Abstract
AIM: An increase in bile ductular structures is observed in diverse human liver diseases, especially in primary biliary cirrhosis (PBC). These structures harbor the progenitor cell component of the liver. Caveolins are cholesterol-binding proteins involved in the regulation of several intracellular processes including cholesterol transport. This study aims to examine the role of caveolin in PBC.
METHODS: Immunohistochemical and Western blotting studies were performed on human liver specimens obtained from patients with PBC and normal liver samples. The expression of caveolin (CAV)-1 and -2 was determined using specific antibodies.
RESULTS: In normal liver, scanty immunostaining for CAV-1 and -2 was observed in bile ductules. In PBC liver samples, the expression levels of CAV-1 and -2 were increased on proliferating bile ductules especially in stage 3 cases, but was sparse on interlobular bile duct in stage 1 specimens. Especially, the regenerating bile ductules at the interface of portal tracts and necrotic areas were immunostained intensely for CAV-1 and -2. These phenomena were confirmed by Western blot.
CONCLUSION: The present results demonstrate increased expression of caveolins in proliferating bile ductules in PBC, which may be related to the homeostasis of cholesterol transport in regenerating bile ductules in PBC liver.
Keywords: Proliferating bile ductule, Caveolin, Immunohis-tochemistry, Primary biliary cirrhosis
INTRODUCTION
Due to the resemblance of “little caves” by transmission electron microscopy, Yamada[1] first coined the term “caveolae intracellulares” in 1955 to describe conspicuous, 50-100 nm invaginations of the plasma membrane found in gallbladder epithelial cells. A few years earlier, Palade[2] also described the presence of such structures in vascular endothelium, although he preferred the term plasmalemmal vesicles to describe the newly discovered cellular organelle “caveolae”.
Caveolin-1 (CAV-1) is a 21-24 ku protein that decorates the cytoplasmic surface of caveolae. CAV-1 was the first discovered member of the caveolin family, and serves as the primary structural component of caveolae[3]. In fact, along with cholesterol and sphingolipids, CAV-1 appears to play a direct role in caveolar biogenesis. The two other members of the caveolin family, caveolin-2 (CAV-2) and caveolin-3 (CAV-3), also target exclusively the caveolar microdomains[4,5]. The expression levels of CAV-1, -2, and -3 fully correlate with the presence of caveolae in cells and tissues. Interestingly, the expression patterns of CAV-1 and -2 are largely distinct from that of CAV-3. While CAV-1 and -2 are expressed at the highest levels in adipocytes, endothelial cells, pneumocytes, and fibroblasts, CAV-3 expression is limited to muscle cells of all types (cardiac, skeletal, and smooth muscle cells)[5,6]. The three proteins overlap in their expression in a few cell types, including smooth muscle cells. Since their discovery, the caveolins have been seen to be intimately involved in all aspects of caveolar function.
Razani et al[7], went on to dissect these effects in primary mouse embryonic fibroblasts and found that CAV-2 is indeed trapped in the golgi compartment and that its expression can be rescued and its localization returned to the plasma membrane following re-introduction of CAV-1. And, this strategy was therefore used to assess the effects of CAV-1 on cellular proliferation and the cell cycle.
Proliferating bile ductular reactions are identified in various human liver diseases, especially in primary biliary cirrhosis (PBC)[8]. The aim of the present study was to clarify the roles of caveolins on the proliferating bile ductules in PBC by conducting immunohistochemistry and Western blotting on liver biopsy specimens obtained from PBC patients.
MATERIALS AND METHODS
Surgical liver biopsy specimens were obtained from 11 female patients with PBC, mean age 54.2 years, range 31-68 years; five cases of Scheur’s stage 1, and six cases of stage 3. PBC was diagnosed clinically and histologically according to the criteria proposed by the Japanese Joint Research Group for Autoimmune Hepatitis[9].
As controls, wedge biopsy specimens from normal portions of the liver were obtained from 10 patients (8 males and 2 females; aged from 44 to 73 years with a mean of 57.3 years) who underwent surgical resection for metastatic liver carcinoma (four colonic carcinoma and four gastric carcinoma).
Immunohistochemistry
Immunostaining was performed on serial sections. Four-micrometer sections were cut from paraffin blocks of formalin-fixed tissue, deparaffinized with xylene, and dehydrated by graded ethanol (70-100%). Deparaffinized sections were autoclaved (120 °C, 2 atm, 15 min) in 10 mmol/L citrate buffer (pH 6.0). Endogenous peroxidase activity was quenched by incubating with 0.3% hydrogen peroxidase. The samples were washed with phosphate buffer saline, and blocked for 10 min with diluted normal goat serum (1:100). Immunostaining was performed using primary antibodies as follows: anti-CAV-1 (polyclonal; Santa Cruz Bio., Santa Cruz, CA) at 1:500 dilution, anti-CAV-2 (monoclonal; Transduction Laboratories, Lexington, KY) at 1:40 dilution. The tissue was incubated with each primary antibody overnight at 4 °C, and then processed by the EnVision method (DAKO, Inc., Tokyo, Japan) at room temperature for 60 min. After repeated washing with PBS, the sections were reacted with diaminobenzidine (DAB) solution containing 0.01% H2O2, and counterstained with hematoxylin for light microscopic study.
Moreover, we conducted double staining with cytokeratin 7 to label the bile ducts. In brief, after DAB reaction, sections were incubated in microwave for 5 min in citrate buffer. Immunostaining was performed by incubating with anti-cytokeratin 7 monoclonal antibody (DAKO, Inc.) at 1:100 dilution for 1 h at room temperature and then processed by EnVision method. After repeated washing with PBS, the sections were reacted with the AEC kit (DAKO) for 2 min.
Western blotting
Western blotting was conducted using fresh control and PBC liver tissues. Briefly, liver tissues were homogenized in 10 volumes of homogenization buffer (20 mmol/L Tris-HCl; pH 7.5, 5 mmol/L MgCl2, 0.1 mmol/L PMSF, 20 μmol/L pepstatin A, and 20 μmol/L leupeptin) using a polytron homogenizer at setting 7 for 90 s. The homogenates were centrifuged at 100000 g for 45 min. The membranes were washed twice, resuspended in 10 volumes of homogenization buffer, homogenized using a Teflon/glass homogenizer, and centrifuged. The membrane proteins thus obtained were used for immunoblotting. Proteins were separated on SDS/PAGE (4-20% gel) (Daiichi-kagaku, Tokyo, Japan) and transferred onto vinylidene difluoride membranes (Millipore, Bedford, MA). The blots were blocked with 50 g/L dried milk in PBS for 30 min, incubated with anti-CAV-1 (1:5000) and anti-CAV-2 (1:200) antibodies, washed with 0.1% Tween 20 in PBS, and incubated with 1:5000 diluted goat anti-rabbit or 1:1000 diluted goat mouse IgG conjugated with horseradish peroxidase (Amersham-Pharmacia, Biotek, Buckinghamshire, UK). Both primary and secondary antibodies were diluted with 0.1% Tween 20 in PBS, and incubation was at room temperature for 1 h. The immunoreactive bands were visualized with the ECL Plus detection system (Amersham-Pharmacia)
RESULTS
Light microscopic distributions of CAV-1 and -2 in control and PBC liver
In control liver specimens, immunoperoxidase-positive substances showing the presence of CAV-1 were observed sparsely on hepatic sinusoidal lining cells and a little immunostaining of CAV-1 and -2 was observed on bile ducts (Figures 1A-1H).
Figure 1.
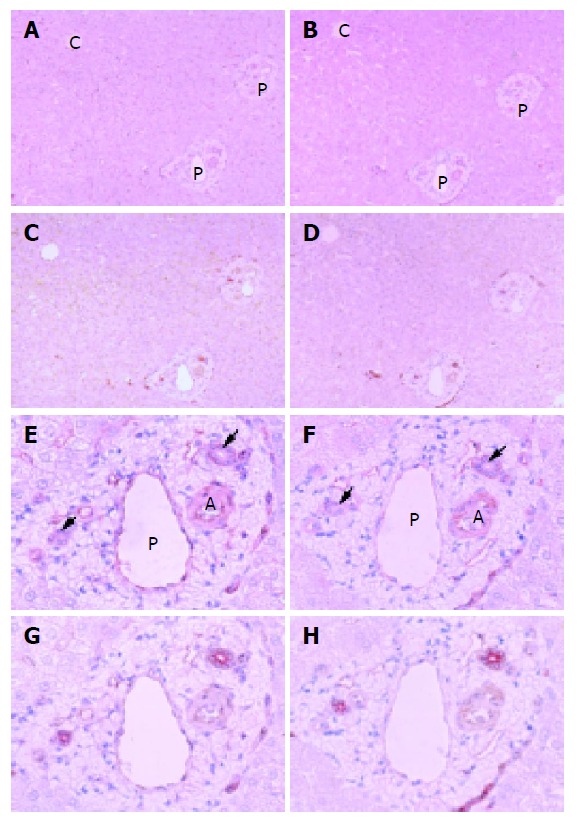
Light microscopic distributions of immunohistochemically stained CAV-1 and -2 in human control liver tissue. A: CAV-1, ×100; B: CAV-2, ×100; C: double staining of CAV-1 and cytokeratin 7, ×100; D: double staining of CAV-2 and cytokeratin 7, ×100; E: CAV-1, ×400; F: CAV-2, ×400; G: double staining of CAV-1 and cytokeratin 7, ×400; H: double staining of CAV-2 and cytokeratin 7, ×400. Hematoxylin counterstain. Immunoperoxidase-positive substances showing the presence of CAV-1 and -2 are observed sparsely on hepatic sinusoidal lining cells and a little immunostaining of CAV-1 and -2 on bile ducts. A: artery, P: portal vein, C: central vein. Arrow denotes bile duct.
In PBC stage 1 liver specimens, there were some immunostaining of CAV-1 and -2 on interlobular bile ducts and some staining on hepatic sinusoidal lining cells (Figures 2A-2D).
Figure 2.

Light microscopic distributions of immunohistochemically stained CAV-1 and -2 in PBC stage 1 liver tissue. A: CAV-1, ×100; B: CAV-2, ×100; C: double staining of CAV-1 and cytokeratin 7, ×100; D: double staining of CAV-2 and cytokeratin 7, ×100. Hematoxylin counterstain. Immunostaining of CAV-1 and -2 is observed to some degree on interlobular bile ducts, intense immunostaining on proliferative bile ductules, and some immunostaining on hepatic sinusoidal lining cells. Asterisks denote interlobular bile ducts.
In PBC stage 3 liver specimens, reaction products showing CAV-1 and -2 were localized abundantly on hepatic sinusoidal lining cells and proliferating bile ductules. Regenerating bile ductules at the interface of portal tracts and necrotic areas were immunostained intensely for CAV-1 and -2, showing significant increase compared to control liver (Figures 3A-3D).
Figure 3.
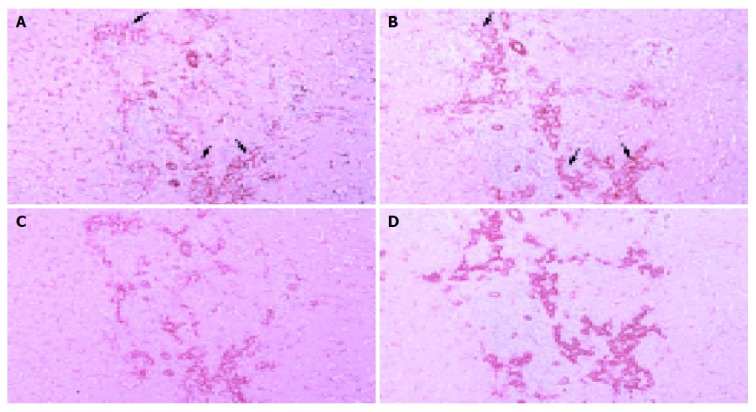
Light microscopic distributions of immunohistochemically stained CAV-1 and -2 in PBC stage 3 liver tissue. A: CAV-1, ×100; B: CAV-2, ×100; C: double staining of CAV-1 and cytokeratin 7, ×100; D: double staining of CAV-2 and cytokeratin 7, ×100. Hematoxylin counterstain. Reaction products showing CAV-1 and -2 are localized abundantly on hepatic sinusoidal lining cells and proliferative bile ductules. Regenerating bile ductules at the interface of portal tracts and necrotic areas are immunostained intensely for CAV-1 and -2, showing significant increase compared to control liver. Arrow denotes proliferative bile ductule.
Western blot analysis of CAV-1 and 2 proteins in control and PBC liver tissues
To confirm the immunohistochemical results, we investigated protein expression of CAV-1 and -2 by Western blotting in normal and PBC liver tissues. CAV-1 and -2 were found in abundance in PBC stage 3 liver specimens and were undetectable in normal liver tissue (Figure 4).
Figure 4.
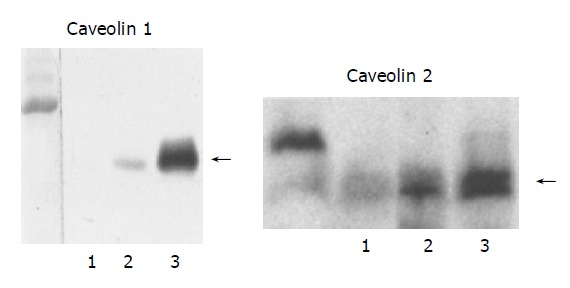
Western blot analysis of the expression of CAV-1 and -2 proteins in human control and PBC liver tissues. Lane 1: normal liver; lane 2: PBC stage 1 liver; lane 3; PBC stage 3 liver. CAV-1 and -2 are found in abundance in PBC stage 3 specimens, less intensely in PBC stage 1, and is undetectable in normal liver tissue. Samples containing 50 μg of membrane protein were subjected to electrophoresis on SDS/PAGE gel (CAV-1 and -2: 4-20%) and analyzed by blotting.
DISCUSSION
Proliferating bile ductular reactions are identified in various human liver diseases. In chronic cholestatic liver diseases, reactive ductules form a labyrinth at the edge of the portal tracts and were thought to form a reservoir in which toxic bile can accumulate[8]. The mechanism of bile ductular proliferation remains unclear. Cholangiocyte proliferation is known to occur in the presence of lipopolysaccharide (LPS). The proliferative response of cholangiocytes to inflammatory mediators such as LPS involves IL-6-mediated activation of the p44/p42 MAPK pathway[10].
Recombinant human CAV-1 and -2 adenoviruses infected mice exhibited a 10- and 7-fold increase in hepatic CAV-1 and -2 protein expression, respectively. Hepatic CAV-1 and/or CAV-2 overexpression significantly increased bile flow and secretion of all biliary lipids[11]. These findings suggest an association between caveolins and bile function.
Caveolins are the defining protein components of caveolae. Interestingly, caveolins bind cholesterol directly. In addition, cholesterol binding may stabilize the formation of caveolin homo-oligomeric complexes. Since liquid-ordered domains are dramatically enriched in cholesterol, caveolins may be attracted or partitioned into the liquid-ordered domains through a direct interaction with cholesterol[12]. The activity of caveolae in free cholesterol homeostasis can be considered as a mechanism of protection against free cholesterol accumulation[13]. Thus, the areas of high expression of caveolin on proliferating bile ductule may be the sites that are protected against cholestasis in PBC.
In this study, caveolins were overexpressed on hepatic lining cells in PBC stage 3 liver tissue. Previously, we reported that CAV-1 expression on sinusoidal lining cells were significantly increased in cirrhotic liver compared to normal liver. CAV-1 expression may be associated with LPS signaling or internalization[14]. In another study, CAV-1 protein expression was increased in liver tissue exposed to bacterial endotoxin, and was localized primarily to vascular tissues in both the pericentric and the periportal areas of the liver[15]. Therefore, inflammatory mediators such as LPS in PBC may stimulate bile ductule proliferation as discussed above[10] and also induced expression of caveolins.
Further elucidation of the cellular mechanisms of caveolin in regulating bile ductule functions in the liver may advance the understanding of cholestatic liver.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK.
References
- 1.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 4.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci U S A. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 6.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 7.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 8.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 9.Ohta Y. Diagnostic criteria for primary biliary cirrhosis. Acta Hepatol Jpn. 1992;33:657. [Google Scholar]
- 10.Park J, Gores GJ, Patel T. Lipopolysaccharide induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology. 1999;29:1037–1043. doi: 10.1002/hep.510290423. [DOI] [PubMed] [Google Scholar]
- 11.Moreno M, Molina H, Amigo L, Zanlungo S, Arrese M, Rigotti A, Miquel JF. Hepatic overexpression of caveolins increases bile salt secretion in mice. Hepatology. 2003;38:1477–1488. doi: 10.1016/j.hep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fielding CJ, Fielding PE. Cholesterol and caveolae: structural and functional relationships. Biochim Biophys Acta. 2000;1529:210–222. doi: 10.1016/s1388-1981(00)00150-5. [DOI] [PubMed] [Google Scholar]
- 14.Lei MG, Morrison DC. Differential expression of caveolin-1 in lipopolysaccharide-activated murine macrophages. Infect Immun. 2000;68:5084–5089. doi: 10.1128/iai.68.9.5084-5089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kresge N, Ashburn JH, Merkel SM, Schrum LW, Clemens MG. Increased caveolin-1 association with eNOS in hepatic vasculature following nonlethal endotoxemia (abstract) Hepatology. 2001;34:347. [Google Scholar]


