Abstract
AIM: Danshen’s capability to induce salivary fluid secretion and its mechanisms were studied to determine if it could improve xerostomia.
METHODS: Submandibular glands were isolated from male Wistar rats under systemic anesthesia with pentobarbital sodium. The artery was cannulated and vascularly perfused at a constant rate. The excretory duct was also cannulated and the secreted saliva was weighed in a cup on an electronic balance. The weight of the accumulated saliva was measured every 3 s and the salivary flow rate was calculated. In addition, the arterio-venous difference in the partial oxygen pressure was measured as an indicator of oxygen consumption. In order to assess the mechanism involved in Danshen-induced fluid secretion, either ouabain (an inhibitor of Na+/K+ ATPase) or bumetanide (an inhibitor of NKCC1) was additionally applied during the Danshen stimulation. In order to examine the involvement of the main membrane receptors, atropine was added to block the M3 muscarinic receptors, or phentolamine was added to block the α1 adrenergic receptors. In order to examine the requirement for extracellular Ca2+, Danshen was applied during the perfusion with nominal Ca2+ free solution.
RESULTS: Although Danshen induced salivary fluid secretion, 88.7 ± 12.8 μL/g-min, n = 9, (the highest value around 20 min from start of DS perfusion was significantly high vs 32.5 ± 5.3 μL/g-min by carbamylcholine, P = 0.00093 by t-test) in the submandibular glands, the time course of that secretion differed from that induced by carbamylcholine. There was a latency associated with the fluid secretion induced by Danshen, followed by a gradual increase in the secretion to its highest value, which was in turn followed by a slow decline to a near zero level. The application of either ouabain or bumetanide inhibited the fluid secretion by 85% or 93%, and suppressed the oxygen consumption by 49% or 66%, respectively. These results indicated that Danshen activates Na+/K+ ATPase and NKCC1 to maintain Cl- release and K+ release for fluid secretion. Neither atropine or phentolamine inhibited the fluid secretion induced by Danshen (263% ± 63% vs 309% ± 45%, 227% ± 63% vs 309% ± 45%, P = 0.899, 0.626 > 0.05 respectively, by ANOVA). Accordingly, Danshen does not bind with M3 or α1 receptors. These characteristics suggested that the mechanism involved in DS-induced salivary fluid secretion could be different from that induced by carbamylcholine. Carbamylcholine activates the M3 receptor to release inositol trisphosphate (IP3) and quickly releases Ca2+ from the calcium stores. The elevation of [Ca2+]i induces chloride release and quick osmosis, resulting in an onset of fluid secretion. An increase in [Ca2+]i is essential for the activation of the luminal Cl- and basolateral K+ channels. The nominal removal of extracellular Ca2+ totally abolished the fluid secretion induced by Danshen (1.8 ± 0.8 μL/g-min vs 101.9 ± 17.2 μL/g-min, P = 0.00023 < 0.01, by t-test), suggesting the involvement of Ca2+ in the activation of these channels. Therefore, IP3-store Ca2+ release signalling may not be involved in the secretion induced by Danshen, but rather, there may be a distinct signalling process.
CONCLUSION: The present findings suggest that Danshen can be used in the treatment of xerostomia, to avoid the systemic side effects associated with muscarinic drugs.
Keywords: Salivary fluid secretion, Xerostomia, Chinese herb, Danshen, Submandibular gland, Oxygen consumption, Extracellular Ca2+
Core tip: Perfusion of rat submandibular glands allowed for the measurement of fluid secretion and oxygen consumption during Danshen stimulation, which induced a large salivary fluid secretion. Ouabain (Na+/K+ ATPase inhibitor) and bumetanide (NKCC1 inhibitor), inhibited fluid secretion and oxygen consumption significantly, indicating that Danshen has a similar basic mechanism for secretion. Receptor blockers indicated that Danshen does not bind with M3 or α1 receptors. These findings suggest that the mechanism for Danshen-induced fluid secretion could be different from that induced by carbamylcholine. Accordingly, Danshen may avoid the systemic side effects caused by muscarinic drugs in the treatment of xerostomia.
INTRODUCTION
Saliva moistens and washes the surface of the oral cavity to maintain the oral environment, and facilitates swallowing and chewing food as a lubricant. The subjective feeling of dry mouth is called xerostomia, which is mainly caused by salivary gland hypofunction (SGH). SGH is a condition in which non-stimulated or stimulated salivary flow is significantly reduced, due to many reasons, such as aging, radiation therapy, medication, and Sjögren’s syndrome. The incidence of SGH in the population over 65 years old is 30%-40%[1]. Xerostomia reduces the quality of life (QOL)[2,3]. The current treatment for xerostomia includes the administration of parasympathomimetic drugs or artificial saliva[4]. Parasympathomimetic drugs, including pilocarpine and cevimeline, activate muscarinic receptors on the salivary glands to stimulate fluid secretion. However, muscarinic receptors exist in many organs of the body, so this medication is accompanied by systemic side effects, for example, nausea, diarrhea and other adverse gastrointestinal reactions[5]. Therefore, most patients tend not to take these drugs, but rather use artificial saliva. However, artificial saliva also has disadvantages. It is difficult to take when the mouth is used continuously, such as during speech. Therefore, there is a clinical requirement for salivary fluid promoters with fewer side effects.
Danshen
Murakami et al[6] examined 20 Chinese herbs regarding their capability to promote salivary fluid secretion, using isolated and vascularly perfused submandibular glands (SMG) of rats. They found 15 herbs that could promote salivary fluid secretion of the gland during muscarinic stimulation with carbamylcholine (carbachol, CCh). Among those 15 herbs, it was found that Danshen (DS) not only promotes salivary fluid secretion, but also that it induces it.
DS is a representative Chinese herb in the category of the agents that promote blood circulation and eliminate stasis[7]. DS is mainly used for coronary atherosclerotic disease of the heart. The main pharmacological functions of DS have been reported as: (1) dilatation of the blood vessels; (2) promotion of microcirculation; (3) anti-coagulant activity; (4) antithrombotic activity; and (5) an anti-inflammatory function. Among the components of the DS extracts, salvianolic acids, phenolic acids and diterpenechinone compounds of the tanshinone type are equally effective[8].
DS has been clinically used to relieve dry mouth in Chinese medicine. However, only a few reports have addressed the direct effect of DS on salivary glands[9] and no studies have been conducted to examine the mechanisms involved related to the induction of salivary secretion. The present study was planned and conducted to confirm the salivary induction due to DS and examine the possible mechanisms involved in DS-induced salivary fluid secretion.
Experimental plan to assess the ability of Chinese herbs to induce salivary fluid secretion
In order to measure fluid secretion in response to Chinese herbs, we employed isolated and arterially perfused rat salivary glands, because this is only the method that makes it possible to measure fluid secretion, except for a method employing in vivo glands. The use of in vivo glands is an ideal model for clinical application, but with this method, we cannot avoid influences from neural and hormonal effects. A previous study showed that five kinds of Chinese herbs, previously known as medicine for xerostomia, could not promote salivary fluid secretion in isolated and perfused glands[6]. In addition, because the epithelial structure is maintained in the isolated perfused SMG, this method makes it possible to observe not only transcellular fluid secretion, but also paracellular fluid secretion[10].
Transcellular fluid secretion is based on Cl- release through the Ca2+ dependent-Cl- channel (TMEM16A) across the luminal plasma membrane[11]. The junctional flow of Na+ is followed by a requirement for electroneutrality to increase luminal osmolarity. The osmotic gradient allows osmosis through aquaporine 5 on the luminal membrane and junctional water flow. For Cl- entry across the basolateral membrane, the Na+/K+/2Cl- cotransporter (NKCC1) is driven by the Na+ electrochemical gradient, which is maintained by Na+/K+ ATPase. Because the double antiport system, including the anion and Na+/H+ exchangers, has a minimal contribution for Cl- entry during HCO3- free perfusion, the experimental system can be simplified by avoiding HCO3- utilization.
The experimental system for the assessment of new secretagogues, including Chinese herbs, was established as follows: (1) Fluid secretion measured by the use of a computer-aided electronic balance in the isolated perfused SMG; (2) Oxygen consumption measured by arterio-venously placed oxygen electrodes, inhibition of fluid secretion and oxygen consumption by ouabain for assessment of the activity of Na+/K+ ATPase; (3) Inhibition of fluid secretion by bumetanide for assessment of NKCC1 for the fluid secretion; (4) Use of atropine and phentolamine to assess the involvement of muscarinic and alpha-adrenergic receptors stimulated by new secretagogues; and (5) The requirement of extracellular Ca2+.
Using the experimental plan shown above, this study was conducted to investigate the salivary fluid secretion induced by DS. In addition, this experimental plan will be useful to check new pharmaceuticals for their effect on salivary fluid secretion.
MATERIALS AND METHODS
Isolation and perfusion of SMG
Adult Wistar male rats (Wistar/ST, 9 wk, 240-280 g) were purchased from Japan SLC Inc. and given a standard pellet diet and water ad libitum. The rats were anesthetized by intraperitoneal injection of pentobarbital sodium at a dose of 30 mg/kg body weight (Somnopentyl, Kyoritsu Seiyaku Co., Japan) after induction with 3 Vol% isoflurane (Forane, AbbVie Inc., Illinois, United States). The SMGs were surgically isolated as previously described[12]. Briefly, the attached sublingual gland and its duct were removed after ligation of both the feeding arteries and the draining vein. The main duct of the SMG was cannulated with a fluorine-fiber tube (0.3 mm ID × 0.5 mm OD, EXLON, Iwase Co. Ltd., Japan) for sampling. The artery distal to the glandular branch was cannulated with a stainless steel catheter connected to the infusion line for perfusion. Then, the vein from the gland was cut free, with exception for measurement of oxygen consumption.
Perfusion of the gland
The isolated SMGs were placed in a humidified chamber at 37 °C and perfused arterially with the aid of a peristaltic pump (Cole-Palmer, 7553-10, United States) at a rate of 1.8 mL/min to supply enough oxygen even without a specific oxygen carrier during the secretory period. Immediately before the excretory duct cannulation, the fluorine-fiber tube was filled with distilled water. The other end of the ductal cannula was placed under water in a small cup on an electronic balance with a minimum digital readout of 0.1 mg (Shimadzu AEG-220, Kyoto, Japan), avoiding any contact with the side and bottom of the cup.
After a control perfusion of buffered salt solution for 20 min, the weight of the secreted saliva was recorded for 5 min prior to the start of the stimulation. Carbamylcholine chloride (CCh, Sigma, MO, United States) was applied at 0.2 μmol/L for 5 min as a control to test the vigor of the gland, and in these experiments, the fluid secretion of CCh at 4.95-5 min was set as 100% to normalize the individual variation. CCh was removed by washing for another 5 min using perfusion fluid. The DS solution was continually perfused for 60 min.
Perfusion fluid
The perfusion fluid was a salt solution with the following composition (in mmol/L): Na+ 140.0, K+ 4.3, Ca2+ 1.0, Mg2+ 1.0, Cl- 148.3, and glucose 5.0. The solution was buffered at pH 7.4 with N-2-hydroxyethyl piperazine-N’-2-ethane sulfonic acid (HEPES, 10 mmol/L) and gassed with 100% O2 at 37 °C. Salts, glucose, HEPES, CCh and phentolamine were purchased from Sigma (MO, United States).
Preparation of the DS solution
The granular extract from the root of the DS was provided by Tian Jiang Pharmaceutical Co. Ltd., Jiang Yin, China. Each gram of this DS granule was concentrated and equivalent to 5 g of the crude DS. The DS stock solution was prepared as follows: (1) Two gram of DS were dissolved in the perfusion fluid by ultrasonic concussion for 5 min. The precipitate was removed twice after each centrifugation at 4000 rpm for 5 min. The clear supernatant was obtained and its pH value was adjusted to 7.4 by sodium hydroxide solution (1 mol/L, Nacalai Tesque, Inc., Kyoto, Japan); (2) The neutral solution was prepared to 0.5 g/mL (each concentration in this study refers to crude DS, rather than the extract of DS) in a buffer solution. This solution was filtered through a filter with a pore size of 0.22 μm (Sterivex-GV, Millipore, MA, United States) to obtain the DS stock solution; (3) Finally, before using, the stock solution was diluted with the perfusate, and prepared into concentrations of 1, 3, 5, 25 and 50 g/L.
Salivary fluid secretion
The cumulative weight was automatically measured every 3 s and the data was transferred to a spreadsheet file in a computer. The fluid secretion rate was calculated from the time differentiation of the cumulative volume of saliva, assuming a saliva specific gravity of 1.0.
Oxygen consumption
The partial oxygen pressure (PO2) of the perfusate and the effluent was measured polarographically at 37 °C by the Dissolved Oxygen Measuring System (Model 203, Instech Laboratories, PA, United States). The artery and the vein of each of the isolated SMGs were cannulated and arterially perfused with perfusion fluid. Clark-type electrodes were placed serially on the arterial and venous sides of the perfusion line. The data were stored every 15 s by computer. The calibration of the system was performed during perfusion with distilled water 100% equilibrated with air. The partial pressure of oxygen was obtained as the value at 100% air saturation at 37 °C and 1 atm. The rate of oxygen consumption was expressed by changes in the AV difference of oxygen pressure from the resting level. Oxygen consumption during DS-induced salivary fluid secretion was measured in the same time course as the salivary fluid secretion induced by 5 g/L DS. Oxygen consumption during DS perfusion with other drugs was simultaneously measured with the fluid secretion.
Ouabain, bumetanide, atropine and phentolamine
Atropine (1 μmol/L; Nacalai Tesque. Inc., Japan) or phentolamine (5 μmol/L; Sigma, MO, United States) was continually perfused and 10 min later, DS (5 g/L) solution was added and continually perfused for 50 min. The salivary fluid secretion and oxygen consumption were recorded simultaneously during experiments that involved ouabain and bumetanide. In addition to the first experiment, ouabain (1 mmol/L; Merck KGaA, Germany) or bumetanide (10 μmol/L; Sankyo, Japan), were respectively added at 10 min after the DS was administered and removed at 15 min.
Removal of extracellular Ca2+
The measurement of the salivary fluid secretion and the perfusion of the SMGs were almost the same as in the first experiment, except that Ca2+ in the perfusate was removed nominally without any chelating agent before the administration of CCh or DS, in order to examine the contribution of extracellular Ca2+ on DS-induced salivary fluid secretion.
Statistical analysis
The values for the salivary fluid flow rates were presented as mean ± SE, and n was the number of glands. Statistically significant differences between the values were determined by double-tailed t-test or ANOVA as indicated in the text, and P values below 0.05 were regarded as statistically significant. We mainly used absolute values at different time points (using the units μL/g-min and mmHg) in each experiment for statistical comparisons using the t-test, whereas, in the figures, the relative values (% controlled to the value at 5 min during CCh stimulation) were used to express the time course of the responses, and also to express the degree of the inhibitions
RESULTS
Time course of the DS-induced salivary fluid secretion
A low dose of CCh (0.2 μmol/L) was used as a control stimulation, to normalize the variation of fluid secretion shown by individual glands. All values for the fluid secretory rate during the experiment were normalized to the fluid secretion at 5 min of the control CCh stimulation. Addition of the CCh immediately induced salivary fluid secretion. In contrast, after the addition of the DS, the fluid secretion did not start immediately, i.e., latency was observed, as shown in Figure 1. This figure shows a relative fluid secretion to the control at 5 min, which averaged at each sampling point.
Figure 1.
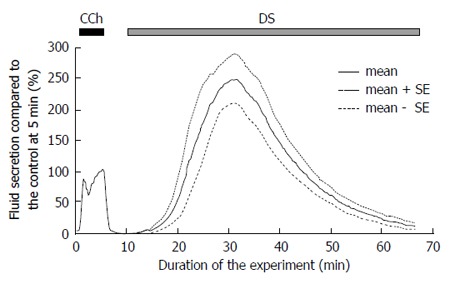
Time course of the Danshen-induced salivary fluid secretion. After control stimulation with 0.2 μmol/L CCh for 5 min, DS (5 g/L) was introduced at 10 min and perfused for 60 min. Respectively, the bold and dotted lines show the average values and the mean ± SE of the salivary fluid secretion of 9 glands. DS: Danshen; CCh: Carbamylcholine.
The DS-induced secretory process had two stages. Initially the fluid secretion increased gradually to reach the highest secretion 88.7 ± 12.8 μL/g-min, n = 9 glands around 20 min from start of DS perfusion. This is a significantly high value compared to that obtained during CCh control (32.5 ± 5.3 μL/g-min, P = 0.00093 by t-test). Thereafter, the fluid secretion declined gradually to the plateau value (6.3 ± 2.1 μL/g-min, 24.1% ± 8.3% of the CCh control) at 60 min. After this decline, the gland did not start another fluid secretion process even during prolonged perfusion with DS.
The latency of the salivary fluid secretion decreased with high doses of DS perfusion
The latency is the time period required for the SMG to start fluid secretion, that is, the time from the application of DS perfusion to the start of fluid secretion. The latency of the salivary secretion was observed at different doses of DS (Figure 2). The latency of the salivary fluid secretion (mean ± SE) decreased with higher doses of DS perfusion: 42.3 ± 5.5 min at 1 g/L DS (n = 6), 10.7 ± 1.0 min at 3 g/L (n = 9), 7.7 ± 1.1 min at 5 g/L (n = 9), 1.3 ± 0.5 min at 25 g/L (n = 6) and 1.8 ± 0.3 min at 50 g/L (n = 8), respectively (n = the number of glands employed for each dose).
Figure 2.

The latency of stimulation with different doses of Danshen. The latency of the fluid secretion was shown as the mean ± SE for different DS doses. The latency reduced as the dose of DS increased. DS: Danshen.
Highest secretory rate of the DS-induced salivary fluid secretion
The fluid secretion induced by DS perfusion was demonstrated in a dose-dependent manner within the range of 1-50 g/L. DS perfusion in the rat SMGs at doses of 1, 3, 5, 25 and 50 g/L induced the highest fluid secretion of 14.7% ± 4.3% of the CCh control fluid secretion, with the various doses showing fluid secretion values of 171.5% ± 36.7%, 308.9% ± 45.1%, 415.3% ± 66.8%, and 364.0% ± 68.7%, respectively (Figure 3). There was no significant difference among the groups with 5, 25 and 50 g/L. Therefore, the 25 g/L dose of DS could have the highest efficiency to promote fluid secretion (415.3% ± 66.8% of the CCh control) among all of the doses tested. The median effective dose (ED50) of DS was 3.6 g/L, calculated by the straight line of regression method (Y = 289.5X + 46.3, r2 = 0.905).
Figure 3.
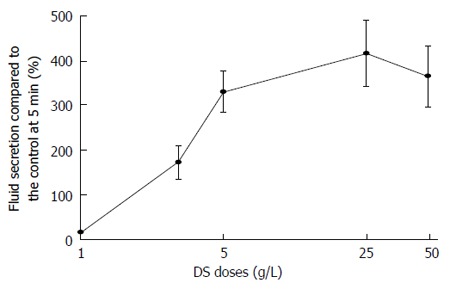
Dose response of the highest Danshen-induced fluid secretion. The highest fluid secretion was shown as mean ± SE and the doses of DS employed were 1, 3, 5, 25 and 50 g/L, respectively. The fluid secretion increased from 1 g/L to 25 g/L, and reached a plateau level at the higher doses. DS: Danshen.
Oxygen consumption during the DS-induced salivary fluid secretion
The oxygen consumption of the isolated SMGs increased to 19.9 ± 2.4 mmHg, n = 5 glands rapidly after the administration of DS without any latency (Figure 4 shows the average of relative values to the control at 5 min). Corresponding to the induction of the fluid secretion, the oxygen consumption increased and reached the highest value at 22-37 min (127.1 ± 12.9 mmHg, n = 5 glands, significantly high vs 69.5 ± 14.6 mmHg during CCh stimulation, P = 0.00017, by t-test), which calculated as 166.7% ± 7.9% of the control stimulation with CCh among the experimental series shown in Figure 4, followed by a gradual decrease.
Figure 4.
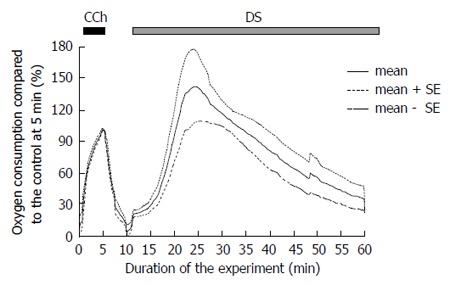
Time course of the A-V difference of partial oxygen pressure during the Danshen perfusion. CCh stimulation was conducted for 5 min. as a control measure. At 10 min., DS was added and perfused for 30 min. This figure shows the average values (bold line, n = 5 glands) and the standard error of the means (mean ± SE, dotted lines). DS: Danshen; CCh: Carbamylcholine.
Ouabain decreased fluid secretion and oxygen consumption
Ouabain, an inhibitor of Na+/K+ ATPase, was applied to assess the activity of the primary active transport during DS stimulation. The fluid secretion was measured by 1 mmol/L ouabain during stimulation with 5 g/L DS (Figure 5A). The fluid secretion decreased to a plateau level of 14.4 ± 4.0 μL/g-min, n = 7 glands, significantly from the highest fluid secretion, 99.2 ± 8.5 μL/g-min, P = 1.075 × 10-6 by t-test. Ouabain decreased the DS-induced fluid secretion by 85% of the highest value. After the removal of ouabain, the fluid secretion showed a slight recovery, and thereafter gradually decreased to reach the zero level.
Figure 5.
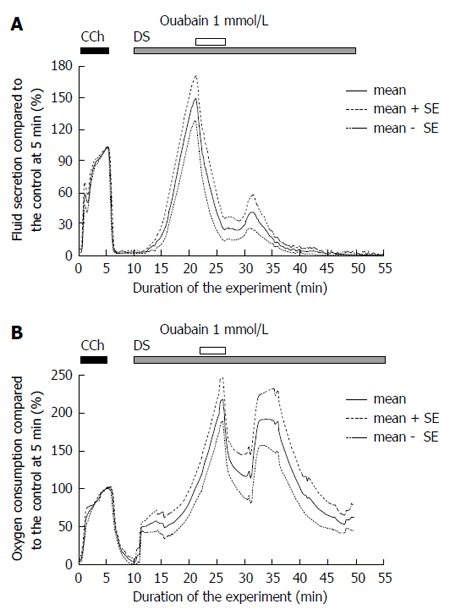
Inactivation of Na+/K+ ATPase by ouabain. A: Danshen-induced salivary fluid secretion. CCh stimulation was conducted for 5 min as control measure. At 20 min ouabain was added for 5 min, and the DS-induced fluid secretion decreased significantly (P < 0.05 vs control). The figure shows the average (bold line, n = 5) and the standard error of the means (mean ± SE, dotted lines); B: Oxygen consumption during DS stimulation. At 25 min ouabain was added for 5 min, and the oxygen consumption decreased significantly (P < 0.05 vs control). This figure shows the average values (bold line, n = 5) and the standard error of the means (mean ± SE, dotted lines). DS: Danshen; CCh: Carbamylcholine.
The addition of ouabain decreased the oxygen consumption to 112.5 ± 27.8 mmHg, n = 8 glands, from the highest level of oxygen consumption, 197.4 ± 26.7 mmHg (n = 13 glands), P = 0.04996 by t-test. After the removal of the ouabain, the oxygen consumption partially recovered, and then gradually decreased (Figure 5B).
Bumetanide decreased fluid secretion and oxygen consumption
Bumetanide was used to block the NKCC1 cotransporter. Bumetanide (10 μmol/L) was administered at 10 min from the start of the DS administration, and then removed at 15 min. Bumetanide significantly decreased the DS-induced fluid secretion, from the pre-blocked level, 25.8 ± 5.5 μL/g-min at 20-21 min (n = 7 glands) to a plateau level, 6.6 ± 2.5 μL/g-min at 25-26 min (n = 7 glands), P = 0.0078 by t-test (Figure 6A). After the removal of the bumetanide, the salivary fluid secretion increased again to another peak, 37.8 ± 7.0 μL/g-min at 30-35 min (n = 7 glands). Thereafter the fluid secretion gradually decreased to the zero level. The decreased level of fluid secretion, 1.97 ± 1.29 μL/g-min at 45-60 min (n = 7 glands), was significantly lower than the recovered peak of the DS-induced salivary fluid secretion, P = 0.0003 by t-test. The residual fluid secretion during inhibition by bumetanide was 6.6 μL/g-min at 25-26 min from the start of the experiment. The inhibition of salivary fluid secretion by bumetanide was calculated as 93% of the maximum secretion (99 μL/g-min) induced by DS. This finding indicates that NKCC1 is activated during DS-induced fluid secretion.
Figure 6.
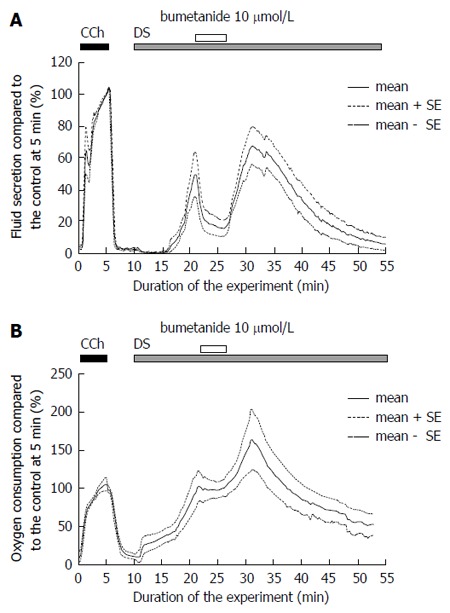
Inactivation of NKCC1 by bumetanide. A: The effects of bumetanide on Danshen-induced salivary fluid secretion. Initially, CCh was added for 5 min as a control measure. At 20 min bumetanide was added for 5 min, and the fluid secretion decreased significantly to a plateau inhibited level (P < 0.05 vs control). This figure shows the average values (bold line, n = 6) and the standard error of the means (mean ± SE, dotted lines); B. The effects of bumetanide on DS-induced oxygen consumption. The oxygen consumption was shown by arterio-venous difference of partial oxygen pressure (mmHg). Initially, CCh was added for 5 min as a control measure. At 20 min bumetanide was added for 5 min. DS: Danshen; CCh: Carbamylcholine.
The increase in oxygen consumption by DS was stopped by bumetanide and stayed at the plateau level of 98.1% ± 2.8% at 22-25 min (Figure 6B). After removal of the bumetanide, the oxygen consumption increased again to the peak.
Comparison of the inhibition between ouabain and bumetanide
Ouabain inhibition of Na+/K+ ATPase decreases both the ATP hydrolysis and the driving force of Na+ entry and K+ release. As a result, the oxygen consumption decreases, the Cl- entry via the cotransporter and antiporters are decreased, and then fluid secretion will be reduced. On the other hand, bumetanide blocks only NKCC1. DS stimulation induced a salivary fluid secretion, accumulating to 1428.0 ± 152.8 μL/g for 60 min. The maximal fluid secretion due to DS (5 g/L) was 3 times larger than that of CCh (0.2 μmol/L). Bumetanide (10 μmol/L) decreased the fluid secretion rate to 16.8% ± 5.5% of the CCh control (n = 7 glands). Because the highest DS fluid secretion was 309 ± 45% of the CCh control, bumetanide inhibited the highest fluid secretion by 95% of the highest DS-induced fluid secretion. In contrast, ouabain decreased the fluid secretion rate to 23.9% ± 10.8% of the CCh control (n = 5 glands), which means that ouabain inhibited the fluid secretion by 90% of the highest DS-induced fluid secretion. The inhibition by bumetanide was larger than that due to ouabain as shown in Figure 7.
Figure 7.
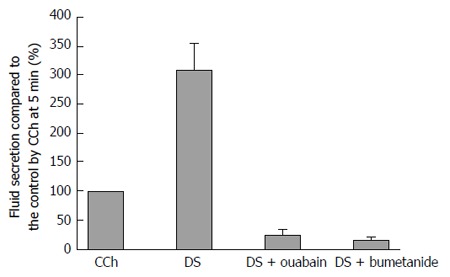
The residual Danshen-induced fluid secretion during inhibition due to ouabain or bumetanide. This figure shows the standard error of the means (mean ± SE) of the salivary fluid secretion. Ouabain (inhibitor of Na+/K+ ATPase) and bumetanide (inhibitor of NKCC1) decreased the fluid secretion due to the DS (n = 9). The DS column indicates the maximum response of the fluid secretion due to DS. DS: Danshen; CCh: Carbamylcholine.
The oxygen consumption was measured as an arterio-venous difference in the partial oxygen pressure (AV difference in PO2, mmHg). Although this value includes the oxygen that escaped from the gland surface and the perfusion line, the AV PO2 difference was normalized (%) by the value at 5 min during CCh stimulation. The AV difference in PO2 was 69.5 ± 14.6 mmHg (n = 5 glands) at 5 min from the start of the CCh stimulation. DS increased the AV PO2 difference to the highest value, 197.4 ± 26.7 mmHg of the CCh control (mean ± SEM, n = 13 glands), at 22-37 min from the start of the experiment, which was an average (284% of the CCh control) from 13 glands and higher than one group of experiments shown in Figure 4. Ouabain (1 mmol/L) was added after about 25 min for 5 min, as shown in Figure 5B. Ouabain decreased the oxygen consumption to 100.0% ± 17.3% of the CCh control at 29-31min, n = 6 glands. Bumetanide (10 μmol/L) stopped the increase in oxygen consumption at 98.1% ± 2.8% (at 22-25 min) of the CCh control. Therefore, as a minimal estimation, ouabain inhibited the oxygen consumption by 49% and bumetanide inhibited it by 66%.
Participation of muscarinic or alpha1 adrenergic receptors in DS-induced fluid secretion
Figure 8 shows the highest secretion rate during perfusion with DS, DS with atropine, and DS with phentolamine, which were shown as the % of the fluid secretion rate due to CCh at 5 min. When atropine (1 μmol/L) or phentolamine (5 μmol/L) was applied separately on the DS perfusion, the salivary fluid secretion decreased slightly, but not significantly, to 263% ± 63% (n = 7) and 227% ± 63% (n = 14), respectively (DS induced secretion at 309 ± 45%, n = 9). The latency and the time course of the fluid secretion remained the same. The inhibition ratios of atropine and phentolamine were 12% and 20%, respectively. The P values obtained by ANOVA were 0.899 between control and atropine, and 0.626 between control and phentolamine, respectively, indicating no statistical differences.
Figure 8.
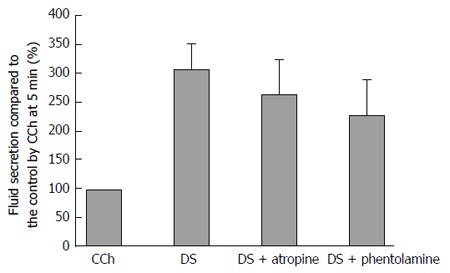
Effects of atropine and phentolamine on the Danshen-induced fluid secretion. The standard error of the means (mean ± SE) of the highest fluid secretion was shown in the DS only group (n = 9), the DS with atropine (n = 7), and the DS with phentolamine (n = 14). n shows the number of the glands employed. The values were expressed as percentage of the fluid secretion rate at 5 min with CCh as control. There was no significant differences (P > 0.5 vs control) among the 3 groups. DS: Danshen; CCh: Carbamylcholine.
Requirement of extracellular Ca2+ for DS-induced fluid secretion
In regard to the physiological stimulation necessary to induce salivary fluid secretion, an increase in the cytosolic Ca2+ level ([Ca2+]i) is necessary for the activation of K+ channels on the basolateral membrane and the Cl- channel on the luminal membrane. After the initial release of Ca2+ from cytosolic stores, the level of [Ca2+]i is maintained by the balance of entry and extrusion of cytosolic Ca2+. In order to examine the Ca2+ entry during DS stimulation, the Ca2+ in the perfusate was removed nominally without any chelating agent. During Ca2+ free perfusion, CCh (0.2 μmol/L) was perfused from 5 min to 10 min, followed by perfusion with DS (25 g/L) for 15 min. DS and CCh could hardly induce any fluid secretion. During the DS perfusion, the highest fluid secretion rate was only 1.8 ± 0.8 μL/g-min at 8.65 min (n = 8 glands) (while during perfusion with DS and calcium the fluid secretory rate was 101.9 ± 17.2 μL/g-min at 8.65 min, n = 6, P = 0.00023 by t-test). These results indicated that extracellular Ca2+ was essential for DS to induce salivary fluid secretion.
DISCUSSION
The application of traditionally used Chinese herbs in clinical medicine has been increasing all around the world, especially for chronic diseases. However, the information available on the mechanisms of their pharmaceutical action is limited. Collaborative experiments conducted by the Nanjing Medical College (China) and the National Institute for Physiological Sciences (Japan) screened the Chinese herbs that promote salivary fluid secretion in isolated perfused rat salivary glands[6]. During that collaborative work, the methods required for screening effective Chinese herbs were developed. As a result, it was discovered that Danshen (DS) induced salivary fluid secretion without other added stimulants. The present work was planned to clarify the mechanism by which DS induces salivary fluid secretion. During this study, a number of different methodologies required to pursue the mechanisms involved in salivary secretion were combined. This set of methodologies will be useful for future studies in the search for new drugs with unknown mechanisms for salivary fluid secretion.
Danshen
DS is obtained from the dried root of Salvia miltiorrhiza, a plant native to China and Japan. In 1934, Nakao and Fukushima[13] first isolated the tanshinones from DS. According to the traditional usage, DS is reduced into a water decoction, which contains more hydrophilic components. Therefore, the phenolic acids from DS have been extracted since the 1980s, and were called the salvianolic acids[14-16]. In the present study, we used Ringer’s solution to dissolve DS, so most phenolic acids and a small amount of tanshinone were dissolved in our DS solution. The plant, Salvia miltiorrhiza, was briefly mentioned in Mabberley’s Plant-Book[17], with a short comment on its use locally for heart conditions. However, DS is famous as a “blood-activating” drug in the field of traditional Chinese medicine (TCM). In TCM, the term “blood-activating” means a treatment of the symptoms caused by the reduction of fluid secretion, such as dry mouth, dry eyes and so on[18,19]. While the TCM reports have not revealed any mechanism for production of saliva, it is well known in the field of physiology that salivary fluid secretion is mainly induced by activation of the muscarinic receptors on salivary acinar cells. Acetylcholine (ACh) is released from parasympathetic nerve endings and binds with this receptor, then links with the elevation of cytosolic Ca2+. The elevation of cytosolic Ca2+ activates the Cl- channel to release Cl- into the lumen. The mechanisms by which DS induces salivary fluid secretion may be hidden within this sequential mechanism.
In a previous study[6], during the screening of Chinese herbs, we used a moderate concentration of CCh, 0.2 μmol/L. Because the concentration 1 μmol/L is a supermaximal concentration for salivary fluid secretion, the moderate concentration was suitable to examine if fluid secretion was promoted or not. In addition, we normalized the values for fluid secretion and oxygen consumption to avoid variations among individual glands. This measure was implemented because the responses to Chinese herbs could vary among the individual rats and the surgical procedures employed were not yet fully developed by the young investigators involved in the study. That collaborative study showed that DS promotes salivary fluid secretion, compared with CCh, and could be a promising drug in treatment for the relief of dry mouth caused by hypofunction of the salivary glands. However, the characteristics and possible mechanisms associated with the sole use of DS have not been studied.
DS-induced salivary fluid secretion
Sole DS stimulation induced fluid secretion in the isolated and perfused SMG of the rat. However, the time course of the secretion induced by DS was different from the CCh-induced fluid secretion. DS started fluid secretion with a time period latency and the secretion gradually increased to reach the highest value, which was 2.5 times higher than the fluid secretion at 5 min due to CCh. This type of high fluid secretion due to DS has not been reported previously. However, the fluid secretion slowly declined from the highest value to the zero level around 60 min from the start of the DS administration. These characteristics suggested that the mechanism for DS-induced salivary fluid secretion was different from that induced by CCh or ACh. CCh and ACh activate the M3 receptor to release IP3 and quickly release Ca2+ from IP3-regulated calcium-stores[20]. The process includes channel activation and also quick osmosis, resulting in a quick onset of fluid secretion. Fluid secretion can be quickly started by activation of the α1 adrenergic receptor[21,22] and neurokinin A receptor[23]. Therefore DS-induced secretion could use a different signalling process, compared with IP3-store Ca2+ release signalling.
The clinical dosage of DS ranges from 10 to 50 g/person because the treatment recipe is usually a mixture of several herbs, and the proportion of DS varies depending on the individual symptoms. For experimental convenience, we adopted an average dose of 25 g/person for the experiment. Assuming that all the DS will move to the blood circulation (5 L for 60 kg body weight), the concentration of DS in the blood will be 5 g/L. We took 5 g/L as a standard concentration of DS in the perfusion fluid. On the other hand, the relationship between DS dose and fluid secretion was examined using a series of doses at 1, 3, 5, 25, and 50 g/L. Fluid secretion increased with the higher doses of DS, while the latency was shorter at the higher doses of DS. The results of the dose of 5 g/L were slightly higher than the ED50. These results suggested that we can control clinically the level of fluid secretion between 5 g/L and 25 g/L, which was also within the safe therapeutic dose. At doses higher than 25 g/L, the effect of the DS would not improve and side effects may appear, such as bleeding. Therefore, the administration of DS requires rigorous guidance and clinical observation. These results may be of some help for studies on the clinical applicability of DS.
The latency decreased as the dose of the DS was increased. This feature was apparently different from the instant reaction shown when the salivary fluid secretion was stimulated by CCh through muscarinic receptors. Our previous study[6] showed that CCh rapidly stimulated salivary fluid secretion by the SMGs through the activation of muscarinic receptors. When perfused with DS, it took a long time to induce salivary fluid secretion, and there was no initial peak effect. However, when CCh was added to the DS perfusion at an early time, a marked superimposed peak in the salivary fluid secretion was shown. The salivary fluid secretion by the SMGs induced by DS decreased gradually after reaching the highest secretion (88.7 ± 12.8 μL/g-min, 309% ± 45% of the CCh control, at 21.5 min), until the secretion stopped. Although continually perfused with DS, the gland did not secrete further after the secretion stopped. However, after washing with buffer solution, salivary fluid secretion was induced again when stimulated by DS. These phenomena indicated that other mechanisms may be involved in the DS-related promotion of salivary fluid secretion, which were also different from that of the muscarinic and α1 receptors.
In summary, at doses over 25 g/L, the effect of DS does not improve and there is a risk of side effects, such as bleeding. Therefore, the administration of DS requires rigorous guidance and clinical observation.
Inhibition of Na+/K+ ATPase during DS stimulation
The increase in oxygen consumption reflects the increased energy metabolism during fluid secretion[24,25]. Because the increment of the oxygen consumption becomes the same as the increase in heat production, this suggests that the energy metabolism is mostly from oxidative phosphorylation in mitochondria. In addition, the increment of the oxygen consumption and the K+ uptake during the post-stimulatory activation of Na+/K+ ATPase were compared, and the results showed that the increase in oxygen consumption during fluid secretion is mostly from the activation of Na+/K+ ATPase[12]. However, the protein synthesis and its secretion contributed less to the increase in the oxygen consumption during the combined stimulation of CCh and isoproterenol (β-adrenergic stimulant)[26]. Finally, we managed to estimate the activation of Na+/K+ ATPase from the decrement of oxygen consumption during the application of ouabain.
Oxygen consumption during latency during DS stimulation
The oxygen consumption of the gland immediately increased after the administration of DS, even though there was no fluid secretion. This indicated that some energy consuming processes were activated by DS. Because the dilation of the capillary bed and thus the promotion of microcirculation occurred simultaneously, the energy metabolism of the uncirculated region was possibly added due to the shunt closure. The fluid secretion started several minutes later, so these processes probably did not include fluid secretion. The promotion of microcirculation could be one of these processes. Another possibility is the activation of the synthesis of secretory proteins. However, protein secretion was not measured in this study.
Oxygen consumption during DS-induced secretion
The time courses of oxygen consumption and salivary fluid secretion were similar during DS stimulation, showing a slow increase and gradual decline. This suggests a close relationship between fluid secretion and the activation of Na+/K+ ATPase. Ouabain (g-strophanthin) is a blocker of Na+/K+ ATPase. Na+/K+ ATPase is located on the basolateral membrane of the salivary acinar cell. According to the mostly accepted model for salivary fluid secretion mechanism[27], cytosolic K+ is continuously released across the basolateral membrane through Ca2+-activated K+ channels. The driving force for K+ release is the electrochemical potential of K+, which is established by Na+/K+ ATPase. Na+/K+ ATPase pumps K+ in the cell and Na+ is pumped out. During the hydrolysis of one ATP, Na+/K+ ATPase extrudes 3 Na+ ions for the uptake of 2 K+ ions, which produces a negative membrane potential. Therefore the enzyme also maintains a Na+ electrochemical potential for Na+ entry, which drives the Na+/K+/2Cl- cotransporter for Cl- uptake. The addition of ouabain blocked Na+/K+ ATPase, while the DS-induced fluid secretion significantly decreased to a plateau level. This decrease in salivary fluid secretion recovered with the removal of ouabain.
In the present experiment, the oxygen consumption due to DS stimulation followed the time course of the fluid secretion. The highest level of oxygen consumption due to DS stimulation increased significantly, compared with that due to CCh. Importantly, ouabain suppressed the fluid secretion by 90%. These findings suggest that DS-induced fluid secretion is maintained by activation of Na+/K+ ATPase and that the increased energy metabolism is mostly supplied for the DS-induced fluid secretion.
Inhibition of Na+/K+/2Cl- cotransporter during the DS stimulation
It has been widely accepted that the Na+/K+/2Cl- cotransporter uptakes Cl- from the basolateral side against the Cl- electrochemical potential, making Cl- the driving force for Cl- release through the luminal Cl- channel (TMEM16A). Bumetanide inhibits the activity of the Na+/K+/2Cl- cotransporter. Bumetanide, at 100 μmol/L, abolished the fluid secretion of the rat SMGs by ACh (1 μmol/L) during perfusion without bicarbonate (Murakami, 1997, unpublished). However, bumetanide decreased the fluid secretion by 33% of the sustained fluid secretion during perfusion with bicarbonate (Murakami, 1997, unpublished). In both cases, the oxygen consumption remained at 70% of the control during stimulation (Murakami, 1997, unpublished). These findings indicate that the Na+/H+ antiporter was not inhibited by bumetanide, and that the Cl-/bicarbonate antiporter can uptake Cl- during perfusion with bicarbonate, but not without bicarbonate. In the present study, DS-induced salivary fluid secretion was decreased to 7% of the highest value by bumetanide (10 μmol/L) during bicarbonate-free perfusion. This indicated that the Na+/K+/2Cl- cotransporter was almost fully activated during the DS stimulation.
Ouabain inhibits the activation of Na+/K+ ATPase. In rodents, including the rat, the susceptibility to ouabain is lower than it is in other animals. Thus, a concentration of 1 mmol/L is not able to completely inhibit the fluid secretion, the oxygen consumption, or the K+ uptake during secretory stimulation[12]. Ouabain decreased the fluid secretion to 26% of control at 1 mmol/L of ouabain and 10% at 10 mmol/L during ACh stimulation at 1 μmol/L. The difference in the inhibition of the fluid secretion by ouabain and bumetanide could be due to the incomplete inhibition by 1 mmol/L ouabain, although the concentration of bumetanide (10 μmol/L) was lower than it was in the previous experiment (100 μmol/L, unpublished).
In the present experiment, we used bicarbonate-free perfusion. The intracellular HCO3- could be less than 1 mmol/L ICF and the Cl- uptake by the Cl-/HCO3- exchanger may have a very minor contribution to the total Cl- uptake from ECF. Thus, NKCC1 plays a major role for Cl- uptake from ECF. The salivary fluid secretion in the NKCC1 knockout mouse dropped over 60% even during perfusion with HCO3-[28]. The oxygen consumption remained at 51% of the control during ouabain inhibition, and at 34% of the control during bumetanide inhibition. This similarity in the inhibition suggests that the remaining oxygen consumption could include the lesser activity of the compensation of the Na+/H+ exchanger. Further studies will be required to clarify the issue, including perfusion with bicarbonate and measurements of the cytosolic pH.
Can DS stimulate muscarinic or α1 adrenergic receptors?
This is the most important question, related to whether DS is a stimulator of the muscarinic or α1-adrenergic receptor, similar to the present therapeutics for xerostomia. The superior salivary nucleus at the medulla oblongata sends the neuron to the SMG for stimulation of the fluid secretion. The M3 muscarinic receptors play a main role in the initiation and maintenance of fluid secretion at the secretory end-piece cells (i.e. acinar cells). The accepted present terminology allows for acinar cells in rat SMGs, but not in human SMGs (secretory end-piece cells are allowed in both). The α1-adrenergic receptor also plays a role in the production of salivary fluid. In addition, substance P and VIP are also physiologically active substances related to salivary fluid secretion. Acetylcholine, nor-adrenaline and substance P bind with the M3 muscarinic receptors, the α1-adrenoreceptors and the substance P receptors, and produce IP3. IP3 binds with the IP3 receptors of the Ca2+ stores, which releases Ca2+ and increases cytosolic Ca2+ levels. Then, the Ca2+ activates K+ and Cl- release from the cell and the water movement starts through the aquaporins. The possible contribution of the muscarinic and α1-adrenergic receptors was denied by the results of the present experiments using atropine or phentolamine, which are potent inhibitors for both receptors. These results suggest that DS does not bind with either the M3 muscarinic or the α1-adrenergic receptors. Therefore we consider that DS is a promising secretagogue which could avoid the systemic side effects induced by the recent muscarinic drugs, such as cevimeline, pilocarpine and so on.
Does DS stimulation require extracellular Ca2+?
Extracellular Ca2+ entry plays a role in the maintenance of salivary fluid secretion in CCh-induced salivary fluid secretion[29]. Cytosolic Ca2+ is the most important signal that activates both the luminal Cl- and basolateral K+ channels, which thus evokes a salivary fluid secretion. The Ca2+ release from stores increases the cytosol Ca2+ level quickly, while it also quickly empties the cytosolic store, which sends a signal for Ca2+ entry from outside of the cell, Capacitative theory[30]. Thus the cytosolic Ca2+ level is maintained to support continuous fluid secretion.
Under calcium-free perfusion, DS stimulation did not induce any salivary fluid secretion, either with or without a Ca2+ chelating agent. This result suggested that Ca2+ entry and a low level of [Ca2+]i increase are essential for DS to induce salivary fluid secretion. Ca2+ entry probably provided a slow but continuous increase in cytosol calcium level, which provides support for DS to induce fluid secretion. The secretory characteristics, the slow increase and then the slow decrease, suggested that there is probably a change in [Ca2+]i during the time course of the DS-induced salivary fluid secretion.
There are many possible mechanisms that may induce salivary fluid secretion, such as aquaporin (AQP5 in the salivary gland[31]). However, no report has been published on the relationship between fluid secretion and the incidence of AQP5. One report indicated that the salivary fluid secretion decreased in AQP5 KO mice[32]. Other studies have confirmed that salivary secretion is also affected by the regulation of other neurotransmitter receptors, such as the vasoactive intestinal polypeptide receptor[33], the tachykinin receptor[34], the purine receptor[35], the vanilloid receptor[36], and the cannabinoid receptor[37]. The issue of whether or not these newly discovered receptors are involved in the role of DS-induced salivary fluid secretion requires further experiments. Further studies are also necessary to elucidate the mechanisms involved, including screening of the membrane calcium channels such as a TRPC1 and Orai1 for store-operated Ca2+ entry (SOCE[38-40]). TRPC4 and TRPV6 are also considered to be candidates for the molecular components of SOCE channels[41,42].
In addition, in the future, the experimental plan employed in this study could be used in order to assess the capability of newly discovered drugs and classical Chinese herbs to promote fluid secretion.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Takanori Narita (Nihon Univ. Sch. Biosci. Res.) and Wei Ding (Nanjing Medical Univ.), for their scientific discussions. We must also thank the Institute of Integrated Traditional and Western Medicine (Nanjing Medical Univ.), for kindly supplying the DS used in our research.
COMMENTS
Background
Xerostomia (dry mouth) is caused by salivary hypofunction (reduction in salivary fluid secretion). Among the 20 Chinese herbs (CHs) currently used in the treatment of xerostomia, Danshen induced salivary fluid secretion. The aim of the present study was to clarify the mechanisms involved in Danshen-induced salivary fluid secretion and assess the possible use of Danshen in the treatment of xerostomia without side effects.
Research frontiers
The present therapeutic procedures for xerostomia are limited and include the supplemental use of artificial saliva and the use of stimulants. Assuring a frequent supply of artificial saliva is difficult during speech, and muscarinic drugs used as stimulants cause systemic side effects due to parasympathetic activities. The mechanism by which Danshen induces salivary fluid secretion has not been studied previously.
Innovations and breakthroughs
The oral intake of decocted Chinese herbs is common in traditional Chinese medicine, but the relationship between dose and salivary fluid secretion has not been clarified. Using isolated perfused glands, the authors clarified that quantitative relationship. That evidence-based relationship makes it possible to estimate fluid secretion from the applied dose.
Applications
The present study presents a set of experimental procedures that can be employed to clarify the unknown mechanisms for other new drugs, including CHs to induce salivary secretion. This information could possibly provide a theoretical guide for choosing herbs to relieve xerostomia in TCM practice.
Terminology
Xerostomia is a subjective feeling of dry mouth and mainly caused by salivary gland hypofunction (SGH). SGH is a condition in which non-stimulated or stimulated salivary flow is significantly reduced due to many reasons. Danshen (DS) is a representative Chinese herb in the category of the agents that promote blood circulation and eliminate stasis. DS is mainly used for coronary atherosclerotic heart disease. The submandibular gland is one of three major salivary glands, along with the parotid gland and the sublingual gland. The submandibular gland secretes 65% of the whole non-stimulated saliva, and the parotid gland secretes 50% of the whole stimulated saliva.
Peer-review
The manuscript by Wei F et al describes the studies on the mechanism by which the Chinese herb Danshen induces salivary fluid secretion in the perfused rat submandibular gland. The series of experiments was systemically planned to examine the mechanism, which is applicable for other CHs and unknown drugs to modify the salivary fluid secretion. The results proposed the possibility of Danshen for therapeutics to induce salivary fluid secretion without systemic side effects due to parasympathetic stimulation.
Footnotes
Supported by Grants from The National Institute for Physiological Sciences and Graduate University for Advanced Studies.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 24, 2014
First decision: September 15, 2014
Article in press: November 19, 2014
P- Reviewer: Maja T, Meyers BM S- Editor: Yu J L- Editor: O’Neill M E- Editor: Wang CH
References
- 1.Sreebny LM. Dry mouth: a common world wide tormentor. Dry mouth, the malevolent symptom. In: Sreebny LM, Vissink A, editors. USA: Wiley & Sons; 2010. pp. 3–9. [Google Scholar]
- 2.Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49:309–326. doi: 10.1016/j.cden.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134:61–69; quiz 118-119. doi: 10.14219/jada.archive.2003.0018. [DOI] [PubMed] [Google Scholar]
- 4.Ge L, Lin M. Physiological studies of saliva and the evaluation of artificial saliva. J Clinical Stomatol. 2008;24:181–184. [Google Scholar]
- 5.Joraku A, Sullivan CA, Yoo JJ, Atala A. Tissue engineering of functional salivary gland tissue. Laryngoscope. 2005;115:244–248. doi: 10.1097/01.mlg.0000154726.77915.cc. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M, Wei MX, Ding W, Zhang QD. Effects of Chinese herbs on salivary fluid secretion by isolated and perfused rat submandibular glands. World J Gastroenterol. 2009;15:3908–3915. doi: 10.3748/wjg.15.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu HJ. Research on pharmacological function of red sage. J Zhejiang Univ TCM. 2008;32:694–695. [Google Scholar]
- 8.Du GH, Zhang JT. The general situation and progress of the modern research of red sage root (Radix Salviae miltiorrhizae) Herald Med. 2004;23:355–360. [Google Scholar]
- 9.Jiang ZX, Jiang LY, Qiu JX, Qian Y, Luo QP. Clinical investigation of traditional Chinese medicine treatment of xerostomia after head and neck cancer postradiotherapy. Acta Acad Med Jiangxi. 2005;45:25–30. [Google Scholar]
- 10.Murakami M, Hashimoto S, Wei M, Hill AE. Morpho-physiological approach to the paracellular route for salivary secretion by isolated perfused submandibular gland. J Med Invest. 2009;56 Suppl:322–324. doi: 10.2152/jmi.56.322. [DOI] [PubMed] [Google Scholar]
- 11.Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl- channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285:12990–13001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Miyamoto S, Imai Y. Oxygen consumption for K+ uptake during post-stimulatory activation of Na+, K(+)-ATPase in perfused rat mandibular gland. J Physiol. 1990;426:127–143. doi: 10.1113/jphysiol.1990.sp018130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakao M, Fukushima T. On the chemical composition of Salvia Miltiorrhiza (Chinese drug Tan-shen) J Pharm Soc Jpn. 1934;54:844–858. [Google Scholar]
- 14.Li LN, Tan R, Chen WM. Salvianolic acid A, a new depside from root of Salvia miltiorrhiza. Planta Med. 1984;50:227. doi: 10.1055/s-2007-969684. [DOI] [PubMed] [Google Scholar]
- 15.Ai CB, Li LN. Sterostructure of Salvianolic acid B and isolation of Salvianolic acid C. J Nat Prod. 1988;51:145. [Google Scholar]
- 16.Ai CB, Li LN. Salvianolic Acids D and E: Two New Depsides from Salvia miltiorrhiza. Planta Med. 1992;58:197–199. doi: 10.1055/s-2006-961428. [DOI] [PubMed] [Google Scholar]
- 17.Mabberley DJ. Mabberley’s Plant-Book. 3rd ed. London, UK: Cambridge Univ Press; 2008. p. 763. [Google Scholar]
- 18.Jiang YP, Liu H. The understanding of prescription of Sjogren’s syndrome. Study J Trad Chinese Med. 2002;20:654. [Google Scholar]
- 19.Zhang YM. Efficacy comparison of Xueshuantong capsules and Danshen tablets in treating older women with dry eyes. Chinese J Mod Drug Appl. 2013;7:49–50. [Google Scholar]
- 20.Menniti FS, Bird GS, Takemura H, Thastrup O, Potter BV, Putney JW. Mobilization of calcium by inositol trisphosphates from permeabilized rat parotid acinar cells. Evidence for translocation of calcium from uptake to release sites within the inositol 1,4,5-trisphosphate- and thapsigargin-sensitive calcium pool. J Biol Chem. 1991;266:13646–13653. [PubMed] [Google Scholar]
- 21.Martinez JR, Quissell DO, Wood DL, Giles M. Abnormal secretory response to parasympathomimetic and sympathomimetic stimulations from the submaxillary gland of rats treated with reserpine. J Pharmacol Exp Ther. 1975;194:384–395. [PubMed] [Google Scholar]
- 22.Bockman CS, Bruchas MR, Zeng W, O’Connell KA, Abel PW, Scofield MA, Dowd FJ. Submandibular gland acinar cells express multiple alpha1-adrenoceptor subtypes. J Pharmacol Exp Ther. 2004;311:364–372. doi: 10.1124/jpet.104.066399. [DOI] [PubMed] [Google Scholar]
- 23.Qi B, Narita T, Satoh K, Guo MY, Katsumata-Kato O, Murakami M, Fujita-Yoshigaki J, Sugiya H. Characteristics of neurokinin A-induced salivary fluid secretion in perfused rat submandibular gland. Arch Oral Biol. 2010;55:737–744. doi: 10.1016/j.archoralbio.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M. Measurement of heat production in dog submandibular gland. Jpn J Physiol. 1979;29:491–507. doi: 10.2170/jjphysiol.29.491. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M. [Heat production, blood flow, O2 uptake and CO2 output in the secretary process of the dog submandibular gland (author’s transl)] Nihon Seirigaku Zasshi. 1981;43:135–147. [PubMed] [Google Scholar]
- 26.Murakami M, Yoshimura K, Segawa A, Loffredo F, Riva A. Relationship between amylase and fluid secretion in the isolated perfused whole parotid gland of the rat. Eur J Morphol. 2000;38:243–247. doi: 10.1076/0924-3860(200010)38:4;1-o;ft243. [DOI] [PubMed] [Google Scholar]
- 27.Catalán MA, Nakamoto T, Melvin JE. The salivary gland fluid secretion mechanism. J Med Invest. 2009;56 Suppl:192–196. doi: 10.2152/jmi.56.192. [DOI] [PubMed] [Google Scholar]
- 28.Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J Biol Chem. 2000;275:26720–26726. doi: 10.1074/jbc.M003753200. [DOI] [PubMed] [Google Scholar]
- 29.Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 30.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Murakami M, Murdiastuti K, Hosoi K, Hill AE. AQP and the control of fluid transport in a salivary gland. J Membr Biol. 2006;210:91–103. doi: 10.1007/s00232-005-0848-2. [DOI] [PubMed] [Google Scholar]
- 32.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich CD, Holtmann M, Miller LJ. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology. 1998;114:382–397. doi: 10.1016/s0016-5085(98)70491-3. [DOI] [PubMed] [Google Scholar]
- 34.Sugiya H, Obie JF, Putney JW. Two modes of regulation of the phospholipase C-linked substance-P receptor in rat parotid acinar cells. Biochem J. 1988;253:459–466. doi: 10.1042/bj2530459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallacher DV. Are there purinergic receptors on parotid acinar cells? Nature. 1982;296:83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- 36.Dunér-Engström M, Fredholm BB, Larsson O, Lundberg JM, Saria A. Autonomic mechanisms underlying capsaicin induced oral sensations and salivation in man. J Physiol. 1986;373:87–96. doi: 10.1113/jphysiol.1986.sp016036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busch L, Sterin-Borda L, Borda E. Expression and biological effects of CB1 cannabinoid receptor in rat parotid gland. Biochem Pharmacol. 2004;68:1767–1774. doi: 10.1016/j.bcp.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 38.Putney JW. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 39.Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 42.Schindl R, Kahr H, Graz I, Groschner K, Romanin C. Store depletion-activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2-aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca(2+) release-activated Ca(2+) channel) channels. J Biol Chem. 2002;277:26950–26958. doi: 10.1074/jbc.M203700200. [DOI] [PubMed] [Google Scholar]


