Abstract
AIM: To investigate the expression of several important molecules involved in major histocompatibility complex (MHC) class I presentation pathway in primary hepatocellular carcinoma (HCC), and to determine whether cytotoxic T lymphocyte (CTL) vaccine therapy was suitable for HCC.
METHODS: Labeled streptavidin biotin (LSAB) method of immunohisto-chemistry was used to study 33 HCC tissue specimens.
RESULTS: Most HCC tissues and adjacent histological normal hepatocytes expressed HLA-I antigens,TAP, and B7, expression of B7 was especially strong, and there was no significant difference between them (P>0.05).
CONCLUSION: The MHC class I presentation pathway in primary hepatocellular carcinoma may not be abnormal or dysfunctional, and CTL could kill these tumor cells. Thus, it is suitable and practicable to design and construct CTL vaccine against HCC.
Keywords: MHC I, Hepatocellular carcinoma
INTRODUCTION
Cell-mediated immunity plays an important role in anti-tumor immune response, especially lysis by cytotoxic T lymphocytes (CTL)[1]. Intact MHC I presentation pathway is crucial for introduction of cellular immunity and killing by CTL. The transporter associated with antigen processing (TAP), human leukocyte antigen (HLA)-I antigens and B7 are important molecules of the MHC I presentation pathway. TAP is a transmembrane transport protein located in endoplasmic reticulum (ER) and its main function is to translocate endogenously processed antigenic peptides from cytosol into ER lumen. There, antigenic peptides and HLA class I molecules assemble into a HLA I molecule-antigenic peptide complex, and then this complex is translocated to the surface of cell and recognized by CD8+T lymphocytes to provide the first signal of T cell activation. B7 is the most important costimulatory molecule of antigen-presenting cells (APCs), and it binds to its partner (CD28) on the surface of T cells to deliver the second signal. Upon the double signal stimulation T cells proliferate and differentiate to initiate cell immunity. At the effective stage, lysis of target cells by CTL is also dependent on recognition of HLA I molecule-antigenic peptide complex on the target cells. Many tumors down-regulate or lose expression of TAP, HLA-I antigens or B7 molecules leading to dysfunction or defect of MHC I presentation pathway to escape from immune surveillance of the host[1-7]. The aim of CTL epitope-based vaccine is to induce and produce specific CTL response. Therefore, it is necessary to investigate the expression of TAP, HLA-I antigens and B7 in human primary hepatocellular carcinoma, and to understand the relationship between the tumor and host before design and construction of CTL epitope-based vaccine against HCC.
MATERIALS AND METHODS
Main reagents
Mouse anti-human B7 mAb and HLA-ABC mAb were purchased from DAKO Corp. Rabbit anti-human TAP pAb was from Chemicon International, Inc. Biotin labeled goat anti-mouse IgG, HRP-labeled streptavidin and avidin biotin blocking system were from Beijing Zhongshan Corp.
Tumor specimens
Thirty-three pathological specimens were obtained from surgically resected tissues of patients with HCC in West China Hospital of Sichuan University, two of them without adjacent histological normal hepatocytes. All specimens were fixed in 40 g/L formaldehyde, embedded in paraffin. Consecutive sections (5 µm) were prepared and attached to loading-slides smeared with APES before. Pathological diagnoses were made based on routinely processed HE sections.
Immunohistochemistry
The sections were dewaxed and rehydrated. Endogenous peroxidase was blocked with 30 mL/L H2O2 for 15 min. After retrieval of antigens, the sections were incubated with normal goat serum for blocking non-specific antigens and subsequently with antibody (anti-TAP, HLA-ABC and B7, respectively) or PBS as control at 37 °C for 1 h, washed and incubated with biotinylated goat anti-mouse IgG or goat anti-rabbit IgG at 37 °C for 30 min. After being washed as before, HRP labeled streptavidin was added. The following incubation and washing were exactly the same as above. Finally, DAK working solution was added for color development. The reaction was stopped with tap water rinse. Then, the sections were counterstained with hematoxylin and mounted for examination.
Statistical analysis
The differences between HCC tissues and adjacent histological normal hepatic tissues were analyzed with χ2-square test. P<0.05 was considered statistically significant.
RESULTS
Expression of HLA-I antigens
Positive staining of HLA-I antigens appeared in brown. The staining was mainly located on the cell membrane, in the cytoplasm and perinuclear area of tumor cells and hepatocytes. The cytoplasmic staining showed a granular pattern. The adjacent hepatic tissues squeezed by tumor were stained more strongly, and there was negative staining in connective tissues between lobes of liver (Figure 1A).
Figure 1.
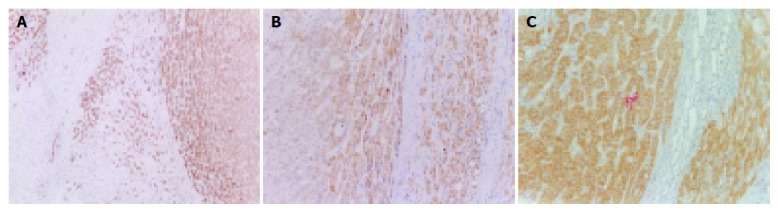
Expression of HLA-I antigens,TAP and B7 in HCC. A: Expression of HLA-I antigens; B: Expression of TAP; C: Expression of B7.
HLA-I antigens expression was strongly positive in all HCC specimens. Thirty of thirty-one (96.8%) adjacent hepatic tissues showed positive staining.
Expression of TAP
Positive staining of TAP appeared in brown, and the staining was mainly located in the cytoplasm and perinuclear area of tumor cells and hepatocytes. The staining showed a granular pattern. The hepatic tissues adjacent to tumor were stained more intensely, and connective tissues between lobes of liver were negatively stained (Figure 1B).
TAP expression was detected in 32 of 33 HCC specimens (97.0%). The positive expression of TAP was present in all 31 normal hepatic tissues adjacent to tumor.
Expression of B7 molecules
The very strongly positive expression of B7 molecules was detected in 33 HCC tissues and 30 adjacent non-neoplastic hepatic tissues. The positive staining located on the cell membrane and filled cytosol, and showed a granular intracytoplasmic pattern. No B7 molecule was detected in connective tissues between lobes of liver (Figure 1C).
Comparison between HCC and adjacent normal hepatic tissues
Expressions of HLA-I antigens, TAP and B7 were strongly positive in 32 of 33 cases of HCC (97.0%).Twenty-nine of thirty-one cases expressed these molecules (93.5%) in adjacent normal hepatic tissues. The statistical analysis showed there was no significant difference in expressions between HCC and adjacent normal hepatic tissues (P>0.05).
DISCUSSION
HCC is the most common malignant tumor of liver, and the second killer cancer in China. Because of unsatisfactory curative effects of surgical therapy, radiotherapy and chemotherapy, and the high rate of recurrence, much effort has been devoted to HCC immunotherapy[1]. Currently, antitumor CTL epitope-based vaccines aimed to induce specific CTL have been widely considered for cancer immunotherapy[8]. Two basic problems need to be solved for the design and construction of CTL vaccines. One is how to induce tumor-specific CTL, the other is killing tumor cells by CTL. Both of them are associated with the function of MHC I presentation pathway in tumor cells. Dysfunction or defect of MHC I presentation pathway could lead to no expression of HLA I molecule-antigenic peptides on the surface of tumor cells, and specific CTL could not recognize the tumor cells and kill them. Therefore, it is necessary to investigate the expression of several important molecules involved in MHC class I presentation pathway in HCC before design and construction of CTL vaccines targeting tumor-associated antigens (TAA) of HCC.
It has been generally acknowledged that tumors usually escape from host immune surveillance by dysfunction or defect of MHC I presentation pathway, such as decrease or defect expression of TAP, HLA-I antigens or B7 molecule[1-7]. But our results showed the majority of HCC tissues and adjacent normal tissues expressed important components of MHC I presentation pathway, which is consistent with the results from human HCC cell lines by other groups. Sung et al[9], and Wadee et al[10], found that several human HCC cell lines strongly express HLA-I antigens by different ways. Kurokohchi et al[11], detected both HLA-I antigen and TAP expression in several liver cancer cell lines by PCR. Furthermore, Butterfield et al[12], obtained three hAFP CTL epitope peptides (hAFP158-166, hAFP325-334, hAFP542-550) from the surface of AFP-producing human HCC cell line HepG2 by acid elution, and this showed that HepG2 had a functional MHC I presentation pathway able to process and present AFP naturally. These results together with our finding indicate that MHC class I presentation pathway may not be abnormal or dysfunctional in most HCCs and adjacent liver tissues, and TAA may be processed and presented naturally in most HCCs. So, specific CTLs are able to recognize and kill HCC cells, the CTL epitope-based vaccine is suitable for immunotherapy of HCC. It also implies that tumor escape is attributed to post-antigen presentation events.
Wei et al[13], proposed that immunotolerance was one of the main mechanisms for tumors to escape from host immune surveillance, and tumors were regarded as a kind of special autoantigens, which are normally tolerable to host immune system. Tumors also escape from immune surveillance through suppressing host immunity by varied ways. Si et al[14], found that B7 molecules were expressed in many cancerous human tissues. Chaperot et al[15], found that malignant B cells from non-Hodgkin’s lymphomas expressed functional B7 molecules. This is consistent with our finding, suggesting that tumor growth is not only attributed to defect of costimulatory molecules in APC. Dendritic cells (DC) are the most potent professional APC of our body, and play an important role in antitumor immunity. The data showed that tumor environment could inhibit activation and maturation, and some tumor infiltrating cells could even secrete IL-10 to suppress host antitumor immunity[16,17]. It was reported that DC in human regional lymph nodes draining cancer exhibited a functional depletion as compared to those from patients without malignancies[18]. Recently, Chen et al[19], reported that neuroblastoma cells could inhibit the immune function of DC. Our studies also showed that DC from peripheral blood of patients with HCC were in a state of low immune function (data unpublished). Taken together, it indicates that the host of tumor is possibly in a state of immunotolerance or immunosuppression. So it is suitable and practicable to design and construct CTL epitope-based vaccines against HCC.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30070855
References
- 1.Melief CJ, Kast WM. Lessons from T cell responses to virus induced tumours for cancer eradication in general. Cancer Surv. 1992;13:81–99. [PubMed] [Google Scholar]
- 2.Ruiz-Cabello F, Garrido F. HLA and cancer: from research to clinical impact. Immunol Today. 1998;19:539–542. doi: 10.1016/s0167-5699(98)01349-8. [DOI] [PubMed] [Google Scholar]
- 3.Cromme FV, Airey J, Heemels MT, Ploegh HL, Keating PJ, Stern PL, Meijer CJ, Walboomers JM. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994;179:335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosch SB, Izbicki JR, Pichlmeier U, Stoecklein N, Niendorf A, Knoefel WT, Broelsch CE, Pantel K. Expression and prognostic significance of immunoregulatory molecules in esophageal cancer. Int J Cancer. 1997;74:582–587. doi: 10.1002/(sici)1097-0215(19971219)74:6<582::aid-ijc4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Fan P, Wang S, Liu X, Zhen L, Wu Z. Major histocompatibility complex class II antigen and costimulatory molecule expression on the surface of breast cancer cells. Zhonghua Zhong Liu Za Zhi. 2002;24:327–330. [PubMed] [Google Scholar]
- 6.Lang S, Whiteside TL, Lebeau A, Zeidler R, Mack B, Wollenberg B. Impairment of T-cell activation in head and neck cancer in situ and in vitro: strategies for an immune restoration. Arch Otolaryngol Head Neck Surg. 1999;125:82–88. doi: 10.1001/archotol.125.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Stopeck AT, Gessner A, Miller TP, Hersh EM, Johnson CS, Cui H, Frutiger Y, Grogan TM. Loss of B7.2 (CD86) and intracellular adhesion molecule 1 (CD54) expression is associated with decreased tumor-infiltrating T lymphocytes in diffuse B-cell large-cell lymphoma. Clin Cancer Res. 2000;6:3904–3909. [PubMed] [Google Scholar]
- 8.Bona CA, Casares S, Brumeanu TD. Towards development of T-cell vaccines. Immunol Today. 1998;19:126–133. doi: 10.1016/s0167-5699(97)01218-8. [DOI] [PubMed] [Google Scholar]
- 9.Sung CH, Hu CP, Hsu HC, Ng AK, Chou CK, Ting LP, Su TS, Han SH, Chang CM. Expression of class I and class II major histocompatibility antigens on human hepatocellular carcinoma. J Clin Invest. 1989;83:421–429. doi: 10.1172/JCI113900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadee AA, Paterson A, Coplan KA, Reddy SG. HLA expression in hepatocellular carcinoma cell lines. Clin Exp Immunol. 1994;97:328–333. doi: 10.1111/j.1365-2249.1994.tb06089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurokohchi K, Carrington M, Mann DL, Simonis TB, Alexander-Miller MA, Feinstone SM, Akatsuka T, Berzofsky JA. Expression of HLA class I molecules and the transporter associated with antigen processing in hepatocellular carcinoma. Hepatology. 1996;23:1181–1188. doi: 10.1002/hep.510230537. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield LH, Meng WS, Koh A, Vollmer CM, Ribas A, Dissette VB, Faull K, Glaspy JA, McBride WH, Economou JS. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol. 2001;166:5300–5308. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 13.Wei YQ. Immunotherapy of tumors with vaccines based on xenogeneic homologous molecules. Anticancer Drugs. 2002;13:229–235. doi: 10.1097/00001813-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Si L, Si H, Chen Y, Sun Y, Wing Y. B7-1 antigen expression in tumor cells from cancerous human tissues. Anal Quant Cytol Histol. 1999;21:521–526. [PubMed] [Google Scholar]
- 15.Chaperot L, Plumas J, Jacob MC, Bost F, Molens JP, Sotto JJ, Bensa JC. Functional expression of CD80 and CD86 allows immunogenicity of malignant B cells from non-Hodgkin's lymphomas. Exp Hematol. 1999;27:479–488. doi: 10.1016/s0301-472x(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 16.Hart DN, Schultze JL, Stewart AK. Presentation of tumor antigens. Semin Hematol. 1999;36:21–25. [PubMed] [Google Scholar]
- 17.Biggs MW, Eiselein JE. Suppression of immune surveillance in melanoma. Med Hypotheses. 2001;56:648–652. doi: 10.1054/mehy.2000.1211. [DOI] [PubMed] [Google Scholar]
- 18.Laguens G, Coronato S, Laguens R, Portiansky E, Di Girolamo V. Human regional lymph nodes draining cancer exhibit a profound dendritic cell depletion as comparing to those from patients without malignancies. Immunol Lett. 2002;84:159–162. doi: 10.1016/s0165-2478(02)00172-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Doffek K, Sugg SL, Shilyansky J. Neuroblastoma cells inhibit the immunostimulatory function of dendritic cells. J Pediatr Surg. 2003;38:901–905. doi: 10.1016/s0022-3468(03)00119-2. [DOI] [PubMed] [Google Scholar]


