Abstract
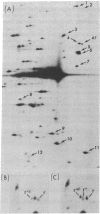
The motility mutant of Chlamydomonas reinhardtii pf14 lacks radial spoke structures in its flagellar axonemes, and 12 proteins present in wild type are missing from a two-dimensional map (isoelectrofocusing/sodium dodecyl sulfate electrophoresis) of its 35S-labeled flagellar proteins. Six of these same proteins are missing in pf1, which lacks spoke-heads. To determine whether any of the missing proteins represent the mutant gene product two experimental approaches have been applied. The first makes use of the fact that gametes of either mutant strain when fused with wild-type gametes to form quadriflagellate dikaryons undergo recovery of flagellar function. Recovery at the molecular level was monitored by prelabeling the mutant proteins with 35S and allowing recovery to occur in the absence of protein synthesis. It is to be expected that the mutant gene product would not be restored as a radioactive protein and that recovery would depend on the assembly of the wild-type counterpart that is not labeled. The second technique makes use of revertants induced by UV irradiation. Dikaryon rescue in the case of pf14 leads to restoration of 11 radioactive components; only protein 3 fails to appear as a radioactive spot. For pf1 only two radioactive proteins are restored; proteins 4, 6, 9, and 10 were not radioactive. Analysis of revertants of pf1 gave evidence (altered map positions) that protein 4 is the mutant gene product. In the case of pf14, analysis of 22 revertants has not provided similar positive evidence that protein 3 is the gene product.
Keywords: cell motility, axonemal structure, protein assembly, mutant gene product
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GIBBONS I. R. STUDIES ON THE PROTEIN COMPONENTS OF CILIA FROM TETRAHYMENA PYRIFORMIS. Proc Natl Acad Sci U S A. 1963 Nov;50:1002–1010. doi: 10.1073/pnas.50.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Rifkin M. R., Luck D. J. Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J Cell Biol. 1977 Jan;72(1):67–85. doi: 10.1083/jcb.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R. P., EBERSOLD W. T. The genetics and cytology of Chlamydomonas. Annu Rev Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- LEWIN R. A. Mutants of Chlamydomonas moewusii with impaired motility. J Gen Microbiol. 1954 Dec;11(3):358–363. doi: 10.1099/00221287-11-3-358. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F. D., Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974 Oct;63(1):35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]