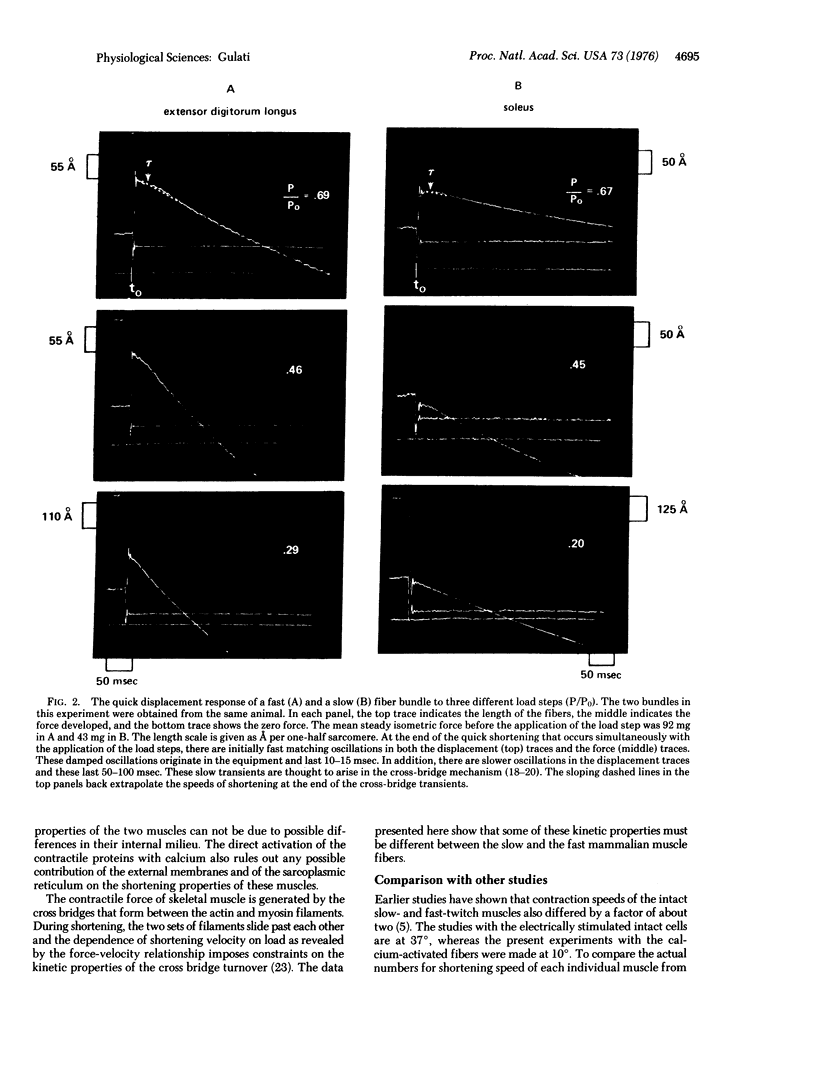
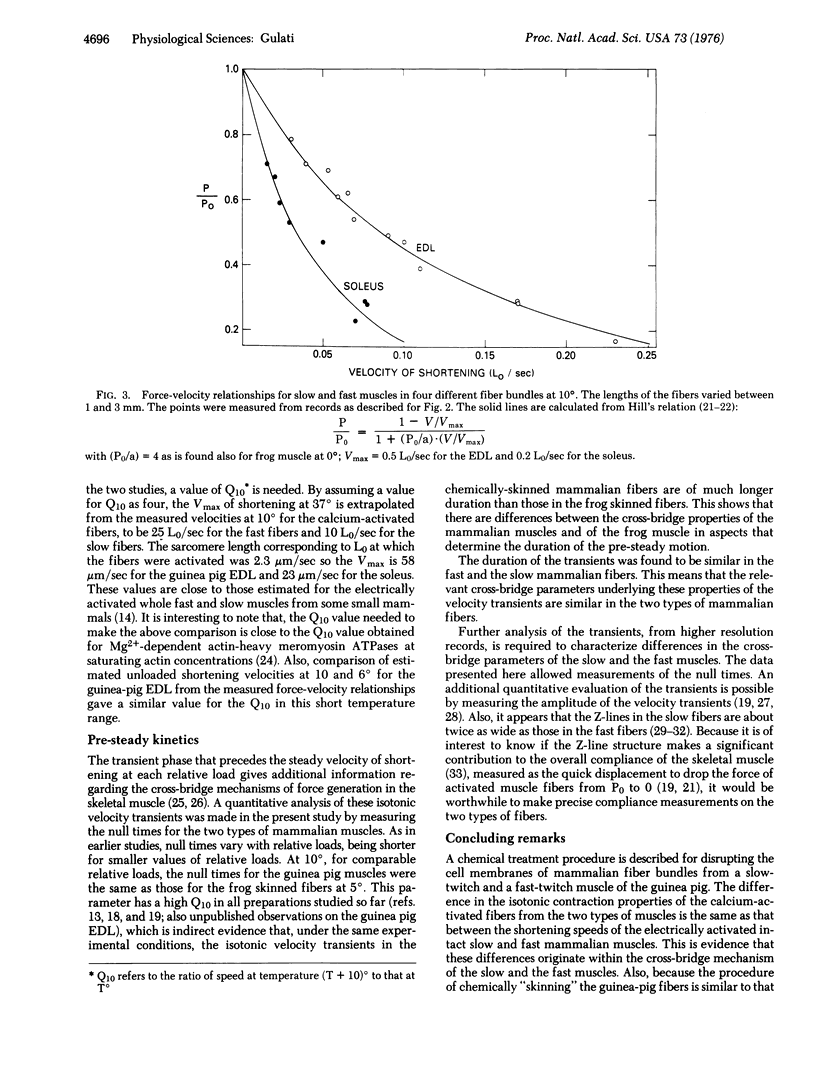
Twitch fiber bundles from a slow (soleus) and a fast (extensor digitorum longus) mammalian muscle after mild chemical treatment were activated with calcium and relaxed in calcium-free solution. Like the electrically activated whole muscles, the force-velocity relationship was such that, at each relative load, the steady speed of shortening for the fast fibers was about two times greater than that for the slow twitch fibers. The duration of pre-steady motion in the two types of fibers was the same. The data provide direct evidence that the difference in the shortening characteristics of the two types of fibers is due to differences in their cross-bridge properties.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., ECCLES J. C., ECCLES R. M. Differentiation of fast and slow muscles in the cat hind limb. J Physiol. 1960 Feb;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch W. W., Moos C. Effect of temperature on actin activation of heavy meromyosin ATPase. Biochim Biophys Acta. 1971 May 11;234(2):183–189. doi: 10.1016/0005-2728(71)90073-9. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M. Discrimination between fiber populations in mammalian skeletal muscle by using ultrastructural parameters. J Ultrastruct Res. 1976 Jan;54(1):76–88. doi: 10.1016/s0022-5320(76)80010-x. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. The activation of striated muscle and its mechanical response. Proc R Soc Lond B Biol Sci. 1971 Jun 15;178(1050):1–27. doi: 10.1098/rspb.1971.0049. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Secrist D., Coby R., Lucas S. Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult. Nature. 1976 Apr 1;260(5550):440–441. doi: 10.1038/260440a0. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Gulati J., Nolan A. C. Contraction transients of skinned muscle fibers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1516–1519. doi: 10.1073/pnas.71.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisin I. L., Gulati J. Cooperative critical thermal transition of potassium accumulation in smooth muscle. Science. 1972 Jun 9;176(4039):1137–1139. doi: 10.1126/science.176.4039.1137. [DOI] [PubMed] [Google Scholar]
- Rowe R. W. The ultrastructure of Z disks from white, intermediate, and red fibers of mammalian striated muscles. J Cell Biol. 1973 May;57(2):261–277. doi: 10.1083/jcb.57.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sréter F. A., Luff A. R., Gergely J. Effect of cross-reinnervation on physiological parameters and on properties of myosin and sarcoplasmic reticulum of fast and slow muscles of the rabbit. J Gen Physiol. 1975 Dec;66(6):811–821. doi: 10.1085/jgp.66.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrový I., Gutmann E. Myosin from fast and slow skeletal and cardiac muscles of mammals of different size. Physiol Bohemoslov. 1975;24(4):325–334. [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek R. J., Asmundson C. R., Cooper R. R., Barnard R. J. Fine structure of fast-twitch and slow-twitch guinea pig muscle fibers. J Morphol. 1973 Jan;139(1):47–65. doi: 10.1002/jmor.1051390104. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- Wise R. M., Rondinone J. F., Briggs F. N. Effect of calcium on force-velocity characteristics of glycerinated skeletal muscle. Am J Physiol. 1971 Oct;221(4):973–979. doi: 10.1152/ajplegacy.1971.221.4.973. [DOI] [PubMed] [Google Scholar]