Abstract
To review the underlying pathophysiology and currently available treatments for pruritis associated with jaundice. English language literature was reviewed using MEDLINE, PubMed, EMBASE and clinicaltrials.gov for papers and trails addressing the pathophysiology and potential treatments for pruritis associated with jaundice. Recent advances in the understanding of the peripheral anatomy of itch transmission have defined a histamine stimulated pathway and a cowhage stimulated pathway with sensation conveyed centrally via the contralateral spinothalamic tract. Centrally, cowhage and histamine stimulated neurons terminate widely within the thalamus and sensorimotor cortex. The causative factors for itch in jaundice have not been clarified although endogenous opioids, serotonin, steroid and lysophosphatidic acid all play a role. Current guidelines for the treatment of itching in jaundice recommend initial management with biliary drainage where possible and medical management with ursodeoxycholic acid, followed by cholestyramine, rifampicin, naltrexone and sertraline. Other than biliary drainage no single treatment has proved universally effective. Pruritis associated with jaundice is a common but poorly understood condition for which biliary drainage is the most effective therapy. Pharmacological therapy has advanced but remains variably effective.
Keywords: Jaundice, Pruritis, Biliary drainage, Bile acids, Lysophosphatidic acid
Core tip: The occurrence of pruritis in association with jaundice has been recognized for many years but its pathogenesis is poorly understood. Recent advances in understanding the neural pathways involved in itch have contributed to the clinical treatment of this important symptom.
INTRODUCTION
Jaundice (from the French jaune meaning yellow), refers to the yellowish discolouration of the skin, sclera and mucous membranes that accompanies deposition of bilirubin in tissues[1,2]. It develops when serum bilirubin levels are elevated above 34 mmol/L (2 mg/dL), with yellow discolouration of the sclera being the site where jaundice is detected earliest due to high elastin content of sclera and its strong binding affinity for bilirubin[3].
Pruritis (from the latin verb prurire, to itch)[4] is defined as an irritating sensation that arouses the desire to scratch to provide at least temporary relief[5,6] and is used synonymously with the word “itch”. The sensation originates in the skin and transitional tissues (oral mucosa, anal mucosa and conjunctiva) and is thought to provide a protective function against irritating stimuli such as insect infestation, stings or chemical irritants. The response to itch is scratching or rubbing the effected area to rid it of the irritant while painful stimuli evoke a withdrawal response. The association between the presence of jaundice and pruritis was first made by the ancient Greek physician Aretæus the Cappadocian in the 2nd century BC[7,8]. Itch is present in 80%-100% of patients presenting with cholestasis and jaundice[9,10] and is the primary presenting symptom in at least 25% of patients with cholestasis[11]. In contrast, less than 10% of patients report pruritic symptoms in community surveys of otherwise healthy individuals[12]. Patients with jaundice often nominate pruritis as their most troublesome symptom to control and the symptom that has the most negative influence on their quality of life[13,14]. The presence of pruritis can cause severe sleep deprivation resulting in lassitude, fatigue, depression and suicidal ideation[8]. This is partly due to the observation that pruritis is often worse at night since it exhibits a circadian rhythm with the highest intensity being reported in the evening and at night[8]. This can make sleep and normal activities impossible and increases the severity of symptoms in summer months or humid tropical climates.
Consequently itch is a significant clinical sign in surgical oncology and in patients with liver disease. However, in spite of this, the pathophysiology of itch is still poorly understood and the available treatment options are not well promulgated. This review summarizes the current understanding of the pathophysiology of pruritis associated with jaundice and the currently available treatment options for the condition.
PHYSIOLOGY OF ITCH
Itch begins with stimulation of skin receptors and nerve endings by pruritogens. This results in activation of polymodal and mechanically insensitive C-fibres (Figure 1). These synapse with secondary neurons in the distal horn which then travel in the contralateral spinothalamic tract and synapse with third order neurones in the thalamus. From the thalamus neurons project to a number of cortical and subcortical areas[15]. However, in spite of the clarification of the anatomical structures involved, the mode of function of the itch pathway is unclear. The specificity theory proposes that there are discrete modality-specific receptors and peripheral nerves that detect itch stimuli for specific areas of skin. This is based on evidence that the application of histamine to skin activated a histamine specific pathway of mechanically insensitive C-fibres[16]. These fibres synapse with a histamine stimulated pathway located in lamina 1 of the spinothalamic tract[17]. Both gastrin-releasing peptide GRP and the gastrin releasing peptide receptors are important in neurotransmission in these neurons[18]. Recently Han et al[19] has demonstrated a group of MrgprA3-positive neurones in the dorsal root ganglion which, when ablated, result in reduction of itch and scratching behaviour.
Figure 1.
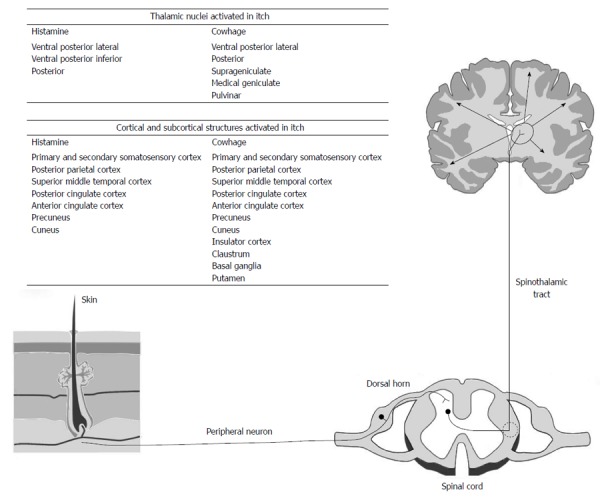
Summary of the peripheral and central neuroanatomy of the itch pathway (adapted from Dhand and Aminoff[15]).
The pattern theory argues that itch, as well as other sensation, is generated by receptors and nerves that are not stimulus specific and the signals are decoded centrally[20]. Consistent with this theory is the observation that the itch pathway may be activated by pain producing stimuli such as capsaicin applied to the skin which will activate mechanically insensitive C-fibres involved in histamine related itch[21] and different pruritogens may activate other neurological pathways. Cowhage are the barbed hairs of the tropical plant Macuna pruriens[22] and their application to skin causes intense itch by stimulating mechanically responsive C-fibres rather than the mechanically insensitive fibres stimulated by the application of histamine. Cowhage stimulated C-fibres innervate different neurons in the spinothalamic tract from histamine[23]. These observations suggest that there are at least two separate itch pathways (histamine and cowhage). Consistent with this is the finding that experienced subjects report different characteristics of the two itch types. Histamine itch is described as “burning” while cowhage itch is described as “stinging”[24].
At cellular level histamine receptors types 1, 3 and 4 are important in the transmission of histamine stimulated itch. Binding of histamine to these receptors activates phospholipase C, phospholipase A2 and transient receptor potential vanilloid 1(TPRV1) resulting in increased intracellular calcium in dorsal root ganglion cells[25]. In contrast cowhage cleaves protease-activated receptor 2 (PAR2) that activates phospholipase C, TRPV1 and transient receptor potential ankyrin 1 (TRPA1) resulting in membrane depolarization[26].
Within the dorsal horn, calcitonin gene-related peptide, gastrin-releasing peptide, substance P and glutamate are important neurotransmitters. There is also a group of inhibitory neurons within the dorsal horn (Bhlhb5 neurons) that synapse between the pain pathway and the histamine stimulated itch pathway. Activation of these neurons by scratching may serve as the cellular basis for how scratching inhibits itch[27]. Scratching may also stimulate inhibitory neurons causing the release of glycine and gamma-amino butyric acid within the central nervous system[28].
Centrally cowhage and histamine stimulated neurons terminate in the contralateral ventral posterior lateral, ventral posterior inferior and posterior nuclei. Cowhage stimulated neurons also project to the contralateral suprageniculate and medial geniculate nuclei[29]. From the thalamus there are projections to the somatosensory cortex, parietal cortex, prefrontal cortex, anterior cingulate gyrus, insula, midbrain and motor cortex[30-32].
PATHOPHYSIOLOGY OF ITCH IN JAUNDICE
Several mechanisms have been proposed to explain the itch that accompanies jaundice. Early theories concentrated on defining a pruritogen released by the liver whose accumulation in skin accounts leads to itch while later theories have concentrated on defining neural circuits involved in the mediation of itch.
Bile salts
Aretaeus, who first recognized the association of jaundice and itch, maintained that itchy skin was due to the presence of “prickly bilious particles” within the skin[7]. This theory remains popular since biliary drainage is usually associated with improvement in itch[33,34]. However, there is often an immediate effect before a fall in plasma bilirubin. In addition itch may precede the appearance of jaundice suggesting that substances other than bilirubin are responsible for pruritis[35]. Consequently bile salts emerged as the primary causative agents in pruritis. This was supported by the observation that feeding bile salts to cholestatic patients worsened pruritis[36], intradermal injection of bile salts in healthy volunteers caused local itching[37,38] and administration of anion exchange resins to bind luminal bile salts decreased itching intensity[39]. However, there is no correlation between the concentration of bile salts in skin or body fluids[40] and the intensity of itch, and the severity of pruritis does not correlate with the severity of cholestasis[41]. Itching in patients with primary biliary cirrhosis may be severe in the early stages of the disease when bile salt concentrations are low but cease to be a significant symptom when liver failure and cholestasis is advanced[42]. In addition many patients with severe cholestasis never experience pruritis[43] while patients with intrahepatic cholestasis of pregnancy all have mild cholestasis but significant symptoms of pruritis[35]. Consequently there is no evidence that bile salts play a direct role in the pathogenesis of itching in jaundiced patients.
Histamine
Histamine is the principle mediator of allergic reactions and is released by mast cells and circulating basophils. Bile salts, particularly chenodeoxycholate and deoxycholate, stimulate the release of histamine from mast cells and plasma histamine concentrations are increased in pruritic patients[44,45]. However, pharmacological doses of bile salts are required to stimulate histamine release from mast cells[46] and histamine antagonists have not been successful in treating pruritic patients[47]. In addition plasma tryptase levels (a marker of mast cell activation) are not elevated in pruritic patients[47], mast cell numbers are not elevated[48], and the typical skin features of histamine release (erythema and swelling) are not seen in pruritic patients[38].
Serotonin
Intradermal injection of serotonin causes itch in healthy volunteers[49] and treatment of pruritic patients with selective serotonin reuptake inhibitors sertraline[50] and paroxetine[51] has been useful in treating pruritis. However, using the 5-HT3-receptor antagonist ondansetron has not been consistently effective in improving itch[52]. This suggests that serotonin may be important in the central nervous system in itch perception or sensory modification but it does not appear to be a direct mediator of pruritis.
Steroids
Steroid hormones may be mediators of pruritis based on the observation that female cholestatic patients often report more intense and prolonged pruritis in comparison with male patients[53], and the itch present in intrahepatic cholestasis of pregnancy typically is most intense in the third trimester when the highest concentrations of steroids and their metabolites are observed. The itch rapidly subsides after delivery and parallels the fall in urinary steroid levels[54]. Steroid hormones may modulate neuronal excitability in cholestatic patients since they act at a number of important neural receptors involved in the itch pathway including TRPV1[55], GABA[56], glycine[57], glutamate[58] and serotonin[59].
Opioids
Endogenous opioids are involved in the mediation of pruritis. Epidurally administered opiates are associated with itching[60] and increased levels of circulating endogenous opioids are seen in animal models of cholestasis and in jaundiced patients[61,62]. Increased expression of preproenkephalin and met-enkephalin are seen in cholestatic livers suggesting that endogenous production is increased[63]. In addition opioid receptors are down regulated in the brains of cholestatic rats suggesting increased exposure to opioid receptor agonists[64]. Finally μ-opioid receptor antagonists (naloxone, naltrexone and nalmefene) exert an anti-pruritic effect[65,66].
However there is no correlation between opioid concentration and itch intensity and opioid concentrations are often similar in patients with intrahepatic cholestasis of pregnancy and with gestation matched controls[67]. Most evidence favours a central role for opioids in the mediation of itch.
Lysophosphatidic acid
Elevated levels of lysophosphatidic acid (LPA) have been found in the plasma of pruritic patients, and intradermal injection is associated with itching[68,69]. LPA is formed from lysophosphatidylcholine by the enzyme autotaxin and is a signaling molecule that acts on a number of specific G-protein coupled receptors present on neuronal cell membranes[68]. Serum LPA concentrations are increased in only pruritic but not in non-pruritic patients with similar levels of cholestasis. In addition autotaxin concentration correlates with itch intensity, with decreased levels seen following biliary drainage and increased levels seen with the recurrence of pruritis[70].
ASSESSMENT OF PRURITIS
Itch is a difficult sensation to quantify. However, there are a number of reported systems used to quantify pruritis and its response to treatment interventions. The most common is a visual analogue scale which was reported by Patrick et al[71] in 1973 and asks the patient to mark the severity of pruritis on a linear analogue scale.
Two other more detailed methods exist. The Eppendorf Itch Questionnaire and the Questionnaire for the Development of pruritis both use a comprehensive list of questions that address sensory and emotional categories to assess the effect of pruritis on the patient’s quality of life. Both questionnaires are detailed but are time consuming to complete[72,73].
TREATMENT
There are a number of reported potential treatments available for patients with pruritis. Our poor understanding of the mechanism underlying pruritis in jaundiced patients has ensured that no single treatment has proved definitive. The evidence base for all reported treatments is variable and most clinicians are aware of the frustrations in finding an effective treatment for the patient with intractable pruritis. The rationale of the reported treatments generally fits into one or more of the following categories: (1) to remove pruritogens from the enterohepatic circulation by non-absorbable anion exchange resins or biliary drainage procedures performed either endoscopically, radiologically or surgically; (2) to alter the metabolism of pruritogens in the liver and/or in the gut; (3) to modify central itch signaling by influencing specific receptors in the central nervous system; and (4) to remove the potential pruritogens from the systemic circulation by invasive methods.
Biliary drainage
Biliary drainage has proven the most effective treatment for pruritis. Generally itching subsides as soon as biliary drainage is obtained and prior to any demonstrable decrease in plasma bilirubin concentrations. For patients with advanced liver disease and bilateral hepatic obstruction unilateral drainage is usually sufficient to palliate pruritis although plasma bilirubin levels and liver function tests may not completely normalize. There are no obvious differences in the effectiveness of available drainage techniques with endoscopic, percutaneous or surgical techniques all successful[33,34,74,75].
Skin care
Skin care in pruritis is often neglected. Twycross (1997) has suggested that appropriate skin care may decrease or eliminate the need for drug therapy[76]. Adequate nutrition is important and a diet that includes protein, carbohydrate, fats and vitamins should be aimed for as well as a fluid intake of two litres of fluid daily.
The aim of skin care is to ensure that the skin does not become dry[13]. Soap should be avoided and replaced with an emollient solution that hydrates the skin[77]. Creams or lotions should be stored in the refrigerator to enhance the cooling effect on application. Heat should be avoided as it enhances local blood flow and exacerbates itching while cool temperatures lower the itch threshold[38]. Consequently soaking in a tepid bath or shower will temporarily reduce itch. After bathing skin should be patted dry with a soft towel and talcum powder and deodorants should be avoided as they may exacerbate itch[13]. Ordinary calamine lotion should be avoided as this has a drying effect on the skin. Oily calamine contains 0.5% phenol and is an effective antipruritic.
Patient clothing and bed linen should be washed in mild detergent and fabric softeners should be avoided. Loose fitting cotton clothing is more comfortable than woollen or synthetic fabrics[13]. If the urge to scratch is overpowering the patient should be encouraged to rub gently or apply pressure at the site of itch instead of scratching. Applying a cold cloth or ice pack will also help. Fingernails should be kept short and clean to avoid skin damage.
Ultraviolet light
Phototherapy using ultraviolet A and ultraviolet B light on the skin has been reported but there is no rationale for its use in jaundice associated pruritis[8,78,79]. Light directed towards the eyes has also been suggested since scratching behaviour often follows a 24 h rhythm. However, a controlled trail of bright light therapy has not been conducted in pruritis associated with cholestasis[78].
Anion exchange resins
This includes cholestyramine, colestipol and colesevelam. These are hydrophilic, water insoluble, non-absorbable and bind bile salts preventing their absorption in the terminal ileum. Cholestyramine is recommended as a 4 g dose one hour before breakfast and this dose can be increased to four times daily[8]. Side effects are common including constipation and malabsorption, and patient compliance can be poor due to the unpleasant taste of the agents[4]. Anion exchange resins also reduce the bioavailability of a number of commonly used agents (digoxin, thyroxine and oral contraceptive agents) and other medications should be taken at least four hours after a dose of one of these agents. Cholestyramine has been assessed in a single blind randomized cross over trial of eight patients and showed that pruritis scores were less in treated patients than in those receiving a placebo[80]. This was confirmed in a double blind placebo controlled trial[81]. Generally improvement in pruritis scores is noted after at least two weeks of treatment[8].
Rifampicin
Rifampicin is an antibiotic and has been used in the treatment of pruritis[11]. Rifampicin induces phase I,II and III biotransformation enzymes and transporters such as CYP3A4, UGT1A1, SULTA1, and MRP2[82,83], enhancing the metabolism of bilirubin and its breakdown products and by modifying the synthesis of secondary bile acids in the intestinal lumen due to its antimicrobial action[4]. However, rifampicin is associated with a number of severe reactions including haemolytic anaemia, renal failure and thrombocytopenic purpura, and regular monitoring of transaminase levels are required due to the possible risk of hepatotoxicity[14]. Rifampicin at a dose of 300 mg/d improves cholestatic pruritis and a meta-analysis performed using five prospective randomized trials confirms its effectiveness in treating pruritis both as an initial therapeutic option and as a treatment following failure of other agents[84].
Ursodeoxycholic acid
Ursodeoxycholic acid has been used in the treatment of primary biliary cirrhosis at a dose of 750 mg/d and improved a number of biochemical parameters but did not improve pruritis[14]. Although it has been effective in treating pruritis in patients with intrahepatic cholestasis of pregnancy possibly due to stimulation of hepatobiliary secretion of progesterone disulfates[85].
Opioid antagonists
A recent review concluded that patients with pruritis due to cholestasis as well as other cause may benefit from treatment with μ-opioid receptor antagonists[86]. Two double blind placebo controlled trials showed improvement in cholestatic pruritis in patients treated with parenteral naloxone at a dose of 0.4 mg followed by an infusion of 0.2 μg/kg. min or oral naltrexone at a dose of 50 mg/d [65,87]. This treatment was associated with a decrease in scratching activity and a decrease in the perception of pruritis[4]. However, administration of opiate antagonists may be associated with an acute withdrawal reaction presenting with abdominal pain, anorexia, and raised blood pressure.
Based on the assumption that opioid induced itch is mediated by activation of μ-opioid receptors and can be suppressed by activation of κ-opioid receptors it was suggested that κ-agonists may be an effective treatment. A recent trial with nalfurafine, a newly described κ-agonist, did effectively reduce itch in haemodialysis patients[88].
Serotonin antagonists
Sertraline is a selective serotonin re-uptake inhibitor. Using a dose of 75-100 mg/d, a randomized controlled trial showed that the treatment was well tolerated and itch scores improved in a group of patients with primary biliary cirrhosis, sclerosing cholangitis, hepatitis C and post necrotic cirrhosis[89]. Sertraline was well tolerated with minor side effects including nausea, dizziness, increased bowel frequency, visual hallucinations and fatigue. Sertraline is contraindicated in patients receiving monamine oxidase inhibitors[14].
Antihistamines
Antihistamines have two potential modes of action in treating pruritis. Firstly they prevent binding of histamine to the H1 receptor and have a second sedating and anticholinergic effect although clinically they are rarely effective[79]. The newer H4 receptor antagonists may have a potential role although this is yet to be formally assessed[90].
Anticonvulsant agents
Anticonvulsants are effective in the treatment of pruritis and probably act at a spinal level by inhibiting transmission. They often do not reach full effectiveness until after 5-6 wk of treatment. Gabapentin (900-2700 mg/d) is currently under investigation although initial analysis of a double blind trial suggested that there was no therapeutic advantage seen over placebo[78,91].
Antidepressants
Antidepressants have been used in the treatment of pruritis. Both paroxetine and setraline are selective serotonin reuptake inhibitors[79]. Mirtazapine and doxepin (both tricyclic antidepressants) have antihistaminic effects and serotonergic effects and have been used to treat pruritis[79].
Immunosuppressants
Cyclosporin (3-5 mg/kg) has a significant anti-pruritic effect within several days of beginning therapy although no trials specifically looking at it use in the treatment of pruritis have been described[79].
Dronabinol
Dronabinol is a psychoactive compound extracted from Cannabis sative and 5 mg administered to patients with intractable cholestasis associated pruritis decreased itch and improved sleep[78]. Dronabinol may act by increasing the threshold to noxious stimuli.
Extracorporeal albumin dialysis
The molecular adsorbent and recirculating system (MARS) is a haemofiltration system that removes albumin-bound substances in patients with liver failure. Although invasive it appears to be effective in controlling pruritis associated with cholestasis[92]. An analysis of patients treated with MARS in three centres showed that MARS was effective in reducing pruritis in 75% of patients[93].
Two case reports indicate that plasmapheresis is a safe therapeutic option and relieves pruritis in pregnant patients with primary biliary cirrhosis[94].
Liver transplantation
Intractable pruritis can become an indication for liver transplantation even if no evidence of cellular hepatic or biliary abnormalities are present[95].
Experimental drug therapies
Propofol 1[96], lidocaine[97], flumecinol[98], stanozolol[99], and butorphanol[100], have been reported in small numbers of patients as having a beneficial effect although none has become part of routine clinical practice.
CURRENT RECOMMENDED TREATMENT
The European Association for the Study of Liver Disease (EASL) guidelines for the drug treatment of pruritis are shown in Table 1 and these are identical to the guidelines of the American Association for the Study of Liver Diseases[14]. These agents are those for which the strongest evidence base exists and have shown the greatest efficacy in the available clinical trials. For patients presenting with biliary obstruction biliary drainage by the most prudent route possible should first be undertaken. The choice of drainage procedure will depend on the nature and site of biliary obstruction and whether further surgical or other active therapy such as chemotherapy and/or radiation therapy is planned. In addition all jaundiced and pruritic patients should be advised of an appropriate skin care regime with regular bathing, careful use of detergents and moisturizers.
Table 1.
Current suggested pharmacological therapy for the management of pruritis associated with jaundice[101]
| Treatment | Agent | Dosage |
| Initial | UDCA | 10-15 mg/kg.d (PO) |
| First line | Cholestyramine | 4-16 g/d (PO) |
| Second line | Rifampicin | 300-600 mg/d (PO) |
| Third line | Naltrexone | 50 mg/d (PO) |
| Fourth line | Sertraline | 100 mg/d (PO) |
UDCA: Ursodeoxycholic acid; PO: Oral administration.
Once biliary drainage has been established and pruritis remains, or in patients where biliary drainage cannot be obtained, implementation of pharmacological therapy using the agents in the order suggested by the EASL should be commenced.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 12, 2014
First decision: September 15, 2014
Article in press: November 19, 2014
P- Reviewer: Shimoyama S, Zhu YL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Chaudhury P, Barkin A, Barkun J. ACS Surgery: Principles and Practice (2010) Available from: http://www.slideshare.net/medbookonline/acs0503-jaundice-2006.
- 2.Briggs CD, Irving GR, Cresswell A, Peck R, Lee F, Peterson M, Cameron IC. Percutaneous transhepatic insertion of self-expanding short metal stents for biliary obstruction before resection of pancreatic or duodenal malignancy proves to be safe and effective. Surg Endosc. 2010;24:567–571. doi: 10.1007/s00464-009-0598-9. [DOI] [PubMed] [Google Scholar]
- 3.Moses RA, Grodzki WJ, Starcher BC, Galione MJ. Elastin content of the scleral spur, trabecular mesh, and sclera. Invest Ophthalmol Vis Sci. 1978;17:817–818. [PubMed] [Google Scholar]
- 4.Ständer S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003;139:1463–1470. doi: 10.1001/archderm.139.11.1463. [DOI] [PubMed] [Google Scholar]
- 5.Ikoma A, Rukwied R, Ständer S, Steinhoff M, Miyachi Y, Schmelz M. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475–1478. doi: 10.1001/archderm.139.11.1475. [DOI] [PubMed] [Google Scholar]
- 6.Weldon D. What lies beneath the surface of the itch in adults? Allergy Asthma Proc. 2007;28:153–162. doi: 10.2500/aap.2007.28.2942. [DOI] [PubMed] [Google Scholar]
- 7.Haas LF. Aretaeus of Cappodocia (130-200) J Neurol Neurosurg Psychiatry. 1991;54:203. doi: 10.1136/jnnp.54.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremer AE, Oude Elferink RP, Beuers U. Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol. 2011;35:89–97. doi: 10.1016/j.clinre.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Huesmann M, Huesmann T, Osada N, Phan NQ, Kremer AE, Ständer S. Cholestatic pruritus: a retrospective analysis on clinical characteristics and treatment response. J Dtsch Dermatol Ges. 2013;11:158–168. doi: 10.1111/j.1610-0387.2012.08028.x. [DOI] [PubMed] [Google Scholar]
- 10.Bergasa NV, Mehlman JK, Jones EA. Pruritus and fatigue in primary biliary cirrhosis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:643–655. doi: 10.1053/bega.2000.0109. [DOI] [PubMed] [Google Scholar]
- 11.Mela M, Mancuso A, Burroughs AK. Review article: pruritus in cholestatic and other liver diseases. Aliment Pharmacol Ther. 2003;17:857–870. doi: 10.1046/j.1365-2036.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 12.Weisshaar E, Dalgard F. Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol. 2009;89:339–350. doi: 10.2340/00015555-0662. [DOI] [PubMed] [Google Scholar]
- 13.Bosonnet L. Pruritus: scratching the surface. Eur J Cancer Care (Engl) 2003;12:162–165. doi: 10.1046/j.1365-2354.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 14.Imam MH, Gossard AA, Sinakos E, Lindor KD. Pathogenesis and management of pruritus in cholestatic liver disease. J Gastroenterol Hepatol. 2012;27:1150–1158. doi: 10.1111/j.1440-1746.2012.07109.x. [DOI] [PubMed] [Google Scholar]
- 15.Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313–322. doi: 10.1093/brain/awt158. [DOI] [PubMed] [Google Scholar]
- 16.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 18.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 19.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 21.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 22.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handwerker HO. Microneurography of pruritus. Neurosci Lett. 2010;470:193–196. doi: 10.1016/j.neulet.2009.06.092. [DOI] [PubMed] [Google Scholar]
- 25.Rossbach K, Nassenstein C, Gschwandtner M, Schnell D, Sander K, Seifert R, Stark H, Kietzmann M, Bäumer W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience. 2011;190:89–102. doi: 10.1016/j.neuroscience.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 31.Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001;92:295–305. doi: 10.1016/s0304-3959(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh JC, Hägermark O, Ståhle-Bäckdahl M, Ericson K, Eriksson L, Stone-Elander S, Ingvar M. Urge to scratch represented in the human cerebral cortex during itch. J Neurophysiol. 1994;72:3004–3008. doi: 10.1152/jn.1994.72.6.3004. [DOI] [PubMed] [Google Scholar]
- 33.VARCO RL. Intermittent external biliary drainage for relief of pruritus in certain chronic disorders of the liver. Surgery. 1947;21:43–45. [PubMed] [Google Scholar]
- 34.Stapelbroek JM, van Erpecum KJ, Klomp LW, Venneman NG, Schwartz TP, van Berge Henegouwen GP, Devlin J, van Nieuwkerk CM, Knisely AS, Houwen RH. Nasobiliary drainage induces long-lasting remission in benign recurrent intrahepatic cholestasis. Hepatology. 2006;43:51–53. doi: 10.1002/hep.20998. [DOI] [PubMed] [Google Scholar]
- 35.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049–2066. doi: 10.3748/wjg.15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahrens EH, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore) 1950;29:299–364. doi: 10.1097/00005792-195012000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kirby J, Heaton KW, Burton JL. Pruritic effect of bile salts. Br Med J. 1974;4:693–695. doi: 10.1136/bmj.4.5946.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herndon JH. Itching: the pathophysiology of pruritus. Int J Dermatol. 1975;14:465–484. doi: 10.1111/j.1365-4362.1975.tb04281.x. [DOI] [PubMed] [Google Scholar]
- 39.van Itallie TB, Hashim SA, Crampton RS, Tennent DM. The treatment of pruritus and hypercholesteremia of primary biliary cirrhosis with cholestyramine. N Engl J Med. 1961;265:469–474. doi: 10.1056/NEJM196109072651004. [DOI] [PubMed] [Google Scholar]
- 40.Ghent CN, Bloomer JR, Klatskin G. Elevations in skin tissue levels of bile acids in human cholestasis: relation to serum levels and topruritus. Gastroenterology. 1977;73:1125–1130. [PubMed] [Google Scholar]
- 41.Ghent CN, Bloomer JR. Itch in liver disease: facts and speculations. Yale J Biol Med. 1979;52:77–82. [PMC free article] [PubMed] [Google Scholar]
- 42.Kremer AE, Beuers U, Oude-Elferink RP, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs. 2008;68:2163–2182. doi: 10.2165/00003495-200868150-00006. [DOI] [PubMed] [Google Scholar]
- 43.Murphy GM, Ross A, Billing BH. Serum bile acids in primary biliary cirrhosis. Gut. 1972;13:201–206. doi: 10.1136/gut.13.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clements WD, O’Rourke DM, Rowlands BJ, Ennis M. The role of mast cell activation in cholestatic pruritus. Agents Actions. 1994;41 Spec No:C30–C31. doi: 10.1007/BF02007753. [DOI] [PubMed] [Google Scholar]
- 45.Gittlen SD, Schulman ES, Maddrey WC. Raised histamine concentrations in chronic cholestatic liver disease. Gut. 1990;31:96–99. doi: 10.1136/gut.31.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quist RG, Ton-Nu HT, Lillienau J, Hofmann AF, Barrett KE. Activation of mast cells by bile acids. Gastroenterology. 1991;101:446–456. doi: 10.1016/0016-5085(91)90024-f. [DOI] [PubMed] [Google Scholar]
- 47.Jones EA, Bergasa NV. Evolving concepts of the pathogenesis and treatment of the pruritus of cholestasis. Can J Gastroenterol. 2000;14:33–40. doi: 10.1155/2000/747495. [DOI] [PubMed] [Google Scholar]
- 48.O’Keeffe C, Baird AW, Nolan N, McCormick PA. Cholestatic pruritus - the role of cutaneous mast cells and nerves. Aliment Pharmacol Ther. 2004;19:1293–1300. doi: 10.1111/j.1365-2036.2004.01997.x. [DOI] [PubMed] [Google Scholar]
- 49.Hägermark O. Peripheral and central mediators of itch. Skin Pharmacol. 1992;5:1–8. doi: 10.1159/000211009. [DOI] [PubMed] [Google Scholar]
- 50.Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98:2736–2741. doi: 10.1111/j.1572-0241.2003.08662.x. [DOI] [PubMed] [Google Scholar]
- 51.Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manage. 2003;26:1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 52.O’Donohue JW, Pereira SP, Ashdown AC, Haigh CG, Wilkinson JR, Williams R. A controlled trial of ondansetron in the pruritus of cholestasis. Aliment Pharmacol Ther. 2005;21:1041–1045. doi: 10.1111/j.1365-2036.2005.02430.x. [DOI] [PubMed] [Google Scholar]
- 53.Lucey MR, Neuberger JM, Williams R. Primary biliary cirrhosis in men. Gut. 1986;27:1373–1376. doi: 10.1136/gut.27.11.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reyes H, Sjövall J. Bile acids and progesterone metabolites in intrahepatic cholestasis of pregnancy. Ann Med. 2000;32:94–106. doi: 10.3109/07853890009011758. [DOI] [PubMed] [Google Scholar]
- 55.Chen SC, Wu FS. Mechanism underlying inhibition of the capsaicin receptor-mediated current by pregnenolone sulfate in rat dorsal root ganglion neurons. Brain Res. 2004;1027:196–200. doi: 10.1016/j.brainres.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 56.Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- 57.Wu FS, Gibbs TT, Farb DH. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol. 1990;37:597–602. [PubMed] [Google Scholar]
- 58.Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- 59.Wetzel CH, Hermann B, Behl C, Pestel E, Rammes G, Zieglgänsberger W, Holsboer F, Rupprecht R. Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor. Mol Endocrinol. 1998;12:1441–1451. doi: 10.1210/mend.12.9.0163. [DOI] [PubMed] [Google Scholar]
- 60.Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- 61.Swain MG, Rothman RB, Xu H, Vergalla J, Bergasa NV, Jones EA. Endogenous opioids accumulate in plasma in a rat model of acute cholestasis. Gastroenterology. 1992;103:630–635. doi: 10.1016/0016-5085(92)90857-u. [DOI] [PubMed] [Google Scholar]
- 62.Thornton JR, Losowsky MS. Opioid peptides and primary biliary cirrhosis. BMJ. 1988;297:1501–1504. doi: 10.1136/bmj.297.6662.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergasa NV, Sabol SL, Young WS, Kleiner DE, Jones EA. Cholestasis is associated with preproenkephalin mRNA expression in the adult rat liver. Am J Physiol. 1995;268:G346–G354. doi: 10.1152/ajpgi.1995.268.2.G346. [DOI] [PubMed] [Google Scholar]
- 64.Bergasa NV, Rothman RB, Vergalla J, Xu H, Swain MG, Jones EA. Central mu-opioid receptors are down-regulated in a rat model of cholestasis. J Hepatol. 1992;15:220–224. doi: 10.1016/0168-8278(92)90040-v. [DOI] [PubMed] [Google Scholar]
- 65.Bergasa NV, Alling DW, Talbot TL, Swain MG, Yurdaydin C, Turner ML, Schmitt JM, Walker EC, Jones EA. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med. 1995;123:161–167. doi: 10.7326/0003-4819-123-3-199508010-00001. [DOI] [PubMed] [Google Scholar]
- 66.Carson KL, Tran TT, Cotton P, Sharara AI, Hunt CM. Pilot study of the use of naltrexone to treat the severe pruritus of cholestatic liver disease. Am J Gastroenterol. 1996;91:1022–1023. [PubMed] [Google Scholar]
- 67.Kremer AE, Martens JJ, Kulik W, Ruëff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018, 1018.e1. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig Dis. 2011;29:66–71. doi: 10.1159/000324131. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51–56. doi: 10.1159/000078632. [DOI] [PubMed] [Google Scholar]
- 70.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, Raap U, van Buuren HR, van Erpecum KJ, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391–1400. doi: 10.1002/hep.25748. [DOI] [PubMed] [Google Scholar]
- 71.Patrick DL, Bush JW, Chen MM. Methods for measuring levels of well-being for a health status index. Health Serv Res. 1973;8:228–245. [PMC free article] [PubMed] [Google Scholar]
- 72.Yosipovitch G, Zucker I, Boner G, Gafter U, Shapira Y, David M. A questionnaire for the assessment of pruritus: validation in uremic patients. Acta Derm Venereol. 2001;81:108–111. doi: 10.1080/00015550152384236. [DOI] [PubMed] [Google Scholar]
- 73.Darsow U, Scharein E, Simon D, Walter G, Bromm B, Ring J. New aspects of itch pathophysiology: component analysis of atopic itch using the ‘Eppendorf Itch Questionnaire’. Int Arch Allergy Immunol. 2001;124:326–331. doi: 10.1159/000053748. [DOI] [PubMed] [Google Scholar]
- 74.Singh MK, Facciuto ME. Current management of cholangiocarcinoma. Mt Sinai J Med. 2012;79:232–245. doi: 10.1002/msj.21298. [DOI] [PubMed] [Google Scholar]
- 75.Hwang SI, Kim HO, Son BH, Yoo CH, Kim H, Shin JH. Surgical palliation of unresectable pancreatic head cancer in elderly patients. World J Gastroenterol. 2009;15:978–982. doi: 10.3748/wjg.15.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilcock A, Twycross R. Symptom management in palliative care: optimizing drug treatment. Br J Hosp Med (Lond) 2006;67:400–403. doi: 10.12968/hmed.2006.67.8.21956. [DOI] [PubMed] [Google Scholar]
- 77.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21; quiz 22-23. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 78.Bergasa NV. The pruritus of cholestasis. J Hepatol. 2005;43:1078–1088. doi: 10.1016/j.jhep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Metz M, Grundmann S, Ständer S. Pruritus: an overview of current concepts. Vet Dermatol. 2011;22:121–131. doi: 10.1111/j.1365-3164.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 80.Duncan JS, Kennedy HJ, Triger DR. Treatment of pruritus due to chronic obstructive liver disease. Br Med J (Clin Res Ed) 1984;289:22. doi: 10.1136/bmj.289.6436.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Padova C, Tritapepe R, Rovagnati P, Rossetti S. Double-blind placebo-controlled clinical trial of microporous cholestyramine in the treatment of intra- and extra-hepatic cholestasis: relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol. 1984;6:773–776. [PubMed] [Google Scholar]
- 82.LeCluyse EL. Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 83.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R, et al. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 2006;26:943–948. doi: 10.1111/j.1478-3231.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- 85.Glantz A, Reilly SJ, Benthin L, Lammert F, Mattsson LA, Marschall HU. Intrahepatic cholestasis of pregnancy: Amelioration of pruritus by UDCA is associated with decreased progesterone disulphates in urine. Hepatology. 2008;47:544–551. doi: 10.1002/hep.21987. [DOI] [PubMed] [Google Scholar]
- 86.Phan NQ, Bernhard JD, Luger TA, Ständer S. Antipruritic treatment with systemic μ-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63:680–688. doi: 10.1016/j.jaad.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 87.Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113:1264–1269. doi: 10.1053/gast.1997.v113.pm9322521. [DOI] [PubMed] [Google Scholar]
- 88.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- 89.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666–674. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- 90.Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 91.Bergasa NV, McGee M, Ginsburg IH, Engler D. Gabapentin in patients with the pruritus of cholestasis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2006;44:1317–1323. doi: 10.1002/hep.21370. [DOI] [PubMed] [Google Scholar]
- 92.Chazouillères O. MARS: The ultimate warrior against pruritus of cholestasis? J Hepatol. 2010;53:228–229. doi: 10.1016/j.jhep.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol. 2010;53:307–312. doi: 10.1016/j.jhep.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 94.Alallam A, Barth D, Heathcote EJ. Role of plasmapheresis in the treatment of severe pruritus in pregnant patients with primary biliary cirrhosis: case reports. Can J Gastroenterol. 2008;22:505–507. doi: 10.1155/2008/969826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–1013. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 96.Borgeat A, Wilder-Smith OH, Mentha G. Subhypnotic doses of propofol relieve pruritus associated with liver disease. Gastroenterology. 1993;104:244–247. doi: 10.1016/0016-5085(93)90858-a. [DOI] [PubMed] [Google Scholar]
- 97.Villamil AG, Bandi JC, Galdame OA, Gerona S, Gadano AC. Efficacy of lidocaine in the treatment of pruritus in patients with chronic cholestatic liver diseases. Am J Med. 2005;118:1160–1163. doi: 10.1016/j.amjmed.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 98.Turner IB, Rawlins MD, Wood P, James OF. Flumecinol for the treatment of pruritus associated with primary biliary cirrhosis. Aliment Pharmacol Ther. 1994;8:337–342. doi: 10.1111/j.1365-2036.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 99.Walt RP, Daneshmend TK, Fellows IW, Toghill PJ. Effect of stanozolol on itching in primary biliary cirrhosis. Br Med J (Clin Res Ed) 1988;296:607. doi: 10.1136/bmj.296.6622.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergasa NV. Medical palliation of the jaundiced patient with pruritus. Gastroenterol Clin North Am. 2006;35:113–123. doi: 10.1016/j.gtc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Beuers UH, Boberg KM, Chapman RW, Chazouilleres O, Invernizzi P, Jones DE. EASL clinical practice guidelines: managment of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]


