Abstract
Since its introduction in 2001 capsule endoscopy opened up the small bowel for diagnostic approaches followed by double balloon enteroscopy which enabled the endoscopic community to perform therapeutic interventions in the whole small intestine. In this review the scientific developments related to indications, diagnostic yield and complications of the last years between the competing devices double ballon enteroscopy, single balloon enteroscopy and spiral enteroscopy are illustrated.
Keywords: Deep enteroscopy, Spiral enteroscopy, Complications, Double balloon enteroscopy, Indications, Single balloon enteroscopy, Diagnostic yield
Core tip: After the first decade of use deep enteroscopy with double balloon enteroscopy, single balloon enteroscopy and spiral enteroscopy are an important part of the armamentarium of modern endoscopy. The review gives an overview of the available literature concerning diagnostic yield, complications and indications of this promising techniques.
INTRODUCTION
Up to the end of the 20th century, the technical options for diagnostic and therapeutic interventions throughout the entire small bowel were limited. The main method, push enteroscopy, requires a special endoscope with an overtube in order to straighten the loop of the endoscope in the stomach, but is nevertheless limited to only a moderate insertion depth[1]. Intraoperative enteroscopy was the second method available for endoscopic examination of the small bowel, but this is an invasive procedure associated with a high rate of complications[2].
When it was introduced in 2001, capsule endoscopy[3] opened up the small bowel for diagnostic approaches, but the method was not able to close the gap for therapeutic interventions in the small bowel.
Yamamoto et al[4] succeeded in overcoming this difficulty with the introduction of the double-balloon technique in 2003. Double-balloon endoscopy (DBE) enables all of the diagnostic and therapeutic interventions used in standard endoscopy - such as biopsy, polypectomy, and dilation - to be carried out anywhere in the entire small bowel. Additional methods have also been introduced in recent years, such as single-balloon enteroscopy (SBE) in 2007[5] and spiral enteroscopy (SE) in 2008[6]. All of these techniques have therefore been summed up under the heading of “device-assisted enteroscopy” (DAE).
AVAILABLE TECHNIQUES
Push enteroscopy
Push enteroscopy allows visualization of the esophagus, stomach, duodenum, and proximal jejunum, using a long endoscope with a standard diameter. Nowadays, the technique is normally used without an overtube to straighten the loop in the stomach or in the colon. The advantage of push enteroscopy that it can rapidly exclude bleeding sources in the proximal jejunum about 50-70 cm behind the pylorus, for example, in patients with suspected mid-gastrointestinal bleeding. However, visualization of the entire small bowel is not possible with this method. Significant advantages for the DBE technique with regard to diagnostic yield and insertion depth were reported in two comparative studies using DBE and push enteroscopy showing that DBE is superior in detecting small bowel polyps whereas the examination time is shorter using SE-technique[1,7].
Double-balloon enteroscopy
When it was introduced in 2003, double-balloon enteroscopy was the first technique that made it possible not only to visualize the small bowel endoscopically but also to carry out therapeutic interventions with the whole armamentarium of therapeutic endoscopy inside the “black box” of the small bowel.
In addition to a 200-cm long endoscope (Fujinon Inc., Wayne, New Jersey, United States) with a separately inflatable balloon at its distal end, the DBE system includes an overtube with the second balloon attached to it. After the instrument has passed the duodenum or ileocecal valve, the small bowel can be retracted using these balloons - leading to a much greater depth of insertion in comparison with push enteroscopy, for example. Diagnostic and therapeutic endoscopes are available for DBE examinations of the small bowel (Fujinon EN-450T5, EN-450P5 and EN-580T; Table 1).
Table 1.
Technical specifications of double-balloon endoscopy and single-balloon enteroscopy devices
| Outer diameter (mm) | Length (cm) | Working channel (mm) | Indications | |
| SBE (Olympus SIF-Q160) | 9.8 | 200 | 2.8 | Small bowel |
| DBE (Fujinon) | ||||
| EN-450P5 | 8.5 | 200 | 2.2 | Small bowel - diagnostic |
| EN-450T5 | 9.4 | 200 | 2.8 | Small bowel - therapeutic |
| EN-450BI5 | 9.4 | 152 | 2.8 | Colonoscopy/DBE-ERCP |
| EN-580T | 9.4 | 200 | 3.2 |
SBE: Single-balloon enteroscopy; DBE: Double-balloon endoscopy; ERCP: Endoscopic retrograde cholangiopancreatography.
The radiographic checking of the endoscope loops that was initially often used does not appear to have any effect on the ability to reach more distal parts of the small bowel. In a prospective evaluation, Manner et al[8] did not identify any effect on the depth of insertion when fluoroscopic control was used. Two small studies that investigated the learning curve in DBE examinations did not find a distinct learning curve[9,10] - possibly because success in performing a DBE examination was defined in these studies as reaching a stable endoscope position in the terminal ileum or reaching the suspected lesion, rather than achieving complete enteroscopy.
For technical aspects of the small-bowel endoscopes that are commercially available, see Table 1.
Single-balloon enteroscopy
The single-balloon endoscope uses one balloon attached to the tip of the overtube, without the balloon attached to the tip of the endoscope. The other specifications are similar to those of therapeutic DBE devices (Table 1).
The main difference in handling between the two types of endoscope is the need to angulate the tip of the SBE device before the pulling maneuver, in order to compensate for reduced stability[11,12]. The angulation maneuver does not have any effect on complication rates during SBE procedures in comparison with DBE examinations. Comparative data for the two techniques with regard to diagnostic yield and therapeutic impact are equivalent, while DBE appears to be more favorable in relation to the complete enteroscopy rate[13-17].
Spiral enteroscopy
During spiral enteroscopy, an overtube with a helical design is positioned on a long endoscope (e.g., a DBE endoscope) and is placed in the duodenum. With slight rotation, the small bowel can be retracted and placed on the overtube. The main advantage of this technique is that it allows rapid advancement of the endoscope to the maximum distance. The spiral overtube is not currently commercially available in Europe.
In addition to the short examination time, highly stable positioning of the endoscope is another advantage; however, spiral enteroscopy appears to be associated with a higher complication rate. Larger prospective comparative trials of complications are lacking at present. DBE provides a significantly greater insertion depth than SE[18]. SE is as yet the least well evaluated technique in the field of small-bowel endoscopy.
Additional technical aspects
The use of carbon dioxide insufflation is generally recommended in small-bowel endoscopy, as it reduces postprocedural pain and helps to achieve a deeper insertion of the scope[19,20].
INDICATIONS FOR DAE
Bleeding
The main indication for DBE and SBE is evaluation of obscure mid-gastrointestinal bleeding, which represents 60%-97% of enrolled cases in studies addressing deep[21-23]. Mid-gastrointestinal bleeding is defined as a bleeding source located between the suspensory muscle of the duodenum (ligament of Treitz) and the ileal papilla (Bauhin valve). Bleeding episodes are classified as overt or occult bleeding with tarry stool and/or hematochezia, as in standard upper or lower gastrointestinal bleeding.
A previous standard endoscopic evaluation with gastroscopy and colonoscopy in optimized conditions is mandatory in all cases. In selected patients, it is advisable to repeat these examinations, as the rate of overlooked bleeding sources within the reach of standard endoscopy has been reported as up to 25%. In this setting, using push enteroscopy may be helpful for detecting bleeding sources in the proximal jejunum.
The diagnostic yields of SBE and DBE in patients with suspected mid-gastrointestinal bleeding are reported to be 47%-77%[12,21,24] and 40%-80%[22,25-27], respectively, with no relevant discrepancies between the two techniques.
Different bleeding sources can be detected relative to the patient’s age. Whereas patients aged 65 or over develop bleeding from angiodysplasia or jejunal diverticula significantly more often[28] (see Figure 1), younger patients suffer from tumors, polyps, inflammatory bowel disease, or bleeding Meckel’s diverticulum (see Figure 2).
Figure 1.
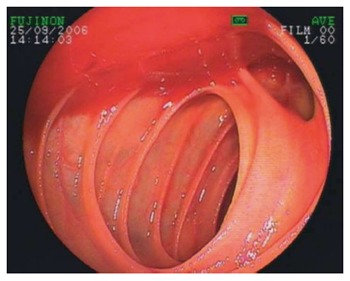
Bleeding from a jejunal diverticulum.
Figure 2.
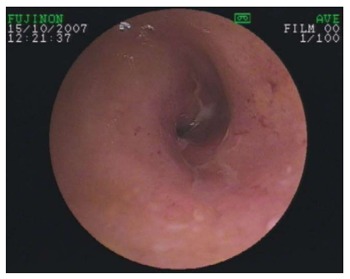
Crohn’s stenosis detected by double balloon enteroscopy.
Another key point in the management of patients with suspected mid-gastrointestinal bleeding is the severity of the blood loss. The more severe the clinical signs of bleeding are, the more invasive and rapid the diagnostic and therapeutic approach should be. Approaches range from elective capsule endoscopy in patients with iron-deficiency anemia to emergency intraoperative enteroscopy in patients with hemodynamically relevant bleeding from jejunal diverticula[2,29]. Another useful tool from the point of view of speed in diagnosing hemodynamically unstable patients with gastrointestinal bleeding is computed-tomographic angiography (CTA), which leads to rapid detection of bleeding sources in patients with active bleeding[30]. In addition to CTA, capsule endoscopy can guide the management in patients with less severe bleeding - helping the endoscopist to select the correct route for balloon-assisted enteroscopy, for example[31-40]. The upper DBE or SBE approach is typically selected for lesions suspected to be located in the upper two-thirds and the retrograde approach for the suspected lower third of the small bowel.
In view of the complex decisions that need to be taken in patients with mid-gastrointestinal bleeding, early transfer to a referral center with expertise in the management of small-bowel diseases is strongly recommended. For details, see an algorithm on bleeding recommended by the authors (Figure 3).
Figure 3.
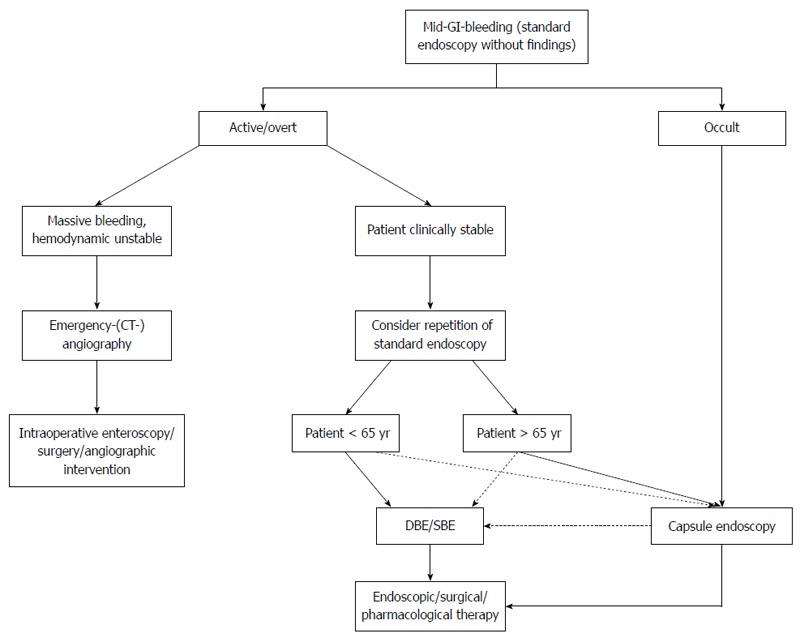
Algorithm mid-gastrointestinal-bleeding. GI: Gastrointestinal; CT: Computed tomography; SBE: Single-balloon enteroscopy; DBE: Double-balloon endoscopy.
The outcome for patients following treatment for a mid-gastrointestinal bleeding episode has been studied in several papers. Whereas Gerson et al[41] reported a re-bleeding rate of 23% after endoscopic treatment for angiodysplasia in 135 patients after 12 mo and a rate of 35% for the need for further iron supplementation or blood transfusions in their patients, Madisch et al[42] did not observe any significant differences in the re-bleeding rate between patients with or without treatment for angiodysplasia. May et al[43] presented a study with a long-term follow-up period of 55 mo including 50 patients who had undergone argon plasma coagulation of angiodysplastic lesions during DBE examinations, and reported a re-bleeding rate of 45%, while the need for further blood transfusions was significantly reduced. These rather disappointing results were confirmed by Samaha et al[44].
Crohn’s disease
Known or suspected Crohn’s disease is a less frequent indication for the use of DAE, accounting for 11%-22% of indications in large databases[22,45]. Crohn’s disease lesions in the small bowel are common and have been detected in 70% of cases when DBE is performed[46].
However, the diagnostic yield and therapeutic impact of DAE on the management of patients with Crohn’s disease depends on whether the condition is only suspected or is a known disease. In a retrospective study of 40 patients with known Crohn’s disease, DBE detected small-bowel lesions in 60% of the patients, leading to a change in the patient’s management in 75% of cases[47]. In a study by Manes et al[48], the diagnostic yield was also 60%, while the diagnostic yield in patients in whom Crohn’s disease was only suspected and who had undergone normal standard upper und lower gastrointestinal endoscopy was 44% for lesions in the small bowel[49].
Nevertheless, DAE including DBE, SBE, and CE is not the ideal tool for the management of Crohn’s patients, due to the invasive nature of DBE and SBE and the risk of capsule retention, which is greater in Crohn’s patients than in others[50]. The main indication is to clarify obscure symptoms in patients with suspected Crohn’s disease and for planned therapeutic interventions such as balloon dilation of small-bowel stenosis detected using other methods (see Figure 2). These interventions have been carried out with high success rates and acceptable complication rates[51,52].
Small-bowel tumors and polyps
All DAE techniques are suitable for detecting small-bowel polyps and tumors, and some can be used to treat these conditions as well. This is due to the ability to carry out biopsy sampling for histological assessment and polypectomy of benign polyps - e.g., in Peutz-Jeghers syndrome.
Polyposis syndromes and tumors of the small bowel are rare conditions, and detection rates of 9.6%-17%[53-55] result from the fact that the patients represent a highly selected group. It is to state that the rate of small bowel tumors in asian series seems to be considerably higher than in western series. Most of the tumors described in the relevant studies are benign, with an emphasis on neuroendocrine tumors in a large group of 1106 patients reported by Cangemi et al[53].
The results of endoscopic polypectomy have been studied in several papers. Gao et al[56] reported on a group of 13 patients with Peutz-Jeghers syndrome and 79 polyps, which were resected without major complications. A success rate of 82% in detecting and removing small-bowel polyps in 22 Peutz-Jeghers patients was reported by Gorospe et al[57]. In a retrospective series, Kopácová et al[58] found no differences in the rates of therapeutic success between DBE and intraoperative enteroscopy in the management of small-bowel polyps, although intraoperative enteroscopy was much more invasive.
Malignant tumors in the small bowel are a rare entity, accounting for 3.6% of cases in a group of 555 patients[59].
In summary, DAE techniques are helpful for detecting, classifying and often also for treating small-bowel tumors and polyps.
DAE AND COMPLICATIONS
Following the introduction of DAE, the complications associated with the techniques became increasingly important. As the complication rate with endoscopic procedures is usually low, large numbers of examinations have to be evaluated in order to determine a reliable complication rate for a new procedure. In addition, new techniques are often initially used mainly by experts, leading to an additional reduction of the complication risks. During the first years of use, complications of DBE examinations were also reported. In addition to known complications such as bleeding, perforation, and complications associated with sedation, a relatively new complication was discovered in the form of post-DBE pancreatitis.
Pancreatitis
Initial case reports on the development of acute pancreatitis following DBE via the oral route were already published in 2006[60,61]. Considerable efforts were then made to assess the risks and mechanisms of developing pancreatitis after DBE, particularly following the documentation of a fatal course of the disease in a patient with pancreatitis in a retrospective cohort of 3894 examinations in a DBE registry in Germany[62]. Also in 2006, Honda et al[63] reported a 47% rate of hyperamylasemia in 13 patients after DBE examinations, while one patient (7%) developed typical clinical signs of pancreatitis. This high incidence of pancreatitis after DBE examinations was not reproduced in larger series. Kopácová et al[64] reported elevated lipase levels in 51% of 31 patients, with only one patient who developed pancreatitis. In subsequent years, several registry data reports and retrospective analyses focusing on complications were published.
In a retrospectively collected database of 10 centers including 2362 examinations, the rate of pancreatitis after DBE examinations was 0.3%[65]. Another retrospective series from centers in the United States, including 2478 patients, reported a pancreatitis rate of 0.2% of cases; this was the first study that documented a case of pancreatitis after DBE via the anal route[66]. The largest prospective data collection to date is the report from the DBE registry in Germany by the German DBE study group, including 1765 patients with 2245 DBE procedures. In this study, the pancreatitis rate was 0.34% after DBE via the oral route[22]. Fortunately, no further deaths have occurred due to pancreatitis.
The pathogenesis of hyperamylasemia and pancreatitis after DBE has been discussed in several papers. Firstly, it is notable that nearly all of the observed cases of pancreatitis have occurred after DBE via the oral route, despite the one case in the series by Gerson et al[66] mentioned. It is therefore arguable that mechanical stress acting on the pancreas or the papilla during the push-and-pull maneuver may play a role in the development of pancreatitis. In an animal model with 20 pigs, Latorre et al[67] observed elevated amylase and lipase levels after examination periods of 90 and 120 min, with no correlations between either the depth of insertion or the duration of the examination and the laboratory tests. However, they observed ischemia-triggered necrosis of pancreatic tissue at autopsy, leading to the conclusion that mechanical stress might be the trigger for tissue damage after DBE.
In a smaller series including 56 patients, Pata et al[68] noted a correlation between pancreatitis and the insertion depth and time between the inflation of the first and second balloons in the duodenum. The authors argued that compression of the papilla of Vater with the balloon may cause the development of pancreatitis. However, the rate of pancreatitis in the study was 12% - much higher than expected from the studies mentioned above, and calling into question the DBE technique and definition of pancreatitis used.
A larger series of 135 patients published by Aktas et al[69] found a good correlation between depth of insertion and numbers of pull maneuvers and the development of hyperamylasemia. The rates of pancreatitis and hyperamylasemia were comparable with previous data in this study.
In summary, a careful examination technique - avoiding mechanical stress to the pancreas through slow retraction of the endoscope and avoiding mechanical stress to the papilla by only using the balloons in deeper parts of the duodenum - is recommended in order to reduce the risk of pancreatitis after DBE examinations.
In view of the potential risk, pancreatitis should be mentioned in the discussion with the patient prior to the examination and should be included in the written informed consent form.
The pancreatitis rates associated with SBE and SE have not yet been adequately investigated. The first report, by Aktas et al[70], only included 105 patients, which is much too low to allow any reliable conclusions to be drawn.
Bleeding and perforation
Bleeding complications have been observed particularly after interventional procedures during DAE. Mensink et al[65] reported an overall bleeding rate of 0.8% in their cohort, but only 0.1% after diagnostic procedures. In other studies, the bleeding rate has ranged from 0.2% to 0.3%, predominantly associated with endoscopic interventions[22,66].
Perforations have been reported after diagnostic as well as therapeutic procedures. Prior abdominal surgery increases the risk of perforation in DAE patients. In the large retrospective and prospective databases, the risk of perforation has been reported as 0.1%-0.3% in diagnostic procedures and 0.8%-2.9% after small-bowel polypectomy[22,65,66]. Due to the potential risk of perforation after interventional DBE and SBE, these techniques should be used only by experts.
Complications related to sedation
As DBE or SBE are time-consuming and technically demanding, the time the patient spends under sedation is longer in comparison with normal upper and lower gastrointestinal endoscopy. The risk of sedation-related complications (SRCs) should therefore be taken into account when treating these patients. However, propofol sedation is usually adequate. When deeper sedation is necessary, the addition of midazolam or pethidine is an option. The routine use of anesthesia is not recommended, but there is a lack of systematic analyses.
SRCs have been documented in 0.5% of cases in the prospective database in Germany[22].
Efforts have been made to examine the safety of DAE in elderly patients (> 75 years), but a higher complication rate was not observed in this group in comparison with younger patients[28,71-73]. DAE can therefore be used with the same level of safety in elderly patients.
Summary on complications
It may be noted that the DBE technique is the best prospectively studied technique in relation to complications and to the diagnostic and therapeutic yield in the field of small-bowel endoscopy (see Table 2). The relatively large number of studies comparing DBE and SBE have not included sufficiently large numbers of patients or examinations to allow valid comparison of complication rates between the two techniques[13,15-17,74]. The least information is available in relation to the complication rate with spiral enteroscopy in comparison with other DAE modalities, with only around a hundred examinations reported[59,75,76].
Table 2.
Complications of double-balloon endoscopy-examinations
| DBE | |
| Pancreatitis | 0.2%-0.34% (-12%) |
| Bleeding | 0.2%-0.8% |
| Perforations | 0.1%-2.9% |
| Sedation related complications | 0.5% |
The data concerning complications of single-balloon enteroscopy and spiral enteroscopy are insufficient for a detailed presentation. DBE: Double-balloon endoscopy.
CONCLUSION
Twelve years after its first introduction in the form of DBE, DAE is now becoming a standard tool for the diagnosis and treatment of small-bowel diseases. In particular, DBE and SBE as the leading techniques have proved their value and safety when used in expert hands in a respectable number of studies. In addition to capsule endoscopy, older techniques such as intraoperative enteroscopy, and imaging techniques such as computed tomography, angiography, and magnetic resonance tomography, the armamentarium available to physicians with endoscopic skills is now appropriate for treating small-bowel diseases.
ACKNOWLEDGMENTS
We thank Michael Robertson for revising the manuscript.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 9, 2014
First decision: September 27, 2014
Article in press: December 1, 2014
P- Reviewer: Casadesus D, Friedland S, Kopacova M, Wang HP, Yoneda M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Matsumoto T, Moriyama T, Esaki M, Nakamura S, Iida M. Performance of antegrade double-balloon enteroscopy: comparison with push enteroscopy. Gastrointest Endosc. 2005;62:392–398. doi: 10.1016/j.gie.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet S, Douard R, Malamut G, Cellier C, Wind P. Intraoperative enteroscopy in the management of obscure gastrointestinal bleeding. Dig Liver Dis. 2013;45:277–284. doi: 10.1016/j.dld.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Appleyard M, Glukhovsky A, Swain P. Wireless-capsule diagnostic endoscopy for recurrent small-bowel bleeding. N Engl J Med. 2001;344:232–233. doi: 10.1056/NEJM200101183440316. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Yano T, Kita H, Sunada K, Ido K, Sugano K. New system of double-balloon enteroscopy for diagnosis and treatment of small intestinal disorders. Gastroenterology. 2003;125:1556; author reply 1556–1557. doi: 10.1016/j.gastro.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Tsujikawa T, Saitoh Y, Andoh A, Imaeda H, Hata K, Minematsu H, Senoh K, Hayafuji K, Ogawa A, Nakahara T, et al. Novel single-balloon enteroscopy for diagnosis and treatment of the small intestine: preliminary experiences. Endoscopy. 2008;40:11–15. doi: 10.1055/s-2007-966976. [DOI] [PubMed] [Google Scholar]
- 6.Akerman PA, Agrawal D, Cantero D, Pangtay J. Spiral enteroscopy with the new DSB overtube: a novel technique for deep peroral small-bowel intubation. Endoscopy. 2008;40:974–978. doi: 10.1055/s-0028-1103402. [DOI] [PubMed] [Google Scholar]
- 7.May A, Nachbar L, Schneider M, Ell C. Prospective comparison of push enteroscopy and push-and-pull enteroscopy in patients with suspected small-bowel bleeding. Am J Gastroenterol. 2006;101:2016–2024. doi: 10.1111/j.1572-0241.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 8.Manner H, May A, Pohl J, Färber M, Ell C. Impact of fluoroscopy on oral double-balloon enteroscopy: results of a randomized trial in 156 patients. Endoscopy. 2010;42:820–826. doi: 10.1055/s-0030-1255727. [DOI] [PubMed] [Google Scholar]
- 9.Tee HP, How SH, Kaffes AJ. Learning curve for double-balloon enteroscopy: Findings from an analysis of 282 procedures. World J Gastrointest Endosc. 2012;4:368–372. doi: 10.4253/wjge.v4.i8.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM, et al. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740–750. doi: 10.1016/j.gie.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura T, Yasuda K, Tanaka K, Uno K, Ueda M, Sanada K, Nakajima M. Clinical evaluation of a newly developed single-balloon enteroscope. Gastrointest Endosc. 2008;68:1112–1116. doi: 10.1016/j.gie.2008.03.1063. [DOI] [PubMed] [Google Scholar]
- 12.Upchurch BR, Sanaka MR, Lopez AR, Vargo JJ. The clinical utility of single-balloon enteroscopy: a single-center experience of 172 procedures. Gastrointest Endosc. 2010;71:1218–1223. doi: 10.1016/j.gie.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Lenz P, Domagk D. Double- vs. single-balloon vs. spiral enteroscopy. Best Pract Res Clin Gastroenterol. 2012;26:303–313. doi: 10.1016/j.bpg.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Efthymiou M, Desmond PV, Brown G, La Nauze R, Kaffes A, Chua TJ, Taylor AC. SINGLE-01: a randomized, controlled trial comparing the efficacy and depth of insertion of single- and double-balloon enteroscopy by using a novel method to determine insertion depth. Gastrointest Endosc. 2012;76:972–980. doi: 10.1016/j.gie.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Takano N, Yamada A, Watabe H, Togo G, Yamaji Y, Yoshida H, Kawabe T, Omata M, Koike K. Single-balloon versus double-balloon endoscopy for achieving total enteroscopy: a randomized, controlled trial. Gastrointest Endosc. 2011;73:734–739. doi: 10.1016/j.gie.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 16.May A, Färber M, Aschmoneit I, Pohl J, Manner H, Lotterer E, Möschler O, Kunz J, Gossner L, Mönkemüller K, et al. Prospective multicenter trial comparing push-and-pull enteroscopy with the single- and double-balloon techniques in patients with small-bowel disorders. Am J Gastroenterol. 2010;105:575–581. doi: 10.1038/ajg.2009.712. [DOI] [PubMed] [Google Scholar]
- 17.Domagk D, Mensink P, Aktas H, Lenz P, Meister T, Luegering A, Ullerich H, Aabakken L, Heinecke A, Domschke W, et al. Single- vs. double-balloon enteroscopy in small-bowel diagnostics: a randomized multicenter trial. Endoscopy. 2011;43:472–476. doi: 10.1055/s-0030-1256247. [DOI] [PubMed] [Google Scholar]
- 18.Messer I, May A, Manner H, Ell C. Prospective, randomized, single-center trial comparing double-balloon enteroscopy and spiral enteroscopy in patients with suspected small-bowel disorders. Gastrointest Endosc. 2013;77:241–249. doi: 10.1016/j.gie.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Hirai F, Beppu T, Nishimura T, Takatsu N, Ashizuka S, Seki T, Hisabe T, Nagahama T, Yao K, Matsui T, et al. Carbon dioxide insufflation compared with air insufflation in double-balloon enteroscopy: a prospective, randomized, double-blind trial. Gastrointest Endosc. 2011;73:743–749. doi: 10.1016/j.gie.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Wang WL, Wu ZH, Sun Q, Wei JF, Chen XF, Zhou DK, Zhou L, Xie HY, Zheng SS. Meta-analysis: the use of carbon dioxide insufflation vs. room air insufflation for gastrointestinal endoscopy. Aliment Pharmacol Ther. 2012;35:1145–1154. doi: 10.1111/j.1365-2036.2012.05078.x. [DOI] [PubMed] [Google Scholar]
- 21.Frantz DJ, Dellon ES, Grimm IS, Morgan DR. Single-balloon enteroscopy: results from an initial experience at a U.S. tertiary-care center. Gastrointest Endosc. 2010;72:422–426. doi: 10.1016/j.gie.2010.03.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möschler O, May A, Müller MK, Ell C. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484–489. doi: 10.1055/s-0030-1256249. [DOI] [PubMed] [Google Scholar]
- 23.Xin L, Liao Z, Jiang YP, Li ZS. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011;74:563–570. doi: 10.1016/j.gie.2011.03.1239. [DOI] [PubMed] [Google Scholar]
- 24.Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Rao GV, Darisetty S. Diagnostic yield and therapeutic impact of single-balloon enteroscopy: series of 106 cases. J Gastroenterol Hepatol. 2009;24:1631–1638. doi: 10.1111/j.1440-1746.2009.05936.x. [DOI] [PubMed] [Google Scholar]
- 25.Mönkemüller K, Weigt J, Treiber G, Kolfenbach S, Kahl S, Röcken C, Ebert M, Fry LC, Malfertheiner P. Diagnostic and therapeutic impact of double-balloon enteroscopy. Endoscopy. 2006;38:67–72. doi: 10.1055/s-2005-921190. [DOI] [PubMed] [Google Scholar]
- 26.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 27.Maaser C, Schmedt A, Bokemeyer M, Kannengiesser K, Ullerich H, Lügering A, Domagk D, Domschke W, Kucharzik T. Long-term efficacy and safety of double balloon enteroscopy--prospective and retrospective data from a single center study. Scand J Gastroenterol. 2010;45:992–999. doi: 10.3109/00365521003710182. [DOI] [PubMed] [Google Scholar]
- 28.Hegde SR, Iffrig K, Li T, Downey S, Heller SJ, Tokar JL, Haluszka O. Double-balloon enteroscopy in the elderly: safety, findings, and diagnostic and therapeutic success. Gastrointest Endosc. 2010;71:983–989. doi: 10.1016/j.gie.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 29.Jakobs R, Hartmann D, Benz C, Schilling D, Weickert U, Eickhoff A, Schoenleben K, Riemann JF. Diagnosis of obscure gastrointestinal bleeding by intra-operative enteroscopy in 81 consecutive patients. World J Gastroenterol. 2006;12:313–316. doi: 10.3748/wjg.v12.i2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. CT enterography in obscure gastrointestinal bleeding: a systematic review and meta-analysis. J Med Imaging Radiat Oncol. 2013;57:263–273. doi: 10.1111/1754-9485.12035. [DOI] [PubMed] [Google Scholar]
- 31.Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, Fleischer DE, Sharma VK. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671–676. doi: 10.1016/j.cgh.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Shishido T, Oka S, Tanaka S, Aoyama T, Watari I, Imagawa H, Yoshida S, Chayama K. Diagnostic yield of capsule endoscopy vs. double-balloon endoscopy for patients who have undergone total enteroscopy with obscure gastrointestinal bleeding. Hepatogastroenterology. 2012;59:955–959. doi: 10.5754/hge12242. [DOI] [PubMed] [Google Scholar]
- 33.Pasha SF, Leighton JA. How useful is capsule endoscopy for the selection of patients for double-balloon enteroscopy? Nat Clin Pract Gastroenterol Hepatol. 2008;5:490–491. doi: 10.1038/ncpgasthep1201. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M, Niwa Y, Ohmiya N, Miyahara R, Ohashi A, Itoh A, Hirooka Y, Goto H. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy. 2006;38:59–66. doi: 10.1055/s-2005-870446. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13:4372–4378. doi: 10.3748/wjg.v13.i32.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arakawa D, Ohmiya N, Nakamura M, Honda W, Shirai O, Itoh A, Hirooka Y, Niwa Y, Maeda O, Ando T, et al. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc. 2009;69:866–874. doi: 10.1016/j.gie.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26:796–801. doi: 10.1111/j.1440-1746.2010.06530.x. [DOI] [PubMed] [Google Scholar]
- 38.Marmo R, Rotondano G, Casetti T, Manes G, Chilovi F, Sprujevnik T, Bianco MA, Brancaccio ML, Imbesi V, Benvenuti S, et al. Degree of concordance between double-balloon enteroscopy and capsule endoscopy in obscure gastrointestinal bleeding: a multicenter study. Endoscopy. 2009;41:587–592. doi: 10.1055/s-0029-1214896. [DOI] [PubMed] [Google Scholar]
- 39.Hadithi M, Heine GD, Jacobs MA, van Bodegraven AA, Mulder CJ. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:52–57. doi: 10.1111/j.1572-0241.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto T, Esaki M, Moriyama T, Nakamura S, Iida M. Comparison of capsule endoscopy and enteroscopy with the double-balloon method in patients with obscure bleeding and polyposis. Endoscopy. 2005;37:827–832. doi: 10.1055/s-2005-870207. [DOI] [PubMed] [Google Scholar]
- 41.Gerson LB, Batenic MA, Newsom SL, Ross A, Semrad CE. Long-term outcomes after double-balloon enteroscopy for obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2009;7:664–669. doi: 10.1016/j.cgh.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Madisch A, Schmolders J, Brückner S, Aust D, Miehlke S. Less favorable clinical outcome after diagnostic and interventional double balloon enteroscopy in patients with suspected small-bowel bleeding? Endoscopy. 2008;40:731–734. doi: 10.1055/s-2008-1077521. [DOI] [PubMed] [Google Scholar]
- 43.May A, Friesing-Sosnik T, Manner H, Pohl J, Ell C. Long-term outcome after argon plasma coagulation of small-bowel lesions using double-balloon enteroscopy in patients with mid-gastrointestinal bleeding. Endoscopy. 2011;43:759–765. doi: 10.1055/s-0030-1256388. [DOI] [PubMed] [Google Scholar]
- 44.Samaha E, Rahmi G, Landi B, Lorenceau-Savale C, Malamut G, Canard JM, Bloch F, Jian R, Chatellier G, Cellier C. Long-term outcome of patients treated with double balloon enteroscopy for small bowel vascular lesions. Am J Gastroenterol. 2012;107:240–246. doi: 10.1038/ajg.2011.325. [DOI] [PubMed] [Google Scholar]
- 45.Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 46.Mensink PB, Aktas H, Zelinkova Z, West RL, Kuipers EJ, van der Woude CJ. Impact of double-balloon enteroscopy findings on the management of Crohn’s disease. Scand J Gastroenterol. 2010;45:483–489. doi: 10.3109/00365520903563774. [DOI] [PubMed] [Google Scholar]
- 47.Mensink PB, Groenen MJ, van Buuren HR, Kuipers EJ, van der Woude CJ. Double-balloon enteroscopy in Crohn’s disease patients suspected of small bowel activity: findings and clinical impact. J Gastroenterol. 2009;44:271–276. doi: 10.1007/s00535-009-0011-4. [DOI] [PubMed] [Google Scholar]
- 48.Manes G, Imbesi V, Ardizzone S, Cassinotti A, Pallotta S, Porro GB. Use of double-balloon enteroscopy in the management of patients with Crohn’s disease: feasibility and diagnostic yield in a high-volume centre for inflammatory bowel disease. Surg Endosc. 2009;23:2790–2795. doi: 10.1007/s00464-009-0518-z. [DOI] [PubMed] [Google Scholar]
- 49.Schulz C, Mönkemüller K, Salheiser M, Bellutti M, Schütte K, Malfertheiner P. Double-balloon enteroscopy in the diagnosis of suspected isolated Crohn’s disease of the small bowel. Dig Endosc. 2014;26:236–242. doi: 10.1111/den.12142. [DOI] [PubMed] [Google Scholar]
- 50.Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Despott EJ, Gupta A, Burling D, Tripoli E, Konieczko K, Hart A, Fraser C. Effective dilation of small-bowel strictures by double-balloon enteroscopy in patients with symptomatic Crohn’s disease (with video) Gastrointest Endosc. 2009;70:1030–1036. doi: 10.1016/j.gie.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Jovanovic I, Vormbrock K, Zimmermann L, Djuranovic S, Ugljesic M, Malfertheiner P, Fry LC, Mönkemüller K. Therapeutic double-balloon enteroscopy: a binational, three-center experience. Dig Dis. 2011;29 Suppl 1:27–31. doi: 10.1159/000331125. [DOI] [PubMed] [Google Scholar]
- 53.Cangemi DJ, Patel MK, Gomez V, Cangemi JR, Stark ME, Lukens FJ. Small bowel tumors discovered during double-balloon enteroscopy: analysis of a large prospectively collected single-center database. J Clin Gastroenterol. 2013;47:769–772. doi: 10.1097/MCG.0b013e318281a44e. [DOI] [PubMed] [Google Scholar]
- 54.Lee BI, Choi H, Choi KY, Byeon JS, Jang HJ, Eun CS, Cheon JH, Shin SJ, Kim JO, Lee MS, et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56:2920–2927. doi: 10.1007/s10620-011-1839-z. [DOI] [PubMed] [Google Scholar]
- 55.Fry LC, Neumann H, Kuester D, Kuhn R, Bellutti M, Malfertheiner P, Monkemuller K. Small bowel polyps and tumours: endoscopic detection and treatment by double-balloon enteroscopy. Aliment Pharmacol Ther. 2009;29:135–142. doi: 10.1111/j.1365-2036.2008.03864.x. [DOI] [PubMed] [Google Scholar]
- 56.Gao H, van Lier MG, Poley JW, Kuipers EJ, van Leerdam ME, Mensink PB. Endoscopic therapy of small-bowel polyps by double-balloon enteroscopy in patients with Peutz-Jeghers syndrome. Gastrointest Endosc. 2010;71:768–773. doi: 10.1016/j.gie.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Gorospe EC, Alexander JA, Bruining DH, Rajan E, Wong Kee Song LM. Performance of double-balloon enteroscopy for the management of small bowel polyps in hamartomatous polyposis syndromes. J Gastroenterol Hepatol. 2013;28:268–273. doi: 10.1111/jgh.12058. [DOI] [PubMed] [Google Scholar]
- 58.Kopácová M, Bures J, Ferko A, Tachecí I, Rejchrt S. Comparison of intraoperative enteroscopy and double-balloon enteroscopy for the diagnosis and treatment of Peutz-Jeghers syndrome. Surg Endosc. 2010;24:1904–1910. doi: 10.1007/s00464-009-0868-6. [DOI] [PubMed] [Google Scholar]
- 59.Partridge BJ, Tokar JL, Haluszka O, Heller SJ. Small bowel cancers diagnosed by device-assisted enteroscopy at a U.S. referral center: a five-year experience. Dig Dis Sci. 2011;56:2701–2705. doi: 10.1007/s10620-011-1640-z. [DOI] [PubMed] [Google Scholar]
- 60.Groenen MJ, Moreels TG, Orlent H, Haringsma J, Kuipers EJ. Acute pancreatitis after double-balloon enteroscopy: an old pathogenetic theory revisited as a result of using a new endoscopic tool. Endoscopy. 2006;38:82–85. doi: 10.1055/s-2005-921179. [DOI] [PubMed] [Google Scholar]
- 61.Honda K, Mizutani T, Nakamura K, Higuchi N, Kanayama K, Sumida Y, Yoshinaga S, Itaba S, Akiho H, Kawabe K, et al. Acute pancreatitis associated with peroral double-balloon enteroscopy: a case report. World J Gastroenterol. 2006;12:1802–1804. doi: 10.3748/wjg.v12.i11.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Möschler O, May AD, Müller MK, Ell C. [Complications in double-balloon-enteroscopy: results of the German DBE register] Z Gastroenterol. 2008;46:266–270. doi: 10.1055/s-2007-963719. [DOI] [PubMed] [Google Scholar]
- 63.Honda K, Itaba S, Mizutani T, Sumida Y, Kanayama K, Higuchi N, Yoshinaga S, Akiho H, Kawabe K, Arita Y, et al. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: an association with the development of pancreatitis. Endoscopy. 2006;38:1040–1043. doi: 10.1055/s-2006-944831. [DOI] [PubMed] [Google Scholar]
- 64.Kopácová M, Rejchrt S, Tachecí I, Bures J. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest Endosc. 2007;66:1133–1138. doi: 10.1016/j.gie.2007.03.1085. [DOI] [PubMed] [Google Scholar]
- 65.Mensink PB, Haringsma J, Kucharzik T, Cellier C, Pérez-Cuadrado E, Mönkemüller K, Gasbarrini A, Kaffes AJ, Nakamura K, Yen HH, et al. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613–615. doi: 10.1055/s-2007-966444. [DOI] [PubMed] [Google Scholar]
- 66.Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O, et al. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177–1182, 1182 e1-3. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Latorre R, Soria F, López-Albors O, Sarriá R, Sánchez-Margallo F, Esteban P, Carballo F, Pérez-Cuadrado E. Effect of double-balloon enteroscopy on pancreas: an experimental porcine model. World J Gastroenterol. 2012;18:5181–5187. doi: 10.3748/wjg.v18.i37.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pata C, Akyüz U, Erzin Y, Mutlu N, Mercan A, Dirican A. Post-procedure elevated amylase and lipase levels after double-balloon enteroscopy: relations with the double-balloon technique. Dig Dis Sci. 2010;55:1982–1988. doi: 10.1007/s10620-009-0956-4. [DOI] [PubMed] [Google Scholar]
- 69.Aktas H, Mensink PB, Haringsma J, Kuipers EJ. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: has the insertion technique improved? Endoscopy. 2009;41:670–673. doi: 10.1055/s-0029-1214976. [DOI] [PubMed] [Google Scholar]
- 70.Aktas H, de Ridder L, Haringsma J, Kuipers EJ, Mensink PB. Complications of single-balloon enteroscopy: a prospective evaluation of 166 procedures. Endoscopy. 2010;42:365–368. doi: 10.1055/s-0029-1243931. [DOI] [PubMed] [Google Scholar]
- 71.Sidhu R, Sanders DS. Double-balloon enteroscopy in the elderly with obscure gastrointestinal bleeding: safety and feasibility. Eur J Gastroenterol Hepatol. 2013;25:1230–1234. doi: 10.1097/MEG.0b013e3283630f1b. [DOI] [PubMed] [Google Scholar]
- 72.He Q, Zhang Q, Li JD, Wang YD, Wan TM, Chen ZY, Pan DS, Cai JQ, Liu SD, Xiao B, et al. Double balloon enteroscopy in the old: experience from China. World J Gastroenterol. 2012;18:2859–2866. doi: 10.3748/wjg.v18.i22.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Byeon JS, Mann NK, Jamil LH, Lo SK. Double balloon enteroscopy can be safely done in elderly patients with significant co-morbidities. J Gastroenterol Hepatol. 2012;27:1831–1836. doi: 10.1111/j.1440-1746.2012.07284.x. [DOI] [PubMed] [Google Scholar]
- 74.Lenz P, Roggel M, Domagk D. Double- vs. single-balloon enteroscopy: single center experience with emphasis on procedural performance. Int J Colorectal Dis. 2013;28:1239–1246. doi: 10.1007/s00384-013-1673-1. [DOI] [PubMed] [Google Scholar]
- 75.Rahmi G, Samaha E, Vahedi K, Ponchon T, Fumex F, Filoche B, Gay G, Delvaux M, Lorenceau-Savale C, Malamut G, et al. Multicenter comparison of double-balloon enteroscopy and spiral enteroscopy. J Gastroenterol Hepatol. 2013;28:992–998. doi: 10.1111/jgh.12188. [DOI] [PubMed] [Google Scholar]
- 76.Frieling T, Heise J, Sassenrath W, Hülsdonk A, Kreysel C. Prospective comparison between double-balloon enteroscopy and spiral enteroscopy. Endoscopy. 2010;42:885–888. doi: 10.1055/s-0030-1255714. [DOI] [PubMed] [Google Scholar]


