Abstract
AIM: To report a patient with C282Y homozygocity, depleted body iron and intestinal atrophy caused by celiac disease (CD) who experienced resolution of the enteropathy with subsequent normalization of iron metabolism upon gluten-free diet.
METHODS: To obtain information on the tissue distribution and quantitative expression of proteins involved in duodenal iron trafficking, we determined the expression of divalent-metal transporter 1 (DMT1), ferroportin 1 (FP1) and transferrin receptor (TfR1) by means of immunohist-ochemistry and real-time PCR in duodenal biopsies of this patient.
RESULTS: Whereas in hereditary hemochromatosis patients without CD, DMT1 expression was up-regulated leading to excessive uptake of iron, we identified a significant reduction in protein and mRNA expression of DMT1 as a compensatory mechanism in this patient with HH and CD.
CONCLUSION: Occult CD may compensate for increased DMT1 expression in a specific subset of individuals with homozygous C282Y mutations in the hemochromatosis (HFE) gene, thus contributing to the low penetrance of HH.
Keywords: Hemochromatosis, Celiac disease, Divalent-metal transporter 1, Transferrin receptor, Iron metabolism
INTRODUCTION
Hereditary hemochromatosis (HH) is an inherited disorder of iron metabolism that is characterized by excessive gastrointestinal iron absorption with subsequent iron deposition in major organs of the body[1,2]. Autosomal recessive transmission of the HH gene and genetic linkage to the HLA complex has been known since the mid-1970s[3,4]. In 1996 a candidate gene for HH, now known as HFE, was detected by positional cloning[5]. Two missense mutations were initially identified in the HFE gene, leading to an exchange of cysteine to tyrosine at position 282 (C282Y) or a change from histidine to aspartate at position 63 (H63D)[5]. Although the homozygous C282Y genotype affects between 1 in 200 and 1 in 400 persons of northern European descent, only a considerably low percentage of these homozygotes develop clinically severe hemochromatosis[6]. Thus, genetic or environmental factors affecting the penetrance of hemochromatosis in patients with HFE mutations still remain to be elucidated.
Iron deficiency anemia in patients with HH is very rarely discovered due to proposed algorithms, which suggest genotyping only in patients with fasting transferrin saturation higher than 45%[1,2]. However, rare cases are documented and may be associated with disorders leading to duodenal atrophy with subsequent iron malabsorption. To date only three patients with HH and iron deficiency anemia associated with celiac disease (CD) have been reported in the world literature[7-9]. In this contribution, we report another patient with C282Y homozygosity, depleted body iron and intestinal atrophy caused by CD. Whereas in HH patients without CD divalent-metal transporter 1 (DMT1) expression is up-regulated leading to excessive uptake of iron, we identified a significant reduction in protein and mRNA expression of DMT1 in duodenal biopsies as a mechanism which modulates iron stores in a patient with HH and CD.
CASE REPORT
A 65-year-old Caucasian female patient presented with chronic diarrhea and weight loss of 10 kg over 4 years. Diarrheal episodes occurred in association with cereals and milk products. An initial lactose-free diet failed to improve symptoms but a recent trial of gluten-free diet brought partial relief at the time of referral. Her history was unremarkable except for the fact that she suffered from hemochromatosis 14 years ago and was treated with phlebotomy over nearly a decade. Two years ago HFE genotyping detected a homozygous C282Y mutation. Nevertheless an oral iron substitution therapy had to be started because she developed an iron deficiency anemia. The initial evaluation revealed the persistence of iron deficiency anemia (Table 1). Endomysium (1:160, reference <1:10), gliadin (IgG 37 kU/L, IgA 740 kU/L, reference <12 kU/L each) and tissue transglutaminase antibodies (253 U, reference <20 U) were strongly positive. Histological examination of small bowel biopsies detected intestinal atrophy with lymphocytic infiltrates suggestive for CD. Within 3 mo of gluten-free diet, diarrhea completely resolved and milk products were tolerated well. Iron substi-tution was stopped when serum iron, ferritin and hemoglobin reached normal values after 4 mo of gluten-free diet (Table 1). She gained 15 kg of weight within 1 year. Control small bowel biopsies showed resolution of the enteropathy, and a breath test with H2 lactose proved resolution of the secondary lactose intolerance.
Table 1.
Clinical chemistry data.
| First visit time of biopsy | End of follow up control biopsy | Reference values | |
| Hemoglobin (g/L) | 112 | 153 | 120-160 |
| Hematocrit | 35 | 45 | 0.37-0.47 |
| Serum iron (µmol/L) | 7.0 | 26.0 | 11.1-31.1 |
| Transferrin (g/L) | 2.5 | 1.9 | 2.0-3.6 |
| Transferrin saturation (%) | <15 | 54 | 16-45 |
| Ferritin (µg/L) | 11 | 78 | 30-150 |
Under iron substitution 100 mg q.d.
Assays
Serum clinical chemistry was performed by standard techniques with an autoanalyzer.
HFE genotyping
Genomic DNA was extracted from whole blood and light cycler (Roche, Mannheim, Germany) amplification of the H63D and C282Y variant fragments with subsequent melting curve analysis was performed using standard cycle conditions. The C187G transversion at codon 63 was monitored with the 3’-fluorescein-labeled anchor 5’-CTTGAAATTCTA-CTGGAAACCCATGGAGTTCGGGGCTCC-3’ and the 5’-LC-Red640-labeled sensor 5’-CACGGCGACTCTCA-TCATCATAGAACACGAACA-3’ probe. The G845A transition at codon 282 was simultaneously analyzed with the 3’-fluorescein-labeled sensor 5’-AGATATACGTAC-CAGGTGGAG-3’ and the 5’-LC-Red640-labeled anchor probe 5’-CCCAGGCCTGGATCAGCCCCTCATTGT-GATCTGGG-3’.
TaqMan real-time PCR
TaqMan real-time PCR primers and TaqMan probe for quantification of DMT1 cDNA and FP1 cDNA were designed as previously described[10]. For the quantification of TfR1 cDNA the following intron spanning primers were used: sense 5’-TCCCAGCAGTTTCTTTCTGTTTT-3’; antisense 5’-CTCAATCAGTTCCTTATAGGTGTCCA-3’. The TaqMan probe 5’-CGAGGACACAGATTATC-CTTATTTGGGTACCACC-3’ was labeled with the reporter fluorescent dye 6-carboxyfluorescein at the 5’ end and with the quencher 6-carboxytetramethyl-rhodamine at the 3’end. For quantification of DMT1, FP1, and TfR1 mRNA levels in duodenal biopsy specimens TaqMan real-time PCR was carried out in the ABIPrism 7700 sequence detector (Applied Biosystems, Vienna, Austria) as previously described[10].
Immunohistochemistry
Routinely formalin-fixed, paraffin-embedded duodenal biopsies were used for immunohistochemical staining performed as previously described[10]. Sections were incubated with 0.1 mL of 300 µg/mL affinity-purified anti-DMT1 (260-275) or anti-FP1 (240-254) antiserum and a biotin-coupled goat anti-rabbit immunoglobulin (Ig) G (Dako, Vienna, Austria) in a 1:500 dilution. Similar immunohistochemical methods, as described for DMT1 and FP1, were used for TfR1, using a monoclonal mouse anti-human TfR1 antibody (Zymed Laboratories, South San Francisco, CA, USA; code 13-6800) diluted 1:200. Sections were incubated with the TfR1 antibody and a biotin-coupled goat anti-mouse immunoglobulin (Ig)G (Dako) in a 1:800 dilution. Subseq-uently, the reaction product was developed as previously described.
Informed consent was obtained for all analyses described in this section.
Outcomes
In order to get an estimate of duodenal iron absorption in this patient who was homozygous for the C282Y mutation (data not shown), we determined DMT-1, FP1 and TfR1 expression by means of immunohistochemistry and TaqMan real-time PCR. To obtain information on the tissue distribution and expression of the proteins, we performed immunohistochemical staining of duodenal biopsy specimens. DMT1, the transmembrane protein, which is centrally involved in absorption of ferrous iron from the intestinal lumen, was expressed at decreased levels at the apical site of enterocytes and recovered significantly during therapy with gluten-free diet (Figures 1A and 1C). In contrast, TfR1 expression was highly increased at the basolateral site before initiation of treatment, when the patient was iron deficient. During follow up, TfR1 expression drastically decreased in line with regeneration of the epithelium as shown in Figures 1B and 1D. FP1 expression was largely unaltered either in atrophic epithelium or during regeneration after therapy (immunohistochemistry not shown).
Figure 1.
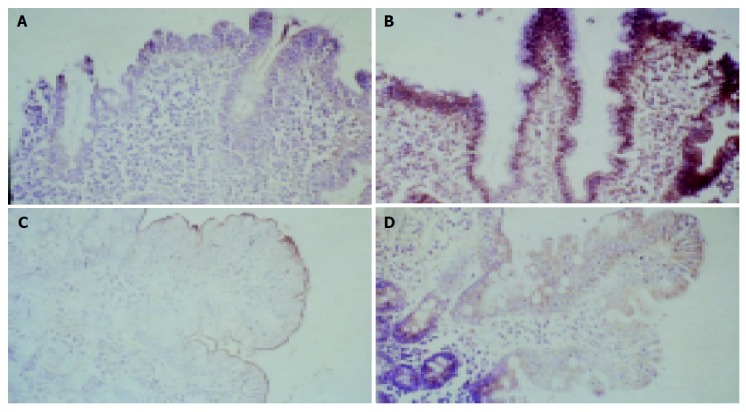
Immunohistochemical staining for DMT1 and TfR1 of duodenal biopsy specimens of the patient taken before (upper panels A and B) and after gluten free diet (lower panels C and D).
To quantify these changes in expression during treatment mRNA levels of DMT1, FP1, and TfR1 were determined by real-time PCR. DMT1 mRNA expression was minimal with subtotal atrophy of the duodenal epithelium but was elevated fourfold after therapy. In contrast, TfR1 expression was inversly related to body iron stores and fell to one-third with regeneration of the epithelium and restoration of iron uptake (Figure 2). Consistent with our immunohistochemistry, FP1 mRNA was only slightly increased during follow-up. These findings indicate that the inverse regulation of basolateral TfR1 expression which is believed to be a sensor for the body’s need for iron, was functional in our patient with HH, whereas DMT1 appeared to be differentially regulated in comparison to other patients with hemochromatosis but no CD.
Figure 2.
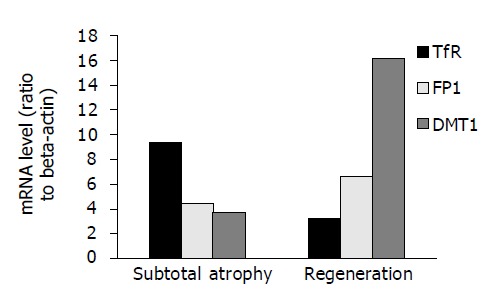
TfR1, FP1 and DMT1 cDNA levels in duodenal biopsy specimens.
DISCUSSION
Over 90% of patients with HH are homozygous for the C282Y mutation of the HFE gene, but the majority of patients, who have been identified as being homozygous for the C282Y mutation, have no evidence of iron overload. The best current estimate of penetrance for C282Y homozygosity is that less than 1% of homozygotes develop frank clinical hemochromatosis[6]. In contrast, another population based study found a higher prevalence of 50%[11], but these data are difficult to interpret since clinical findings are not matched to a control group. Overall population prevalence for clinical HH and HH-related death appears to be far less than expected from C282Y allele frequency and heterozygosity frequency[12].
Normal HFE protein is expressed in cryptal enterocytes of the small intestine and acts to facilitate the iron-sensing function of these cells by a physical association with the transferrin receptor (TfR1) at a strategic site to influence TfR1-mediated iron transport[13]. This facilitory effect is lost by C282Y mutant HFE resulting in a ‘relative’ iron deficiency of enterocytes and an uncoupling of iron uptake regulation[2,14]. This uncoupling leads to increased expression of the divalent-metal transporter 1 (DMT1) and ferroprotein 1 (FP1)[2,14,17]. Whereas DMT1 represents a transmembrane transporter in the apical membrane of duodenal enterocytes which facilitates iron uptake from the intestinal lumen into the enterocytes. FP1 is involved in basolateral iron export into the portal circulation[2,14].
Iron transport across the basolateral plasma membrane of villus enterocytes involves intact epithelia with a sufficient amount of basolateral and apical transport proteins[2,14]. Atrophy of the duodenal mucosa in chronic inflammatory disorders of the small bowel such as CD leads to iron deficiency[15]. Our immunohistochemistry data implicate that a marked reduction of basolateral DMT1 in the atrophic intestinal epithelium causes secondary malabsorption of iron and prevents clinical iron overload. A control biopsy taken on gluten-free diet showed resolved duodenal enteropathy paralleled by ferritin levels within the normal range. Restored iron uptake was accompanied with increased expression of DMT1, which was higher than levels usually present in healthy controls in both immunohistochemical staining and real-time PCR. This pattern of transporter expression after therapy meets with previous data from patients with HH, which show an increased expression of DMT1[10,16,17]. In contrast, FP1 expression was not restored during the observation period but showed a tendency to increase similar to HH patients without CD[10,16,17]. The data clearly demonstrate that the impaired expression of iron transporters in enteropathic mucosa is different from patients with HH and iron overload and compensates for the uncoupling of iron uptake in homozygous C282Y HFE mutants. DMT1 down-regulation in patients with both HH and CD may cause even iron deficiency and anemia although the transferrin receptor is still physiologically regulated by the body’s iron store. Increased duodenal tissue levels of transferrin receptor expression during epithelial atrophy rather favor a selective regulatory process leading to DMT1 down-regulation than an unselective loss of specialized epithelium. Based on these quantitative data and the clinical recovery of our patient from iron deficiency on a gluten-free diet, we hypothesize that CD masks overt HH due to a reversible reduction in the iron transporter DMT1 and therefore could prevent the penetrance of HH.
CD accounts for up to 8.5% of iron deficiency anemia of unknown etiology, especially when refractory to oral supplementation[18], and iron deficiency appears to be the most frequent and sometimes only extraintestinal symptom in CD[19]. Recovery from iron deficiency anemia in CD usually occurs between 6 and 12 mo on gluten-free diet alone as a consequence of normalization of histological alterations on the intestinal mucosa[15]. The prevalence of this disease is difficult to ascertain, because many patients have atypical symptoms or none at all[20], but it seems more common than previously considered and ranges between 0.5% in the general population[21] and 1.6% among patients undergoing endoscopy[22]. CD and hemochromatosis are common HLA defined conditions with surprisingly high frequencies in populations of Northern Europe commonly attributed to survival advantages. A genetic association between CD and the HLA-D locus has emerged and it has been shown that over 95% of patients express the DQα1*0501 DQβ1*0201 heterodimer (HLA DQ2)[20,23]. This locus on chromosome 6p is in close proximity to the HFE locus[24]. Recently, HFE gene mutations have been found to be common and in linkage disequilibrium with different HLA alleles in CD patients compared with controls[25]. A disease specific haplotype that carries both the C282Y HFE gene mutation and HLA DQ2 has been suggested but the origins of the genetic linkage still remain to be investigated in detail[25]. The HFE gene may have spread due to the protection of heterozygotes against iron deficiency[26] and the same might be true for CD which diminishes iron overload. We hypothesize that the genetic predisposition for either disease ameliorates the manifestation of the other thereby leading to a marked extent of unidentified disease. Occult CD may prevent increased DMT1 expression in a specific subset of individuals with homozygous C282Y mutations in the HFE gene thus contributing to the low penetrance of HH.
Footnotes
Science Editor Wang XL Language Editor Elsevier HK
References
- 1.Bacon BR. Hemochromatosis: diagnosis and management. Gastroenterology. 2001;120:718–725. doi: 10.1053/gast.2001.21913. [DOI] [PubMed] [Google Scholar]
- 2.Philpott CC. Molecular aspects of iron absorption: Insights into the role of HFE in hemochromatosis. Hepatology. 2002;35:993–1001. doi: 10.1053/jhep.2002.33466. [DOI] [PubMed] [Google Scholar]
- 3.Simon M, Bourel M, Fauchet R, Genetet B. Association of HLA-A3 and HLA-B14 antigens with idiopathic haemochromatosis. Gut. 1976;17:332–334. doi: 10.1136/gut.17.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon M, Bourel M, Genetet B, Fauchet R. Idiopathic hemochromatosis. Demonstration of recessive transmission and early detection by family HLA typing. N Engl J Med. 1977;297:1017–1021. doi: 10.1056/NEJM197711102971901. [DOI] [PubMed] [Google Scholar]
- 5.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Ellis MC, Fullan A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 6.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G--& gt; A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359:211–218. doi: 10.1016/S0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- 7.Morris WE. Hemochromatosis and celiac sprue. Case report. J Fla Med Assoc. 1993;80:243–245. [PubMed] [Google Scholar]
- 8.Heneghan MA, Feeley KM, Stevens FM, Little MP, McCarthy CF. Precipitation of iron overload and hereditary hemochromatosis after successful treatment of celiac disease. Am J Gastroenterol. 2000;95:298–300. doi: 10.1111/j.1572-0241.2000.01556.x. [DOI] [PubMed] [Google Scholar]
- 9.Turcu A, Lévêque L, Bielefeld P, Besancenot JF, Hillon P. Adult celiac disease and hemochromatosis. Am J Gastroenterol. 2000;95:3661–3662. doi: 10.1111/j.1572-0241.2000.03403.x. [DOI] [PubMed] [Google Scholar]
- 10.Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]
- 11.Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med. 1999;341:718–724. doi: 10.1056/NEJM199909023411002. [DOI] [PubMed] [Google Scholar]
- 12.Jackson HA, Carter K, Darke C, Guttridge MG, Ravine D, Hutton RD, Napier JA, Worwood M. HFE mutations, iron deficiency and overload in 10,500 blood donors. Br J Haematol. 2001;114:474–484. doi: 10.1046/j.1365-2141.2001.02949.x. [DOI] [PubMed] [Google Scholar]
- 13.Parkkila S, Niemelä O, Britton RS, Fleming RE, Waheed A, Bacon BR, Sly WS. Molecular aspects of iron absorption and HFE expression. Gastroenterology. 2001;121:1489–1496. doi: 10.1053/gast.2001.29617. [DOI] [PubMed] [Google Scholar]
- 14.Pietrangelo A. Physiology of iron transport and the hemochromatosis gene. Am J Physiol Gastrointest Liver Physiol. 2002;282:G403–G414. doi: 10.1152/ajpgi.00404.2001. [DOI] [PubMed] [Google Scholar]
- 15.Annibale B, Severi C, Chistolini A, Antonelli G, Lahner E, Marcheggiano A, Iannoni C, Monarca B, Delle Fave G. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am J Gastroenterol. 2001;96:132–137. doi: 10.1111/j.1572-0241.2001.03463.x. [DOI] [PubMed] [Google Scholar]
- 16.Zoller H, Pietrangelo A, Vogel W, Weiss G. Duodenal metal-transporter (DMT-1, NRAMP-2) expression in patients with hereditary haemochromatosis. Lancet. 1999;353:2120–2123. doi: 10.1016/S0140-6736(98)11179-0. [DOI] [PubMed] [Google Scholar]
- 17.Rolfs A, Bonkovsky HL, Kohlroser JG, McNeal K, Sharma A, Berger UV, Hediger MA. Intestinal expression of genes involved in iron absorption in humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G598–G607. doi: 10.1152/ajpgi.00371.2001. [DOI] [PubMed] [Google Scholar]
- 18.Corazza GR, Valentini RA, Andreani ML, D'Anchino M, Leva MT, Ginaldi L, De Feudis L, Quaglino D, Gasbarrini G. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol. 1995;30:153–156. doi: 10.3109/00365529509093254. [DOI] [PubMed] [Google Scholar]
- 19.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–651. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 20.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 21.Volta U, Bellentani S, Bianchi FB, Brandi G, De Franceschi L, Miglioli L, Granito A, Balli F, Tiribelli C. High prevalence of celiac disease in Italian general population. Dig Dis Sci. 2001;46:1500–1505. doi: 10.1023/a:1010648122797. [DOI] [PubMed] [Google Scholar]
- 22.Dickey W. Diagnosis of coeliac disease at open-access endoscopy. Scand J Gastroenterol. 1998;33:612–615. doi: 10.1080/00365529850171882. [DOI] [PubMed] [Google Scholar]
- 23.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong F, McCombs CC, Olson JM, Elston RC, Stevens FM, McCarthy CF, Michalski JP. An autosomal screen for genes that predispose to celiac disease in the western counties of Ireland. Nat Genet. 1996;14:329–333. doi: 10.1038/ng1196-329. [DOI] [PubMed] [Google Scholar]
- 25.Butterworth JR, Cooper BT, Rosenberg WM, Purkiss M, Jobson S, Hathaway M, Briggs D, Howell WM, Wood GM, Adams DH, et al. The role of hemochromatosis susceptibility gene mutations in protecting against iron deficiency in celiac disease. Gastroenterology. 2002;123:444–449. doi: 10.1053/gast.2002.34778. [DOI] [PubMed] [Google Scholar]
- 26.Motulsky AG. Genetics of hemochromatosis. N Engl J Med. 1979;301:1291. doi: 10.1056/NEJM197912063012319. [DOI] [PubMed] [Google Scholar]


