Abstract
AIM: To investigate the expression pattern of clusterin in colorectal adenoma-carcinoma-metastasis series, and to explore the potential role of clusterin in multistage colorectal tumorigenesis and progression.
METHODS: A colorectal carcinoma (CRC)-tissue microarray (TMA), which contained 85 advanced CRCs including 43 cases of Dukes B, 21 of Dukes C and 21 of Dukes D tumors, were used for assessing the expression of clusterin (clone 41D) and tumor cell apoptotic index (AI) by immunohist-ochemistry and TUNEL assay, respectively. Moreover the potential correlation of clusterin expression with the patient’s clinical-pathological features were also examined.
RESULTS: The positive staining of clusterin in different colorectal tissues was primarily a cytoplasmic pattern. Cytoplasmic overexpression of clusterin was detected in none of the normal colorectal mucosa, 17% of the adenomas, 46% of the primary CRCs, and 57% of the CRC metastatic lesions. In addition, a significant positive correlation between overexpression of clusterin and advanced clinical (Dukes) stage was observed (P<0.01). Overexpression of cytoplasmic clusterin in CRCs was inversely correlated with tumor apoptotic index (P<0.01), indicating the anti-apoptotic function of cytoplasmic clusterin in CRCs.
CONCLUSION: These data suggests that overexpression of cytoplasmic clusterin might be involved in the tumorigenesis and/or progression of CRCs. The anti-apoptotic function of cytoplasmic clusterin may be responsible, at least in part, for the development and biologically aggressive behavior of CRC.
Keywords: Clusterin, Colorectal tumorigenesis
INTRODUCTION
Colorectal carcinoma (CRC) is one of the most common human cancers and a major cause of cancer-related death in the developed countries[1]. The incidence of CRC in China including urban Shanghai, is increasing rapidly particularly in the last two decades[2]. At present, CRC is the fourth leading cause of cancer-related death in China. Although CRC has been widely studied and the development of this cancer is most probably a multistep process involving multiple genetic changes, the understanding of the precise pathogenic mechanisms by which normal colorectal epithelial cells become malignant cells, is far from being thoroughly understood.
Clusterin, also known as apolipoprotein J (ApoJ), is a heterodimeric highly conserved and secreted glycoprotein being expressed in a wide variety of tissues and found in many body fluids. Clusterin has been implicated in diverse normal biological processes such as sperm maturation, lipid transportation, tissue remodeling, membrane recycling, cell-cell and cell-substratum interactions and cell apoptosis[3-7]. Recently, a potential oncogenic role of clusterin in the development and/or progression of several human cancers has been examined. Increased expression of clusterin was detected in prostate, renal cell and lung cancer[8,9]. Expression of clusterin has been positively associated with the aggressive nature of breast cancer, while the expression of clusterin in normal breast epithelial cells has been undete-ctable[10].
Moreover, increased expression of clusterin in murine and human intestinal neoplasia has been reported[11]. However, to date, the expression pattern of clusterin and its oncogenic role in the development of human CRC are unclear. In the present study, the expression dynamics of clusterin in an array of human colorectal tissue, normal and pathological, non-neoplastic and neoplastic, were investigated using a tissue microarray (TMA) assay. The potential correlation between clusterin expression and critical clinical/pathological features of CRC were also assessed.
MATERIALS AND METHODS
Patients and tissues
Eighty-five surgically resected CRC specimens were obtained from the First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China as previously described[12]. The age of these patients ranged from 36 to 85 years at the time of surgery (mean age, 59.6 years) and the male/female ratio was 1.2:1. All cases selected were moderately differentiated adenocarcinomas. According to Dukes staging system, the distribution for clinical stage of these CRCs were as follows: Dukes B, 43 cases; Dukes C, 21 cases; Dukes D, 21 cases. A previously constructed TMA block containing all 85 CRC cases was used for immunohistochemical (IHC) and TUNEL assay[12]. In this CRC-TMA, six samples from different pathologic and progressive loci were selected from each of the 85 advanced CRC cases. Tissue samples from adjacent non-neoplastic mucosa, primary carcinomas in inner layers (confined to the layer of mucosa and/or submucosa), muscularis layer and serosa layer of the bowel wall were selected for all CRC cases. Tissue samples from matched adenomatous polyps were obtained from 22 cases (tubular, n = 12; tubulo-villous, n = 3; and villous, n = 7) and tissues from matched lymph node and distant metastases were selected from 21 cases.
IHC staining
IHC studies were performed using a standard streptavidin-biotin-peroxidase complex method[12]. In brief, TMA sections were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 20 min. For antigen retrieval, CRC-TMA slides were microwave-treated in 10 mmol/L citrate buffer (pH 6.0) for 10 min. Nonspecific binding was blocked with 10% normal rabbit serum for 10 min. The TMA slides were incubated with a monoclonal anti-clusterin (Clone 41D; Upstate Biotechnology, Lake Placid, NY, USA, 1:200 dilution) and a monoclonal anti-Ki-67 (Dako, Glostrup, Denmark, 1:100 dilution) for 60 min at 37 °C in a moist chamber, respectively. The slides were then sequentially incubated with a biotinylated rabbit anti-mouse immunoglobulin at a concentration of 1:100 for 30 min at 37 °C and subsequently reacted with a streptavidin-peroxdase conjugate for 30 min at 37 °C and 3’-3’ diaminobenzidine as a chromogen substrate. The nucleus was counterstained using Meyer’s hematoxylin. The slides were dehydrated and coverslipped. Negative controls were performed by replacing the primary antibody with mouse IgG. Known immunostaining positive slides of ovarian cancer were used as positive controls.
For evaluation of the clusterin IHC staining in different colorectal tissues, the positive expression of clusterin in non-malignant and malignant tissues was primarily a cytoplasmic pattern (Figure 1). Both staining intensity and positive areas were recorded. A staining index (values 0-12), obtained as the intensity of clusterin positive staining (negative = 0, weak = 1, moderate = 2, or strong = 3 scores) and the proportion of immunopositive cells of interest (<25% = 1; 25-50% = 2; >50-75% = 3; >75% = 4 scores), was calculated. The status of Ki-67 nuclear expression was counted as the percentage of Ki-67 positive staining cells in each case. The immunostained slides of TMA were reviewed independently twice and intra-observer disagreements (<10%) were reviewed a third time, followed by a conclusive judgment.
Figure 1.
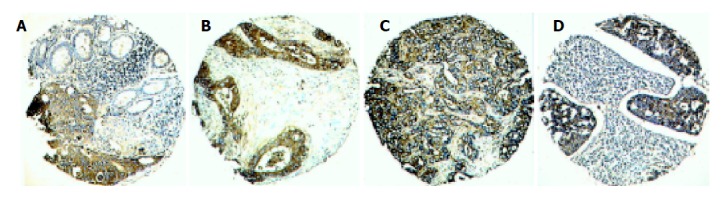
Expression of clusterin in different pathological loci of one Dukes C colorectal carcinoma with matched lymph node metastasis. Strong cytoplasmic staining of clusterin was observed in primary carcinoma in the mucosa layer of the bowel wall, while its adjacent normal mucosa showed a weak positivity (A). Strong cytoplasmic staining of clusterin was also observed in carcinomas in muscularis propria (B), and serosa layer (C) and its matched lymph node metastasis (D) in the same primary carcinoma (original magnification ×100).
TUNEL assay
The fluorescent TUNEL staining was performed using a death detection kit (Roche Diagnostic GmbH, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, the rehydrated TMA section was microwave-treated in 10 mmol/L citrate buffer (pH 6.0) for 5 min. After washing in phosphate-buffered saline (PBS), the specimen was incubated with a mixture of TdT solution (enzyme solution) and FITC labeled dUTP solution (label solution) in a humidified chamber in the dark at 37 °C for 60 min. After washing, the slide was examined with a Zeiss Axiophot fluorescence microscope. Negative controls were obtained by replacing the TdT solution with distilled water. The presence of clear nuclear staining (TUNEL-positive, green color) was indicative of apoptotic cells (Figure 2). Apoptotic bodies were defined as TUNEL-positive, single, relatively large (≥ 4 µm diameter) and rounded bodies existing in extra- or intra-tumor cells with intense staining. The number of TUNEL-positive tumor cell nuclei was counted and the apoptotic index (AI) was determined as the percentage of apoptotic cells in the tumor. Because the mean value of AI for all informative samples in this study was 1.9, tumors were classified into two groups according to their AI: low AI group (AI≤1.9) and high AI group (AI>1.9).
Figure 2.
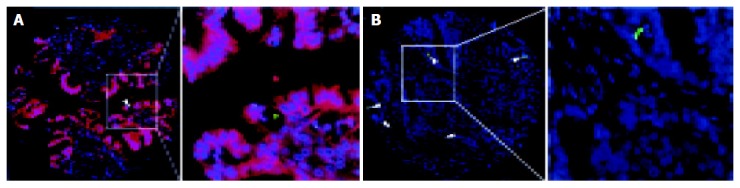
Double fluorescent staining of clusterin and TUNEL in colorectal carcinoma. A: A colorectal carcinoma with overexpression of cytoplasmic clusterin (red) showed low apoptotic index (AI), where apoptosis (green signal indicated with arrows) was observed in only one tumor cell; B: High AI was observed in a CRC with negative expression of cytoplasmic clusterin, where several TUNEL positive cells (green nuclei indicated with arrows) were observed. (Left figure: original magnification ×100; Right figure: ×400).
To investigate the correlation of clusterin expression and cell apoptosis, a simultaneous IHC staining with anti-clusterin antibody and fluorescent TUNEL staining was performed. First, clusterin immunostaining was performed as described above. The second antibody was used with a Cy3 (orange) labeled goat anti-mouse polyclonal IgG (SC-20009, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:100 dilution) and incubated with the section, in the dark, at 37 °C for 45 min. The slide was washed with PBS and then counterstained with 1 µg/mL DAPI in an anti-fade solution, then examined with a Zeiss Axiophot fluorescence microscope equipped with a dual band pass filter for simultaneous visualization of FITC and Spectrum Orange signals using ×10 and ×40 objectives.
Statistical analysis
For statistical evaluation, χ2 test for trend was used to assess the differential expression of clusterin in different colorectal tissues. χ2 test was used to assess the statistical significance of the association of the expression of clusterin with the patient’s clinico-pathological parameters and its correlation with apoptotic indices. Unpaired t test was used to assess the statistical significance of the differential expression of Ki-67 between groups with and without clusterin overexpression. P values lesser than 0.05 were considered significant.
RESULTS
Clusterin expression in colorectal tissues
The expression of clusterin was investigated by IHC in a CRC-TMA which contained adjacent normal colorectal mucosa and different colorectal lesions including premalignant colorectal adenoma, primary CRC, and metastatic CRC. The antibody (clone 41D) used in this study was a monoclonal anti-human clusterin, which recognizes the a subunit of the clusterin heterodimer. The expression pattern of clusterin in epithelial cells of different colorectal tissues was heterogeneous with different staining indices in the cytoplasm. Because the staining index of cytoplasmic expression of clusterin in all 76 informative normal colorectal mucosa was less or equal to 6, we designated the staining index of 0-6 as the normal expression of clusterin. Overexpression of clusterin was defined when staining index was more than 6 (Figure 1B-1D). Using this designation, the overexpression of cytoplasmic clusterin was detected in 4/20 (17%) informative colorectal adenomas, 39/85 (46%) primary CRCs, and 20/35 (57%) lymph node and/or distant metastatic lesions, respectively. The increasing frequencies of clusterin overexpression from normal colorectal mucosa, to benign adenomas and primary CRC, and to CRC metastatic lesion were significant (P<0.01, χ2 trend test, Table 1). However, no heterogeneous expression of clusterin was observed among the different layers of the bowel wall of CRC.
Table 1.
Expression of clusterin in normal colorectal mucosa and in benign and malignant colorectal tumors1.
| Informative cases |
Clusterin |
||
| Normal expression | Overexpression | ||
| Normal mucosa | 76 | 76 (100%) | 0 (0) |
| Adenoma | 20 | 16 (83%) | 4 (17%) |
| Primary carcinoma | 85 | 46 (54%) | 39 (46%) |
| Metastases | 35 | 15 (43%) | 20 (57%) |
1Values are n (%). A significant increasing frequency of overexpression of clusterin was observed in adenoma, in primary carcinoma and in metastatic lesion (P<0.01, χ2 test for trend.
Association of clusterin expression with clinico-pathological features
The association of cytoplasmic expression of clusterin and patient’s clinico-pathological features were further studied. The results showed that cytoplasmic overexpression of clusterin in primary CRC was significantly associated with the patient’s clinical stage. Overexpression of clusterin was detected in 25/42 (60%) CRCs in late clinical stages (Dukes C or D stage), which was significantly higher than that in early stage (Dukes B) (14/43, 33%) (P<0.05, χ2 test). But no significant association was found between clusterin expression and other clinico-pathological features, such as patient’s gender, age, and tumor location (data not shown).
Correlation of clusterin expression with cell apoptosis
Because clusterin has been associated with the process of cell apoptosis[7], the TUNEL assay was used to study the status of apoptosis in these CRC cohorts. A high apoptotic index was detected in 32/83 (39%) of the informative CRCs. The results showed that the frequency of high apoptotic index was significantly higher in tumors with a normal expression of clusterin (24/46 cases, 52%) than that in cases with overexpression of clusterin (8/37, 22%) (P<0.01, Table 2). In addition, the double fluorescent staining with clusterin and TUNEL showed that apoptosis was more likely to occur in tumor cells with low level expression of clusterin, but not in tumor cells with high level of expression of clusterin (Figure 2A). In general, cell apoptosis in CRC was inversely correlated with the overexpression of clusterin.
Table 2.
Correlation of expression of clusterin and apoptotic index (AI) in colorectal carcinomas.
| Clusterin |
Apoptotic index (AI) |
|||
| Informative case | High | Low | P1 | |
| Overexpression (%) | 37 | 8 (22) | 29 (78) | <0.01 |
| Normal expression (%) | 46 | 24 (52) | 22 (48) | |
1χ2 test.
Correlation of clusterin expression with cell proliferation
To identify whether or not the overexpression of clusterin was associated with cell proliferation in colorectal cancer, we further analyzed the potential correlation between the expression of clusterin and cell proliferation (via monoclonal anti-ki-67). For the 39 cases with overexpression of clusterin, an average of 36.7% of the tumor cells was positively stained with Ki-67 antibody, which was similar to that (average of 38.1% cells) in the remaining 46 tumors with normal expression of clusterin (P>0.05, unpaired-t test).
DISCUSSION
Most CRCs arise from adenomas through an archetypal pathogenic pathway, the adenoma-carcinoma-metastasis sequence. CRC is a well-studied example of multistep tumorigenesis and, therefore, offers an excellent model to investigate the accumulation of genetic alterations during this neoplastic process. In the present study, we used a previously constructed TMA-CRC series[12], which was composed of multiple colorectal tissues from different pathological loci of the same patient samples. These samples represented the full spectrum of CRC pathogenesis, including adjacent normal mucosa, benign adenoma, primary carcinomas and lymph node and distant metastases. Overall, overexpression of clusterin was observed to increase from the earliest detectable stage of abnormal growth, adenoma, to primary carcinoma and to local lymph node or distant metastases. These findings provide evidence that the increased expression of clusterin is involved in CRC tumorigenesis and progression. In addition, we found that the overexpression of cytoplasmic clusterin in primary CRCs was strongly associated with patients’ Dukes stage, i.e., overexpression of clusterin in cytoplasm was more frequently detected in later Dukes stages (C and D) when compared to that in Dukes B. These results suggest that overexpression of cytoplasmic clusterin in CRC may facilitate cancer cell invasion and metastasis. Previous studies have also documented that increased expression of clusterin was involved in the develop-ment and progression of several types of carcinomas, including breast, prostate and kidney carcinomas[8-10]. Taken together, clusterin expression may be an important prognostic factor of aggressive nature of several human cancers, including CRC.
Although some hypothetical indirect tumorigenic functions of clusterin could be speculated due to its implication in cell-cell and cell-substratum interactions, and in a cytoprotective role in plasma membrane[13], the biological role in tumorigenesis remains unclear. Trougakos and colleagues have reported that clusterin knockdown in human cancer cell, by using small interfering RNA, may induce higher rates of spontaneous apoptosis and significantly reduce tumor cell growth [14]. Other groups have reported a significant increase in cell death after transfection of antisense oligonucleotides into clusterin[15]. Some authors have suggested that the anti-apoptotic activity of clusterin may account for the genesis and biologically aggressive behavior of several cancer cells[10,16]. In the present study, a significant inverse correlation of clusterin expression and apoptosis was revealed in our CRC cohorts, i.e., a lower apoptotic index was more likely to be observed in CRCs with overexpression of cytoplasmic clusterin than in those with normal expression of this protein. Furthermore, tumor cells undergoing apoptosis were often found with a lower expression level of cytoplasmic clusterin when compared to their adjacent tumor cells without apoptosis. These results demonstrate as well an anti-apoptotic function of clusterin in CRCs.
Clusterin has been implicated in apoptosis as a pro-or anti-apoptotic molecule in various models under different circumstances, in which clusterin may exert different biological functions[13]. Recently, it has become increasingly clear that the clusterin gene codes for a family of different protein isoforms[17], which are derived, by alternative post-translational processes, from the same precursor of 53 ku protein[18,19]. Different isoforms of clusterin have been previously reported to occur, in apoptotic and surviving cells, in the regressing rat ventral prostate[4]. Nuclear clusterin has been suggested as a cell death protein[20]. Recently, data from in vivo and in vitro studies of clusterin in colon tumorigenesis have demonstrated that nuclear clusterin was predominantly expressed in normal mucosa of colon and may act as a pro-apoptosis protein, while cytoplasmic clusterin may function as an anti-apoptosis protein[16]. In the colorectal tumors of the present study, the antibody (clone 41D) used was a monoclonal anti-human clusterin, which recognizes the α subunit of the clusterin heterodimer. The staining of clusterin in colorectal epithelial cells was only a cytoplasmic pattern and nuclear staining was not observed in colorectal epithelial tissues, non-neoplastic and neoplastic. Although further studies are needed to determine the exact function of the different isoforms of clusterin and how the signaling pathways through clusterin gene are regulated, the present study has demonstrated: (1) a cytoplasmic expression pattern of clusterin in the full spectrum of CRC pathogenesis, from normal mucosa, to adenoma, to carcinoma, to metastasis; and (2) an increased level of cytoplasmic clusterin was directly associated with decreased cell apoptosis, increased aggres-siveness and metastasis, suggesting that this protein may play an important role in the multistage development and/or progression of human CRCs.
Footnotes
Supported by the Natural Science Foundation of China, No. 30300401, Guangdong Natural Science Foundation, No. 04009327, and the Leung Kwok Tze Foundation of Hong Kong, China
Co-correspondents: Dan Xie
References
- 1.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 2.Jin F, Devesa SS, Chow WH, Zheng W, Ji BT, Fraumeni JF, Gao YT. Cancer incidence trends in urban shanghai, 1972-1994: an update. Int J Cancer. 1999;83:435–440. doi: 10.1002/(sici)1097-0215(19991112)83:4<435::aid-ijc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BF, Kirszbaum L, Walker ID, d'Apice AJ. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988;81:1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronow BJ, Lund SD, Brown TL, Harmony JA, Witte DP. Apolipoprotein J expression at fluid-tissue interfaces: potential role in barrier cytoprotection. Proc Natl Acad Sci USA. 1993;90:725–729. doi: 10.1073/pnas.90.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratelli M, Galli G, Minto M, Pasinetti GM. Role of clusterin in cell adhesion during early phases of programmed cell death in P19 embryonic carcinoma cells. Biochim Biophys Acta. 1996;1311:71–76. doi: 10.1016/0167-4889(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem. 1999;274:6875–6881. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- 7.O'Sullivan J, Whyte L, Drake J, Tenniswood M. Alterations in the post-translational modification and intracellular trafficking of clusterin in MCF-7 cells during apoptosis. Cell Death Differ. 2003;10:914–927. doi: 10.1038/sj.cdd.4401254. [DOI] [PubMed] [Google Scholar]
- 8.Parczyk K, Pilarsky C, Rachel U, Koch-Brandt C. Gp80 (clusterin; TRPM-2) mRNA level is enhanced in human renal clear cell carcinomas. J Cancer Res Clin Oncol. 1994;120:186–188. doi: 10.1007/BF01202200. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg J, Oyasu R, Lang S, Sintich S, Rademaker A, Lee C, Kozlowski JM, Sensibar JA. Intracellular levels of SGP-2 (Clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res. 1997;3:1707–1711. [PubMed] [Google Scholar]
- 10.Redondo M, Villar E, Torres-Muñoz J, Tellez T, Morell M, Petito CK. Overexpression of clusterin in human breast carcinoma. Am J Pathol. 2000;157:393–399. doi: 10.1016/S0002-9440(10)64552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Halberg RB, Ehrhardt WM, Torrealba J, Dove WF. Clusterin as a biomarker in murine and human intestinal neoplasia. Proc Natl Acad Sci USA. 2003;100:9530–9535. doi: 10.1073/pnas.1233633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie D, Sham JS, Zeng WF, Lin HL, Che LH, Wu HX, Wen JM, Fang Y, Hu L, Guan XY. Heterogeneous expression and association of beta-catenin, p16 and c-myc in multistage colorectal tumorigenesis and progression detected by tissue microarray. Int J Cancer. 2003;107:896–902. doi: 10.1002/ijc.11514. [DOI] [PubMed] [Google Scholar]
- 13.Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34:1430–1448. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 14.Trougakos IP, So A, Jansen B, Gleave ME, Gonos ES. Silencing expression of the clusterin/apolipoprotein j gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64:1834–1842. doi: 10.1158/0008-5472.can-03-2664. [DOI] [PubMed] [Google Scholar]
- 15.Sensibar JA, Sutkowski DM, Raffo A, Buttyan R, Griswold MD, Sylvester SR, Kozlowski JM, Lee C. Prevention of cell death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin) Cancer Res. 1995;55:2431–2437. [PubMed] [Google Scholar]
- 16.Pucci S, Bonanno E, Pichiorri F, Angeloni C, Spagnoli LG. Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene. 2004;23:2298–2304. doi: 10.1038/sj.onc.1207404. [DOI] [PubMed] [Google Scholar]
- 17.Wong P, Pfeffer BA, Bernstein SL, Chambers ML, Chader GJ, Zakeri ZF, Wu YQ, Wilson MR, Becerra SP. Clusterin protein diversity in the primate eye. Mol Vis. 2000;6:184–191. [PubMed] [Google Scholar]
- 18.Lakins J, Bennett SA, Chen JH, Arnold JM, Morrissey C, Wong P, O'Sullivan J, Tenniswood M. Clusterin biogenesis is altered during apoptosis in the regressing rat ventral prostate. J Biol Chem. 1998;273:27887–27895. doi: 10.1074/jbc.273.43.27887. [DOI] [PubMed] [Google Scholar]
- 19.Wong P, Ulyanova T, Organisciak DT, Bennett S, Lakins J, Arnold JM, Kutty RK, Tenniswood M, vanVeen T, Darrow RM, et al. Expression of multiple forms of clusterin during light-induced retinal degeneration. Curr Eye Res. 2001;23:157–165. doi: 10.1076/ceyr.23.3.157.5463. [DOI] [PubMed] [Google Scholar]
- 20.Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;278:11590–11600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]


