Abstract
AIM: To evaluate the role of CDX2 homeobox protein as a predictor for cancer progression and prognosis as well as its correlation with MUC2 expression. CDX2 represents a transcription factor for various intestinal genes (including MUC2) and thus an important regulator of intestinal differentiation, which could previously be identified in gastric carcinomas and intestinal metaplasia.
METHODS: Formalin-fixed and paraffin-embedded tissues from 190 gastric carcinoma patients were stained with monoclonal antibodies recognizing CDX2 and MUC2, respectively. Immunoreactivity was evaluated semiquantitatively and statistical analyses including χ2 tests, uni- and multi-variate survival analyses were performed.
RESULTS: CDX2 was mostly expressed in a nuclear or supranuclear pattern, whereas MUC2 showed an almost exclusive supranuclear reactivity. Both antigens were present in >80% of areas exhibiting intestinal metaplasia. An immunoreactivity in >5% of the tumor area was observed in 57% (CDX2) or in 21% (MUC2) of the carcinomas. The presence of both molecules did not correlate with WHO, Laurén and Goseki classification (with the exception of a significantly stronger MUC2 expression in mucinous tumors). CDX2 correlated with a lower pT and pN stage in the subgroups of intestinal and stage I cancers and was associated with MUC2 positivity. A prognostic impact of CDX2 or MUC2 was not observed.
CONCLUSION: CDX2 and MUC2 play an important role in the differentiation of normal, inflamed, and neoplastic gastric tissues. According to our results, loss of CDX2 may represent a marker of tumor progression in early gastric cancer and carcinomas with an intestinal phenotype.
Keywords: CDX2, MUC2, Monoclonal antibody, Gastric carcinoma, Prognosis
INTRODUCTION
CDX2 (caudal type homeobox 2) belongs to the group of homeobox (hox) genes and is characterized by structural and functional similarities to the homeobox gene caudal expressed in Drosophila melanogaster[1-3]. All homeobox genes code for a so-called homeodomain, a typical amino acid sequence (of about 60 amino acids), which binds DNA and controls the transcription of several genes. Thereby, especially the morphologic diversification along the anterior-posterior axis of the body is determined[4,5]. Mice with a homozygote cdx2 knockout die within 5 d after conception, whereas cdx2 heterozygosity leads to malformations of the skeleton and the development of multiple adenomatous polyps, especially in the proximal colon within the first three months of life[6]. During the embryogenesis of intestinal tissues, CDX2 is also involved in the process of proliferation and differentiation[2,7]. In this context CDX2 regulates the expression of sucrase-isomaltase, lactase, phospholipase A/lysophospholipase in the small bowel[2,8] and carboanhydrase 1 in the colon[9].
The distribution of CDX2 in human gastrointestinal tissues was investigated by Mizoshita et al[10]. They observed the highest levels of cdx2 mRNA in the cecum and colon, lower levels in other tracts of the intestine, and a lack of expression in the stomach. However, in cdx2-transgenic mice, gastric epithelia are transformed into intestinal ones[11]. In humans, an infection with Helicobacter pylori induces a CDX2 expression[12], which is characterized by a cytoplasmic or supranuclear staining[13,14]. A nuclear CDX2 expression could be demonstrated in intestinal metaplasia as well as in gastric carcinomas of the intestinal type according to Laurén[13,15]. A positivity of gastric mucosa exhibiting intestinal metaplasia was observed in about 90% of the cases, whereas about 50% of the carcinomas showed a CDX2 immunoreactivity[14,15]. Differentiated adenocarcinomas are characterized by a higher CDX2 expression compared with undifferentiated tumors[15], and correlating with a stronger reactivity in the intestinal vs diffuse phenotype[15,16]. On the other hand, Almeida et al[14], did not observe a significant correlation of CDX2 and the histopathological tumor type, coinciding with the hypothesis that diffuse-type carcinomas may exhibit features of intestinal differentiation[17,18]. Recent studies reported an inverse correlation between CDX2 expression and the depth of invasion as well as lymph node metastasis[15,16]. In a series of 40 patients, those with CDX2 positive tumor had a significantly higher survival probability[15].
Interestingly, CDX2 also binds to the promoter of the intestinal-type mucin MUC2 and thereby activates MUC2 translation and expression[19]. Whereas normal gastric mucosa does not express MUC2[20-24], intestinal metaplasia is characte-rized by a reduction of gastric mucin types (MUC5AC and MUC6) and an ectopic MUC2 production[20,24-26]. In metaplasia[16,26] and in gastric carcinomas[14], CDX2 and MUC2 are co-expressed. However, previous results regarding correlations of MUC2 expression in gastric cancer with clinico-pathological parameters and prognosis are contradictory[18,24,25,27-30]. Therefore, we studied the expression of CDX2 and MUC2 proteins immunohistochemically in a series of 190 patients suffering from gastric adenocarcinomas. The staining results were correlated with each other, various clinical and pathological factors as well as survival data.
MATERIALS AND METHODS
Patients
The study comprised 190 patients, which underwent a potentially curative total or partial gastrectomy between 1982 and 1991. One hundred patients were male, 90 were female. The mean age was 61.1 years (SD±13.0) with a median of 61.3 years. Patients who died within 4 wk after the surgical intervention were excluded from the study (post-operative mortality). A (neo-)adjuvant radio-or chemotherapy was not performed. Surviving patients were followed-up for at least 5 years. All carcinomas were classified pathologically according to the classifications of the UICC[31], WHO[32], Laurén[33], and Goseki et al[34]. The distribution is shown in Table 1.
Table 1.
Correlation of CDX2 and MUC2 expression with clinico-pathological parameters and classifications.
| Parameter | n | CDX2 positive cases (%) | MUC2 positive cases (%) |
| Age (yr) | |||
| ≤60 yr | 87 | 48 (55.2) | 14 (16.1) |
| >60 yr | 103 | 61 (59.2) | 27 (26.2) |
| P | 0.57 | 0.09 | |
| Gender | |||
| Female | 90 | 57 (63.3) | 21 (23.3) |
| Male | 100 | 52 (52) | 20 (20) |
| P | 0.11 | 0.58 | |
| WHO classification | |||
| Mucinous | 4 | 3 (75) | 4 (100) |
| Signet-ring cell | 85 | 49 (57.6) | 13 (15.3) |
| Tubular/papillary | 80 | 47 (58.8) | 20 (25) |
| Undifferentiated | 21 | 10 (47.6) | 4 (19) |
| P | 0.71 | 0.0007 | |
| Lauren classification | |||
| Diffuse | 105 | 61 (58.1) | 19 (18.1) |
| Intestinal | 70 | 40 (57.1) | 18 (25.7) |
| Unclassified/mixed | 15 | 8 (53.3) | 4 (26.7) |
| P | 0.94 | 0.43 | |
| Goseki classification | |||
| Type I | 54 | 30 (55.6) | 14 (25.9) |
| Type II | 22 | 16 (72.7) | 6 (27.3) |
| Type III | 20 | 9 (45) | 3 (15) |
| Type IV | 94 | 54 (57.4) | 18 (19.1) |
| P | 0.33 | 0.60 | |
| pTNM stages | |||
| I | 53 | 29 (54.7) | 7 (13.2) |
| II | 58 | 41 (70.7) | 18 (31) |
| III | 54 | 27 (50) | 10 (18.5) |
| IV | 25 | 12 (48) | 6 (24) |
| P | 0.09 | 0.13 | |
| pT stages | |||
| pT1 | 33 | 16 (48.5) | 4 (12.1) |
| pT2 | 112 | 69 (61.6) | 28 (25) |
| pT3 | 32 | 18 (56.3) | 6 (18.8) |
| pT4 | 13 | 6 (45.2) | 3 (23.1) |
| P | 0.46 | 0.44 | |
| pN stages | |||
| pN0 | 52 | 31 (59.6) | 8 (15.4) |
| pN1 | 76 | 48 (63.2) | 21 (27.6) |
| pN2 | 52 | 25 (48.1) | 9 (17.3) |
| pN3 | 10 | 5 (50) | 3 (30) |
| P | 0.36 | 0.28 |
Tissue preparation, monoclonal antibodies, and immunohistochemical method
Tumor and corresponding normal tissues were fixed in 3.7% formaldehyde and embedded in paraffin. After cutting of 5-µm thick sections, de-paraffinization was performed according to standard pathological procedures, followed by a microwave pre-treatment (2 min×5 min at 600 W in citrate buffer, pH 6.0). Afterwards, sections were washed in cold water for about 15 min. Endogenous peroxidase activity was blocked by 0.3% H2O2 in methanol (20 min, RT). After washing once with water and twice with tris-buffered saline (TBS), pH 7.6, the incubation with monoclonal antibodies directed against CDX2 (Biogenex, San Ramon, USA) or MUC2 (Novocastra, Newcastle-upon-Tyne, UK) followed. Both were incubated in a dilution of 1:100 (v/v) in antibody dilution buffer (Zymed, San Francisco, USA) overnight at 4 °C and TBS was used as negative control. After washing twice with TBS, the sections were incubated with EnVisionTM+HRP (DAKO, Hamburg, Germany) for 30 min at RT. The sections were washed twice again with TBS followed by 3-amino-9-ethylcarbazol solution (DAKO, Hamburg, Germany) in order to visualize the reaction (30 min, RT). Counterstaining with hematoxylin and embedding in glycerol jelly concluded the immunohistochemical staining procedure.
Microscopic scoring and statistical analyses
A semiquantitative microscopic evaluation was performed by two pathologists independently. Nuclear, supranuclear and cytoplasmic staining was scored according to the percentage of positive tumor cells as follows: score 0: 0-5% positive tumor cells; score 1: >5-35% positive tumor cells; score 2: >35-65% positive tumor cells; score 3: >65% positive tumor cells. Different scores were observed in less than 10% of the cases, a consensus could always be achieved. Cases with score 0 were regarded as negative, cases with score 1-3 as positive.
The data were analyzed statistically using the software (version 4.57) StatView for Windows (Abacus, Berkeley, CA, USA). The relationship between CDX2 and MUC2 immunoreactivity, respectively, and clinico-pathological parameters was evaluated using χ2 tests at a significance level of 5%. Univariate survival analysis was performed according to Kaplan-Meier[35], applying the log-rank (Mantel Cox) test.
RESULTS
Immunohistochemical staining patterns of CDX2 and MUC2 in normal gastric mucosa, intestinal metaplasia, and carcinomas
Non-neoplastic gastric mucosa was present in most cases (n = 104) in the neighborhood of the carcinomas. It was mostly reactively altered (inflammatory reactions) and exhibited a supranuclear CDX2 staining in the majority of the cases (54.8%), whereas others showed a cytoplasmic or nuclear reactivity only and 18.3% was CDX2 negative. CDX2 was mostly found in the deep parts of the gastric glands. Only few cases (8.6%) of intestinal metaplasia (n = 58) were completely negative for CDX2 and in the positive areas, a nuclear or mixed staining in several cell compartments predominated. Among the carcinomas, 42.6% did not express CDX2, and most of the positive cases were reactive in the nuclear and supranuclear cell compartments (Figure 1 and Table 2). On the other hand, MUC2 was only detected in a supranuclear pattern. Whereas most normal (93.3%) and carcinomatous (78.4%) tissues remained negative, intestinal metaplasia was immunoreactive in 84.5% of the cases (Figure 1 and Table 2).
Figure 1.
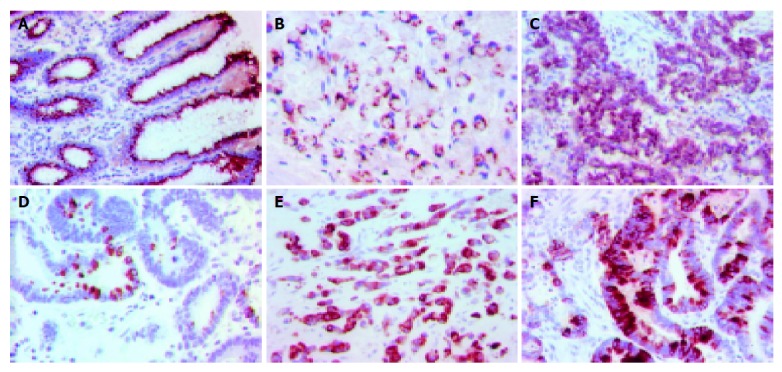
Supranuclear CDX2 immunostaining in non-neoplastic gastric mucosa (A), signet-ring cell carcinoma (B) and tubular adenocarcinoma (C) of the stomach. MUC2 expression in non-neoplastic gastric tissue (D), signet-ring cell (E) and tubular adenocarcinoma (F).
Table 2.
Expression of CDX2 and MUC2 in non-tumorous gastric mucosa, intestinal metaplasia, and carcinomas.
| Localization of staining | Score | Gastric mucosa (%) | Intestinal metaplasia (%) | Carcinoma (%) |
| CDX2 | 0 1 | 9 (18.3) | 5 (8.6) | 81 (42.6) |
| Nuclear | 1 | 2 (1.9) | 3 (5.2) | 6 (3.2) |
| 2 | 1 (1) | 5 (8.6) | 9 (4.7) | |
| 3 | 3 (2.9) | 12 (20.7) | 8 (4.2) | |
| Supranuclear | 1 | 17 (16.3) | 5 (8.6) | 14 (7.4) |
| 2 | 18 (17.3) | 2 (3.4) | 18 (9.5) | |
| 3 | 22 (21.2) | 6 (10.3) | 31 (16.3) | |
| Cytoplasmic | 1 | 10 (9.6) | 0 | 3 (1.6) |
| 2 | 4 (3.8) | 0 | 2 (1.1) | |
| 3 | 3 (2.9) | 0 | 2 (1.1) | |
| Mixed | 1 | 0 | 0 | 2 (1.1) |
| 2 | 3 (2.9) | 1 (1.7) | 2 (1.1) | |
| 3 | 2 (1.9) | 19 (32.8) | 12 (6.3) | |
| MUC2 | 0 | 97 (93.3) | 9 (15.5) | 149 (78.4) |
| Supranuclear | 1 | 5 (4.8) | 19 (32.8) | 22 (11.6) |
| 2 | 2 (1.9) | 14 (24.1) | 13 (6.8) | |
| 3 | 0 | 16 (27.6) | 6 (3.2) |
Correlation of CDX2 and MUC2 immunoreactivity with clinico-pathological parameters
CDX2 or MUC2 positivity or negativity, respectively, was correlated with various clinico-pathological parameters applying χ2 tests. Whereas the status of CDX2 immunoreactivity did not show any association with these variables, MUC2 was significantly stronger in mucinous compared with other types of adenocarcinomas (Table 1). However, if carcinomas of the intestinal type according to Laurén were separately analyzed, a correlation between CDX2 and pTNM as well as pN staging was observed (Table 3). In addition, female and older (>60 years) patients with intestinal type gastric carcinoma exhibited a higher rate of CDX2 positive cases (Table 3) (within this subgroup). On the other hand, MUC2 expression was present in 10.7% of younger (≤60 years) and 26.5% of older patients in the subgroup of diffuse carcinomas according to Laurén (P = 0.04).
Table 3.
Correlation of CDX2 expression with clinico-pathological parameters and classifications in intestinal carcinomas according to Laurén.
| Parameter | n | CDX2 positive cases (%) |
| Age (yr) | ||
| ≤60 yr | 26 | 10 (38.5) |
| >60 yr | 44 | 30 (68.2) |
| P | 0.02 | |
| Gender | ||
| Female | 29 | 21 (72.4) |
| Male | 41 | 19 (46.3) |
| P | 0.03 | |
| pTNM stages | ||
| I | 21 | 13 (61.9) |
| II | 23 | 18 (78.3) |
| III | 17 | 4 (23.5) |
| IV | 9 | 5 (55.6) |
| P | 0.01 | |
| pN stages | ||
| pN0 | 22 | 14 (63.6) |
| pN1 | 26 | 18 (69.2) |
| pN2 | 20 | 6 (30) |
| pN3 | 2 | 2 (100) |
| P | 0.02 |
Correlation of CDX2 and MUC2 immunoreactivity
In χ2 tests, a positive correlation between CDX2 and MUC2 immunoreactivity could be observed in the total group of patients under study, as well as in the following subgroups: intestinal and diffuse type according to Laurén, stage I cancers according to the UICC classification (Table 4).
Table 4.
Correlation of CDX2 and MUC2 expression.
| n | CDX2 negative | CDX2 positive | ||
| All cases | 190 | 81 | 109 | |
| MUC2 negative | 149 | 72 | 77 | |
| MUC2 positive | 41 | 9 | 32 | |
| P | 0.003 | |||
| Intestinal type (Laurén) | 70 | 30 | 40 | |
| MUC2 negative | 52 | 26 | 26 | |
| MUC2 positive | 18 | 4 | 14 | |
| P | 0.04 | |||
| Diffuse type (Laurén) | 105 | 44 | 61 | |
| MUC2 negative | 86 | 41 | 45 | |
| MUC2 positive | 19 | 3 | 16 | |
| P | 0.01 | |||
| Stage I (pTNM) | 53 | 24 | 29 | |
| MUC2 negative | 46 | 24 | 22 | |
| MUC2 positive | 7 | 0 | 7 | |
| P | 0.01 |
Analysis of the prognostic relevance of CDX2 and MUC2
The immunoreactivity of both antigens was tested with regard to a possible prognostic importance according to the univariate survival analysis as described by Kaplan and Meier[35]. CDX2 and MUC2 did not show any significant association with patients’ survival probability (Table 5). The same result was obtained, when subgroups according to Laurén and UICC classification were investigated separately (data not shown).
Table 5.
Univariate survival analysis with regard to the CDX2 and MUC2 expression.
| CDX2 expression | Observations | Uncensored | Censored | Survival (yr) | SD | P (log-rank) |
| Negative | 81 | 57 | 24 | |||
| Q1 (25%) | 0.799 | 0.216 | ||||
| Median | 1.884 | 0.062 | ||||
| Q2 (75%) | 0 | 0 | ||||
| Mean | 3.606 | 0.369 | ||||
| Positive | 109 | 72 | 37 | |||
| Q1 (25%) | 1.060 | 0.069 | ||||
| Median | 2.968 | 1.108 | ||||
| Q2 (75%) | 11.524 | 0 | ||||
| Mean | 5.184 | 0.461 | 0.28 | |||
| MUC2 expression | ||||||
| Negative | 149 | 100 | 49 | |||
| Q1 (25%) | 0.942 | 0.102 | ||||
| Median | 2.171 | 0.368 | ||||
| Q2 (75%) | 11.524 | 0 | ||||
| Mean | 4.966 | 0.394 | ||||
| Positive | 41 | 29 | 12 | |||
| Q1 (25%) | 1.024 | 0.361 | ||||
| Median | 2.557 | 1.284 | ||||
| Q2 (75%) | 0 | 0 | ||||
| Mean | 2.788 | 0.315 | 0.61 |
DISCUSSION
In the present study, a CDX2 immunoreactivity was observed in the majority of gastric mucosal tissue under study. A supra-nuclear staining pattern was prevalent, but a cytoplasmic or nuclear expression was also present, as described earlier[13,14]. Such an expression may be induced by inflammatory reactions caused by H pylori infection and may precede the development of an intestinal phenotype[12,13,15]. Intestinal metaplasia exhibited a stronger CDX2 staining compared with carcinomas (91.4% vs 57.4%). Analogous results of other groups[14-16] are confirmed by our data. Since CDX2 exerts an important function regarding differentiation and maintenance of an intestinal phenotype[2,7], its loss may lead to an uncontrolled proliferation, as observed in the colon[36] and lung[37]. Some authors observed a stronger CDX2 expression in differentiated compared with undifferentiated tumor of the stomach[15,16], whereas we and others[14,36] failed to confirm this finding. With regard to possible correlations with the histopathological tumor type according to Laurén, a stronger CDX2 reactivity in intestinal vs diffuse type cancers was reported[15,16]. Our data are retrieved from a greater series of patients and confirm the results of other authors[14,36], who did not describe such associations. A correlation of CDX2 expression and tumor progression as reflected by staging and lymph node metastasis was present in intestinal-type cancers in our study. In the total patient series a similar tendency was found, which was not significant, as opposed to previous reports[15,16]. Analogously, Seno et al[15], observed a significantly better prognosis of CDX2 positive vs. negative carcinomas in a series of 40 patients. Our data show a similar tendency, which was statistically insignificant, however.
In morphologically normal or reactively altered gastric mucosa, only few glands contained MUC2 positive cells. Such a pattern presumably reflects an initial step of intestinal differentiation, since normal gastric mucosa does not express significant amounts of MUC2[20,24], whereas MUC2 mRNA may be elevated even in the absence of intestinal metaplasia[38]. However, the overwhelming majority of areas exhibiting intestinal metaplasia is MUC2 positive, as observed earlier[24-27]. MUC2 expression in gastric carcinomas varied significantly in dependence from the histological subtype. All mucinous carcinomas under study were immunoreactive, followed by tubular and papillary (25%), undifferentiated (19%) and signet-ring cell (15%) carcinomas according to the WHO classification. A correlation with subtypes according to the Laurén classification was not demonstrated. These data confirm previous observations in part[25,39], whereas other groups reported contradictory results[24,29,38,40,41] especially with regard to associations between MUC2 expression and mucinous differentiation or intestinal vs diffuse type. A significant correlation with pTNM staging and MUC2 expression could not be found, as published by Akyürek et al[24]. Previous data suggesting associations between a positive MUC2 status and a better prognosis[24,28], at least in the group of intestinal carcinomas[25], could not be confirmed in the present study. However, a different monoclonal antibody and another immunohistochemical detection system were applied.
As described earlier[14], CDX2 and MUC2 expression correlated positively. This association was independent from the histopathological subtype (Laurén classification) and an analogous result was obtained in stage I tumors. Considering the results of Satoh et al[13], it is tempting to speculate that gastric mucosa cells exhibiting nuclear CDX2 represent a transient form resulting in intestinally differentiated metaplastic cells. This hypothesis fits in with the role of CDX2 as a transcription factor of intestinal gene products like MUC2. Confirming such a functional relationship, binding of CDX2 to a special sequence of the MUC2 promoter was demonstrated in cdx2-transfected COS-7 cells[19]. This sequence contains a CDX2 protein binding site TTTA(C/T), which was also described in the context of other typical intestinal genes like sucrase-isomaltase[2], lactase[42], and guanylyl cyclase-C[43].
In conclusion, our data confirm that CDX2 represents a transcription factor which is involved in different aspects of gastric pathogenesis. It may be of special importance in inflammatory conditions resulting in the development of intestinal metaplasia. However, CDX2 expression is reduced in gastric carcinomas as compared with intestinal metaplasia. On the other hand, it is associated with tumor progression in the subgroup of intestinal carcinomas according to Laurén as well as early cancers (stage I). A significant correlation with survival probability was not observed at all. In addition, CDX2 expression is positively associated with MUC2 levels. The latter is strongly detectable in mucinous adenocarcinomas but does not exhibit correlations with other relevant clinico-pathologically parameters of gastric carcinomas.
Footnotes
Supported by the Cologne Fortune Program
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Macdonald PM, Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986;324:537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- 2.Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond F, Putt W, Fox M, Edwards YH. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet. 1997;61:393–400. doi: 10.1046/j.1469-1809.1997.6150393.x. [DOI] [PubMed] [Google Scholar]
- 4.Gehring WJ, Müller M, Affolter M, Percival-Smith A, Billeter M, Qian YQ, Otting G, Wüthrich K. The structure of the homeodomain and its functional implications. Trends Genet. 1990;6:323–329. doi: 10.1016/0168-9525(90)90253-3. [DOI] [PubMed] [Google Scholar]
- 5.Cillo C. HOX genes in human cancers. Invasion Metastasis. 1994;14:38–49. [PubMed] [Google Scholar]
- 6.Chawengsaksophak K, James R, Hammond VE, Köntgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 7.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troelsen JT, Mitchelmore C, Spodsberg N, Jensen AM, Norén O, Sjöström H. Regulation of lactase-phlorizin hydrolase gene expression by the caudal-related homoeodomain protein Cdx-2. Biochem J. 1997;322(Pt 3):833–838. doi: 10.1042/bj3220833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond F, Sowden J, Morrison K, Edwards YH. The caudal-type homeobox protein Cdx-2 binds to the colon promoter of the carbonic anhydrase 1 gene. Eur J Biochem. 1996;236:670–681. doi: 10.1111/j.1432-1033.1996.t01-1-00670.x. [DOI] [PubMed] [Google Scholar]
- 10.Mizoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T, Kato T, Joh T, Itoh M, Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa--with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer. 2001;4:185–191. doi: 10.1007/pl00011741. [DOI] [PubMed] [Google Scholar]
- 11.Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, Osawa H, Kaneko Y, Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 12.Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, Sugano K. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:94–100. doi: 10.1007/s005350200002. [DOI] [PubMed] [Google Scholar]
- 13.Satoh K, Mutoh H, Eda A, Yanaka I, Osawa H, Honda S, Kawata H, Kihira K, Sugano K. Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: effect of eradication of Helicobacter pylori. Helicobacter. 2002;7:192–198. doi: 10.1046/j.1523-5378.2002.00080.x. [DOI] [PubMed] [Google Scholar]
- 14.Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36–40. doi: 10.1002/path.1246. [DOI] [PubMed] [Google Scholar]
- 15.Seno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T, Taketo MM. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int J Oncol. 2002;21:769–774. doi: 10.3892/ijo.21.4.769. [DOI] [PubMed] [Google Scholar]
- 16.Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, Kenji Yagi O, Saitoh K, Takeshita K, Iwai T, et al. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47–55. doi: 10.1016/s0304-3835(01)00753-4. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro F, Moutinho-Ribeiro M, David L, Seixas M, Sansonetty F, Soares P, Serrano A, Sobrinho-Simões M. Signet ring cell carcinoma of the stomach: a morphometric, ultrastructural, and DNA cytometric study. Ultrastruct Pathol. 1992;16:603–614. doi: 10.3109/01913129209023750. [DOI] [PubMed] [Google Scholar]
- 18.Reis CA, David L, Carvalho F, Mandel U, de Bolós C, Mirgorodskaya E, Clausen H, Sobrinho-Simões M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48:377–388. doi: 10.1177/002215540004800307. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300:813–818. doi: 10.1016/s0006-291x(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 20.Carrato C, Balague C, de Bolos C, Gonzalez E, Gambus G, Planas J, Perini JM, Andreu D, Real FX. Differential apomucin expression in normal and neoplastic human gastrointestinal tissues. Gastroenterology. 1994;107:160–172. doi: 10.1016/0016-5085(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 21.De Bolós C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 22.Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681–2690. [PubMed] [Google Scholar]
- 23.Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Akyürek N, Akyol G, Dursun A, Yamaç D, Günel N. Expression of MUC1 and MUC2 mucins in gastric carcinomas: their relationship with clinicopathologic parameters and prognosis. Pathol Res Pract. 2002;198:665–674. doi: 10.1078/0344-0338-00318. [DOI] [PubMed] [Google Scholar]
- 25.Baldus SE, Zirbes TK, Engel S, Hanisch FG, Mönig SP, Lorenzen J, Glossmann J, Fromm S, Thiele J, Pichlmaier H, et al. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int J Cancer. 1998;79:133–138. doi: 10.1002/(sici)1097-0215(19980417)79:2<133::aid-ijc6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 26.Reis CA, David L, Correa P, Carneiro F, de Bolós C, Garcia E, Mandel U, Clausen H, Sobrinho-Simões M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 27.Filipe MI, Linehan JM, Durrant LG, Price MR, Smeeton NC, Pathak S, Swallow DM. Expression of a peptide epitope of the colonic mucin MUC2 in precursor lesions to gastric carcinoma. Eur J Cancer Prev. 1996;5:287–295. doi: 10.1097/00008469-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998;4:2605–2614. [PubMed] [Google Scholar]
- 29.Sakamoto H, Yonezawa S, Utsunomiya T, Tanaka S, Kim YS, Sato E. Mucin antigen expression in gastric carcinomas of young and old adults. Hum Pathol. 1997;28:1056–1065. doi: 10.1016/s0046-8177(97)90059-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer. 2001;92:1427–1434. doi: 10.1002/1097-0142(20010915)92:6<1427::aid-cncr1466>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Sobin C, Wittekind C TNM Classification of Malignant Tumours. 5th edition. New York: Wiley; 1997. [Google Scholar]
- 32.Hamilton SR, Aaltonen LA. WHO classification of tumours: Pathology and genetics of tumours of the digestive system. Lyon: IARC Press, 2000 [Google Scholar]
- 33.Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 34.Goseki N, Takizawa T, Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut. 1992;33:606–612. doi: 10.1136/gut.33.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan EL, Meier J. Nonparametric estimations from incomplete observations. J Am Assoc Stat. 1958;53:457–481. [Google Scholar]
- 36.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol. 2001;159:2239–2248. doi: 10.1016/S0002-9440(10)63074-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsuuchi M, Hinoda Y, Itoh F, Endo T, Satoh M, Xing PX, Imai K. Expression of MUC2 gene in gastric regenerative, metaplastic, and neoplastic epithelia. J Clin Lab Anal. 1999;13:259–265. doi: 10.1002/(SICI)1098-2825(1999)13:6<259::AID-JCLA2>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto-de-Sousa J, David L, Reis CA, Gomes R, Silva L, Pimenta A. Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch. 2002;440:304–310. doi: 10.1007/s00428-001-0548-y. [DOI] [PubMed] [Google Scholar]
- 40.Taylor KL, Mall AS, Barnard RA, Ho SB, Cruse JP. Immunohistochemical detection of gastric mucin in normal and disease states. Oncol Res. 1998;10:465–473. [PubMed] [Google Scholar]
- 41.Gürbüz Y, Kahlke V, Klöppel G. How do gastric carcinoma classification systems relate to mucin expression patterns? An immunohistochemical analysis in a series of advanced gastric carcinomas. Virchows Arch. 2002;440:505–511. doi: 10.1007/s00428-002-0620-2. [DOI] [PubMed] [Google Scholar]
- 42.Fang R, Santiago NA, Olds LC, Sibley E. The homeodomain protein Cdx2 regulates lactase gene promoter activity during enterocyte differentiation. Gastroenterology. 2000;118:115–127. doi: 10.1016/s0016-5085(00)70420-3. [DOI] [PubMed] [Google Scholar]
- 43.Park J, Schulz S, Waldman SA. Intestine-specific activity of the human guanylyl cyclase C promoter is regulated by Cdx2. Gastroenterology. 2000;119:89–96. doi: 10.1053/gast.2000.8520. [DOI] [PubMed] [Google Scholar]


