Abstract
AIM: We report a case with a prolonged course of hepatitis A, with alanine aminotransferase (ALT) higher than 500 IU/L for more than 2 mo.
METHODS: A middle-aged woman had an elevated IgG level of more than 2000 mg/dL, positive anti-nuclear antibodies (ANA) and anti-smooth muscle antibodies (ASMA), but no evidence of persistent hepatitis A virus (HAV) infection. Liver biopsy findings were compatible with prolonged acute hepatitis, although acute onset of autoimmune hepatitis could not be ruled out.
RESULTS: It was assumed that she developed a course of hepatitis similar to autoimmune hepatitis triggered by HAV infection. Ursodeoxycholic acid (UDCA) treatment was initiated and a favorable outcome was obtained.
CONCLUSION: We describe a case of a middle-aged woman who showed a prolonged course of acute hepatitis A mimicking autoimmune hepatitis. Treatment with UDCA proved to be effective.
Keywords: Prolonged, IgG, Hepatitis A, Autoimmune hepatitis
INTRODUCTION
Hepatitis A is a common cause of viral hepatitis in humans and is usually a self-limiting disease that resolves within a few weeks after onset. Occasionally a biphasic or relapsing form of hepatitis A occurs[1]. Even in this form, prognosis is good and chronic hepatitis does not occur. Nevertheless, the development of autoimmune hepatitis after HAV infection, albeit rare, has been reported[2-7]. Such reports have described that HAV infection seemed to trigger the development of autoimmune chronic hepatitis.
In this report we describe a case of a middle-aged woman who showed a prolonged course of acute hepatitis A mimicking autoimmune hepatitis.
CASE REPORT
In June 2001, a 48-year-old woman presented with fatigue and fever for a few days, followed by the onset of jaundice. She was admitted to a local hospital. Serum levels of total bilirubin (T-bili), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 7.4 mg/dL, 2780 IU/L and 5560 IU/L respectively. IgM anti-HAV was positive, establishing the diagnosis of acute hepatitis A. During a 4-wk period there was gradual improvement of symptoms and biochemical parameters. Serum levels of T-bili, AST and ALT declined to 2.2 mg/dL, 78 IU/L and 265 IU/L respectively (Figure 1). However, 6 wk after the first onset of jaundice, serum transaminase levels elevated again, and continued at high levels (over 500 U/L) for 2 mo. She was then referred to our department at Chiba University Hospital on October 5, 2001.
Figure 1.
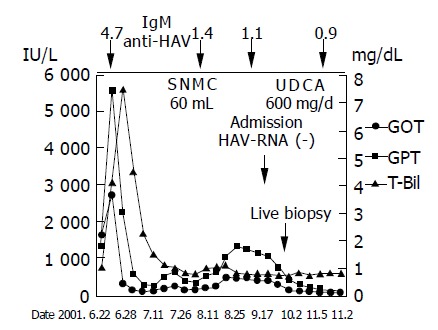
Clinical course of the patient. IgM anti-HAV titers are shown at the top. SNMC: Stronger Neo-Minophagen C; UDCA: ursodeoxycholic acid.
On admission, serum levels of AST, ALT and T-bili were 398 IU/L (normal, <40 IU/L), 1143 IU/L (normal, <40 IU/L) and 0.8 mg/dL (normal, 0.2-1.2 mg/dL) respectively (Table 1). Her IgG level was 2040 mg/dL. Anti-nuclear antibodies (ANA) and anti-smooth muscle antibodies (ASMA) were positive, showing titers of 1:160 and 1:40 respectively. IgM anti-HAV, HAV-RNA, HBsAg, IgM anti-HBc, and anti-HCV were negative. She had no history of alcohol or drug abuse. Abdominal ultrasonography revealed mild splenomegaly without ascites. Liver needle biopsy was performed on October 16 (Figure 2). The general architectonic structure of the liver was preserved. Portal spaces were slightly enlarged with mild fibrosis and were occupied with infiltrates including a few plasma cells. Focal necrosis of the parenchyma was also observed. The limiting plate was mostly preserved. These findings were compatible with prolonged acute hepatitis, although acute onset of autoimmune hepatitis could not be excluded. Treatment with ursodeo-xycholic acid (UDCA) at 600 mg/d was then begun, and rapid improvement of serum transaminase levels was noted. Six months later, the serum levels of transaminase and IgG were within normal limits. UDCA was discontinued 12 mo after initiation and transaminase levels remained within normal range (Figure 1).
Table 1.
Laboratory data on admission.
| Peripheral blood | Blood chemistry | Serology | |||
| WBC | 4 600 /L | T.Bili | 0.8 mg/dL | IgG | 2040 mg/dL |
| RBC | 438 x 10‚S/L | GOT | 398 IU/L | IgA | 274 mg/dL |
| Hb | 13.6 g/dL | GPT | 1143 IU/L | IgM | 158 mg/dL |
| Ht | 39.5% | LDH | 283 IU/L | CRP | 0.1 mg/dL |
| MCV | 90.0 fl | ALP | 317 IU/L | Immunology | |
| MCH | 31.0 pg | -GTP | 349 IU/L | ANA | × 160 |
| MCHC | 34.4% | T-CHO | 170 mg/dL | ASMA | × 40 |
| Plt | 27.8 x 104 /L | TG | 73 mg/dL | AMA | (-) |
| Coagulation | Glu | 95 mg/dL | anti-LKM-1 Ab | (-) | |
| PT | 12.7 s (96%) | BUN | 15 mg/dL | HLA-DR6, DR9 | |
| APTT | 32.2 s | Cr | 0.54 mg/dL | Viral markers | |
| TP | 8.0 mg/dL | IgM HA-Ab | 1.1 | ||
| ESR | 4 mm/1h | Alb | 4.5 g/dL | HAV-RNA | (-) |
| 14 mm/2h | TSH | 0.95 IU/mL | HBs-Ag | (-) | |
| FreeT3 | 3.04 pg/mL | HCV-Ab | (-) | ||
| FreeT4 | 1.16 ng/mL | HCV-RNA | (-) | ||
Abbreviations: ANA, anti-nuclear antibodies; ASMA, anti-smooth muscle antibodies; AMA, anti-mitochondrial antibody; anti-LKM-1 Ab, anti-liver kidney microsome-1 antibody; HLA, human lymphocyte antigens.
Figure 2.
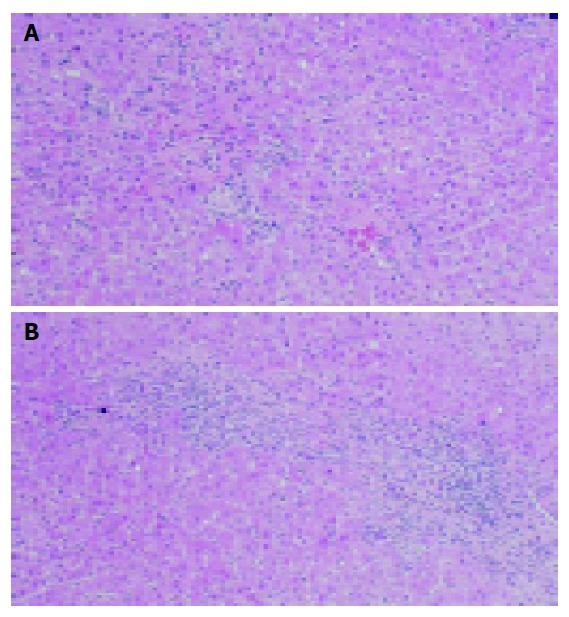
Liver biopsy. A: Focal necrosis of the parenchyma was observed; B: Portal spaces were slightly enlarged with mild fibrosis and were occupied with infiltrates including a few plasma cells. The limiting plate was mostly preserved. Hematoxylin-eosin staining; original magnification ×200.
DISCUSSION
This case showed a prolonged course of acute hepatitis A, with ALT higher than 500 IU/L for more than 2 mo. Schiff described that a biphasic or relapsing form of viral hepatitis A occurred in 6-10% of acute hepatitis A cases, and that the full duration of illness was 16-40 wk in these cases[1]. He also described that, characteristically, there was a persistence of IgM anti-HAV positivity throughout the entire course, and HAV RNA had been detected in the serum during the relapse[1]. In the present case, when serum transaminase re-elevated after initial improvement, IgM anti-HAV continued to decrease, and at admission to our hospital, IgM anti-HAV and HAV RNA were negative. Therefore, it is assumed that the relapse of hepatitis A in the present case was likely not due to persistent HAV infection, but rather the possible involvement of some other factor.
As the patient was a middle-aged woman, the IgG level was elevated to more than 2000 mg/dL, and ANA and ASMA were positive, we could only conclude that the patient developed a course similar to autoimmune hepatitis triggered by HAV infection. Using the international scoring system for the diagnosis of autoimmune hepatitis[8], this patient fulfilled the criteria for a probable diagnosis of AIH. The pathological findings were compatible with acute hepatitis, although acute onset of AIH could not be ruled out.
Obviously, HAV infection is a very rare initiating factor of AIH. There are six reports describing AIH triggered by acute hepatitis A[2-7] (Table 2). In all cases, steroid therapy was initiated resulting in a rapid decline in serum transaminase levels. Recently, the effectiveness of UDCA in the treatment of AIH with no apparent adverse effects, was reported[9]. In the present case, because of a probable diagnosis of AIH, treatment with UDCA was begun, and a gradual improvement in serum transaminase levels was achieved. Six months after UDCA was started, serum transaminase and IgG were within normal limits, and UDCA treatment was discontinued 6 mo later. At present, the patient is well without therapy, and there has been no evidence of recurrence.
Table 2.
Reports of AIH triggered by HAV infection.
| Age | Sex | Interval (wk) | IgG (mg/dL) | Therapy | |
| Vent S, et al (1991) | 13 | F | 17 | N.D. | Steroid |
| Vent S, et al (1991) | 18 | M | 19 | N.D. | Steroid |
| Rahaman SM, et al (1992) | 55 | F | 10 | 2070 | Steroid |
| Huppertz HI, et al (1993) | 7 | M | 10 | 3700 | Steroid |
| Oshikata S, et al (1996) | 70 | M | 28 | 2313 | Steroid |
| Hilzenrat N, et al (1999) | 55 | F | N.D. | N.D. | Steroid |
| Tamura T, et al (2000) | 39 | F | 8 | 2219 | Steroid |
| Present case | 48 | F | 16 | 2040 | UDCA |
N.D.: not described. Interval: from hepatitis A infection to onset of AIH (wk).
In this report, we described a case of prolonged acute hepatitis A mimicking AIH. Although not a definite case of AIH based on the international scoring system, this case may suggest the occurrence of the same pathophysiologic state as AIH triggered by HAV infection.
Footnotes
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10 Suppl 1:S18–S20. doi: 10.1016/0264-410x(92)90534-q. [DOI] [PubMed] [Google Scholar]
- 2.Vento S, Garofano T, Di Perri G, Dolci L, Concia E, Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991;337:1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- 3.Rahaman SM, Chira P, Koff RS. Idiopathic autoimmune chronic hepatitis triggered by hepatitis A. Am J Gastroenterol. 1994;89:106–108. [PubMed] [Google Scholar]
- 4.Huppertz HI, Treichel U, Gassel AM, Jeschke R, Meyer zum Büschenfelde KH. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol. 1995;23:204–208. doi: 10.1016/0168-8278(95)80336-x. [DOI] [PubMed] [Google Scholar]
- 5.Oshikata S, Miyanaga O, Kikuchi I, Mihara K, Ishibashi H. A case of autoimmune hepatitis presumably induced by acute hepatitis type A. Acta Hepatol Japonica. 1996;37:738–743. [Google Scholar]
- 6.Hilzenrat N, Zilberman D, Klein T, Zur B, Sikuler E. Autoimmune hepatitis in a genetically susceptible patient: is it triggered by acute viral hepatitis A? Dig Dis Sci. 1999;44:1950–1952. doi: 10.1023/a:1026645629103. [DOI] [PubMed] [Google Scholar]
- 7.Tamura T, Suzuki S, Yamato A, Sudou I, Harada Y, Motiduki M. Autoimmune hepatitis diagnosed by the opportunity of hepatitis a virus infection. Nihon Shokakibyo Gakkai Zasshi. 2000;97:1043–1047. [PubMed] [Google Scholar]
- 8.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Yoneda M, Yokohama S, Tamori K, Sato Y, Aso K, Aoshima M, Hasegawa T, Makino I. Efficacy of ursodeoxycholic acid in Japanese patients with type 1 autoimmune hepatitis. J Gastroenterol Hepatol. 1998;13:490–495. doi: 10.1111/j.1440-1746.1998.tb00674.x. [DOI] [PubMed] [Google Scholar]


