Abstract
AIM: To compare the clinical outcomes of uncovered and covered self-expandable metal stent placements in patients with malignant duodenal obstruction.
METHODS: A total of 67 patients were retrospectively enrolled from January 2003 to June 2013. All patients had symptomatic obstruction characterized by nausea, vomiting, reduced oral intake, and weight loss. The exclusion criteria included asymptomatic duodenal obstruction, perforation or peritonitis, concomitant small bowel obstruction, or duodenal obstruction caused by benign strictures. The technical and clinical success rate, complication rate, and stent patency were compared according to the placement of uncovered (n = 38) or covered (n = 29) stents.
RESULTS: The technical and clinical success rates did not differ between the uncovered and covered stent groups (100% vs 96.6% and 89.5% vs 82.8%). There were no differences in the overall complication rates between the uncovered and covered stent groups (31.6% vs 41.4%). However, stent migration occurred more frequently with covered than uncovered stents [20.7% (6/29) vs 0% (0/38), P < 0.05]. Moreover, the overall cumulative median duration of stent patency was longer in uncovered than in covered stents [251 d (95%CI: 149.8 d-352.2 d) vs 139 d (95%CI: 45.5 d-232.5 d), P < 0.05 by log-rank test] The overall cumulative median survival period was not different between the uncovered stent (70 d) and covered stent groups (60 d).
CONCLUSION: Uncovered stents may be preferable in malignant duodenal obstruction because of their greater resistance to stent migration and longer stent patency than covered stents.
Keywords: Duodenal obstruction, Stents, Palliative treatment, Gastric outlet obstruction, Neoplasms
Core tip: Malignant duodenal obstruction is a terminal event in patients with pancreatic, hepatobiliary, duodenal, and metastatic cancer. In these patients, maintenance of oral food intake is crucial because it is essential to their quality of life. However, comparison of clinical outcomes between uncovered and covered stent placements have been not well evaluated in malignant duodenal obstruction. Our results show that uncovered stents may be preferable in patients with malignant duodenal obstruction because of their greater resistance to stent migration and longer overall duration of stent patency than those of covered stents.
INTRODUCTION
Malignant duodenal obstruction is a late complication of pancreatic, hepatobiliary, duodenal, and metastatic cancer, which is usually diagnosed at an advanced stage when curative resection is impossible[1,2]. The median survival rate of patients with unresectable malignant duodenal obstruction varies widely and can be up to 6-12 mo[3]. Malignant duodenal obstruction leads to nausea, vomiting, and cachexia, resulting in progressive deterioration of the patient’s quality of life[4,5]. The main goal of treatment in these patients is palliation of obstructive symptoms, thereby improving the quality of life. Although palliative surgical procedures have traditionally been performed, they are associated with a high rate of morbidity and mortality due to advanced disease and poor general condition[6-8]. Self-expandable metal stent (SEMS) placement is associated with a higher clinical success rate, shorter delay of oral intake following the procedure, lower incidence of delayed gastric emptying, and shorter hospital stay than palliative surgery[9,10].
Although SEMS insertion has an excellent technical and clinical success rate for relieving gastric outlet obstruction symptoms, recurrent obstruction of the uncovered SEMS due to progressive tumor ingrowth through the wire mesh of the stent is a significant problem[11-16]. To overcome the increased rate of recurrent obstruction associated with uncovered stents, a covered stent has recently been used in the palliative treatment of malignant gastric outlet obstruction[17,18]. However, the use of covered stents has been plagued by stent migration[4,19]. Moreover, previous studies have focused on the clinical outcomes of stent placement in patients with peripyloric obstruction caused by gastric cancer. However, a limited number of clinical studies have compared the clinical outcomes between uncovered and covered metal stents in patients with malignant duodenal obstruction. Therefore, the present study aimed to compare the technical success rate, clinical success rate, complication rate, stent patency, and survival between uncovered and covered stents.
MATERIALS AND METHODS
Patients
We retrospectively reviewed our hospital’s database to identify all patients with malignant duodenal obstruction who underwent endoscopic SEMS placement at Seoul National University Boramae Hospital between January 2003 and June 2013. All the patients had symptomatic obstruction characterized by nausea, vomiting, reduced oral intake, and weight loss. In patients with suspected biliary obstruction-based on liver function tests and computed tomography (CT) scan-endoscopic biliary stenting using a metallic stent was always attempted prior to duodenal stenting.
All patients underwent CT to determine the extent of the tumor and to evaluate the site, degree, and length of the obstructive lesion. The histopathological diagnosis was confirmed by endoscopic biopsy that was performed before or during stent placement.
The inclusion criteria were acute malignant gastric outlet obstruction, diagnosed based on the clinical obstructive symptoms and a radiologic examination. The exclusion criteria included asymptomatic patients with duodenal obstruction, evidence of perforation or peritonitis, concomitant small bowel obstruction, or duodenal obstruction caused by benign strictures.
The present study was performed in accordance with the guidelines of our institutional review board, which approved this study.
Duodenal SEMS placement
The stent length was selected by allowing for an additional 2-4 cm to be exposed distally and proximally to the obstructive lesion. The 3 stent types used in our study were as follows: (1) the Niti-S D-type pyloric/duodenal stent (uncovered SEMS; Taewoong Medical, Seoul, Korea); (2) the Niti-S Head-type pyloric/duodenal stent (covered SEMS; Taewoong Medical); and (3) the newly developed Niti-S Comvi-type pyloric/duodenal stent (covered SEMS; Taewoong Medical). The uncovered Niti-S D-type stent was made from a mesh of a single strand of nitinol wire formed into a cylindrical shape without flared ends. The covered Niti-S Head-type stent had a silicone covering with uncovered flared ends. The covered Niti-S Comvi pyloric/duodenal stent had a polytetrafluoroethylene (PTFE) covering with uncovered ends. The inserted stents had diameters of 18 mm, 20 mm, 22 mm, and 24 mm and lengths of 60 mm, 80 mm, 100 mm, and 120 mm.
Two expert endoscopists (Jeong JB, Kim JW) inserted all the stents using an endoscopic approach. For the endoscopic stent placement, the endoscope (GIF-2T240 and CF-260, Olympus, Co., Tokyo, Japan) was carefully inserted into the lesion site. One or two clippings were subsequently performed > 2 cm proximal to the lesion. A 0.035-inch guide wire (Trace metro, Cook, United States) was passed across the obstruction and into the distal part of the obstruction. After a 5F biliary catheter was passed through the guide wire across the obstruction, the length of the obstructive lesion was measured by the injection of water-soluble contrast dye (Gastrograffin) through a 5F biliary catheter. A stent delivery system was advanced over the guide wire through the working channel of the endoscope and was inserted in the obstruction by fluoroscopic guidance. The stent was deployed at the stricture site while pulling back the outer sheath, under combined fluoroscopic and endoscopic guidance.
After stent placement, plain radiographs of the abdomen was obtained at 24 h, 48 h, and 72 h, and we evaluated the position of the stent and the relief of the gastric outlet obstruction.
Outcomes
Technical success was defined as successful stent placement across the obstructive lesion. Clinical success was defined as an increase based on the gastric outlet obstruction scoring system (GOOSS), with a score of > 1 point and/or reduction of poor oral intake or vomiting 1 wk after SEMS placement. GOOSS assigns a point score depending on the patient’s level of oral intake (none, 0; liquids only, 1; soft solids, 2; low-residue or full diet, 3)[20]. Complications after stent insertion were defined as adverse events that induced the recurrence of obstructive symptoms, such as stent occlusion, stent migration, perforation, or biliary obstruction. Stent patency was defined by the period between the initial stent placement and loss of stent function as a result of the complications.
Follow-up and stent patency duration
In patients with palliative stent placement, clinical follow-up or telephone interviews regarding the recurrence of obstructive symptoms were conducted at an interval of 1-3 mo.
The duration of stent patency was defined as the time from stent placement to stent-related complications. When no stent-related complication occurred, the duration of stent patency was considered equal to the survival duration.
Statistical analysis
Patients were divided into 2 groups according to stent type (uncovered and covered). Differences between the groups were analyzed using the Mann-Whitney U test for continuous variables and the χ2 or Fisher exact test for categorical variables. Wilcoxon signed-rank tests were used to assess improvements in the GOOSS scores. Overall stent patency and patient survival were estimated using the Kaplan-Meier method and compared using the log-rank test. The putative prognostic factors for stent patency were analyzed by the multivariate Cox proportional hazard model with forward stepwise selection. The following potential prognostic factors were included in this model: age, sex, patient performance status, underlying malignancy, stage of malignancy, obstruction site, stent type, length and diameter of stent, chemotherapy before and after stent placement, and biliary drainage before stent placement. The Factors with substantial impacts (P < 0.2) in the univariate analysis were subsequently evaluated with multivariate analysis. All statistical analyses were conducted using the IBM SPSS Statistics software, version 20 (IBM Corp., United States). A value of P < 0.05 was considered significant.
RESULTS
Patient characteristics
SEMS placement was conducted in 67 patients with malignant duodenal obstruction. Twenty-nine of the patients were male. Our patients were aged 68.7 ± 10.5 years (mean ± SD; range, 37 years-93 years). The causes of malignant obstruction were pancreatic cancer (46.3%), bile duct cancer (17.9%), gallbladder cancer (13.4%), ampulla of Vater cancer (7.5%), duodenal cancer (6%), others cancer including colon, bladder, and cervical cancer (6%), and hepatocellular carcinoma (3%). The median duration from tumor diagnosis to duodenal intervention was 116 d (range, 1 d-2930 d).
The baseline characteristics did not differ significantly between the 2 groups (Table 1). Of the 67 patients, 38 patients (56.7%) received uncovered SEMS, whereas 28 (41.8%) received covered SEMS, with the exception of one case of technical failure (n = 1). Three patients in the uncovered stent group required 2 overlapping stents because of a long stricture at the time of diagnosis. Before the duodenal procedure, a considerable number of patients [29 (76.3%) and 15 (51.7%) in the uncovered and covered stent groups, respectively] had undergone biliary drainage (percutaneous transhepatic biliary drainage, endoscopic retrograde biliary drainage, and surgery). Sixteen (23.9%) of the 67 patients received various palliative chemotherapeutic agents after metal stent placement. Twelve patients with advanced pancreatic cancer were administered the single agent gemcitabine (n = 2) or gemcitabine-based combination chemotherapy (n = 12). Two patients with gallbladder cancer were treated with a combination of gemcitabine and cisplatin. One patient with intrahepatic cholangiocarcinoma was managed with a combination of TS-1 and cisplatin. One patient with hilar cholangiocarcinoma was managed with a combination of gemcitabine and cisplatin.
Table 1.
Baseline characteristics
|
Stent type |
P value | ||
| Uncovered (n = 38) | Covered (n = 29) | ||
| Age (yr) | 68.9 ± 10.2 | 68.5 ± 11.2 | 0.860 |
| Sex (M/F) | 18/20 (47.4/52.6) | 11/18 (37.9/62.1) | 0.440 |
| ECOG PS (0/1/2/3/4) | 0/9/16/9/4 | 0/5/10/8/6 | 0.2031 |
| Previous treatment | |||
| Operation | 6 (9.0) | 5 (7.5) | 1.000 |
| Chemotherapy | 10 (14.9) | 5 (7.5) | 0.377 |
| Previous biliary drainage | |||
| PTBD | 5 (13.2) | 7 (24.1) | 0.246 |
| ERBD | 19 (50.0) | 8 (27.6) | 0.064 |
| PTBD and ERBD | 5 (13.2) | 0 (0.0) | 0.064 |
| Underlying malignancy | 0.4231 | ||
| Pancreatic cancer | 22 (32.8) | 9 (13.4) | |
| Bile duct cancer | 4 (6.0) | 8 (11.9) | |
| Gallbladder cancer | 4 (6.0) | 5 (7.5) | |
| Ampullary cancer | 3 (4.5) | 2 (3.0) | |
| Duodenal cancer | 1 (1.5) | 3 (4.5) | |
| Hepatocellular carcinoma | 1 (1.5) | 1 (1.5) | |
| Others | 3 (4.5) | 1 (1.5) | |
| Cancer stage | 0.3351 | ||
| I | 1 (2.6) | 1 (3.4) | |
| II | 3 (7.9) | 5 (17.2) | |
| III | 2 (5.3) | 1 (3.4) | |
| IV | 32 (84.2) | 22 (75.9) | |
| Stent diameter (mm) | 19.8 ± 0.55 | 19.4 ± 0.92 | 0.699 |
| Stent length (mm) | 99.5 ± 17.1 | 101.4 ± 14.3 | 0.688 |
| Stent number | 0.256 | ||
| 1 stent | 35 (53.0) | 28 (42.4) | |
| 2 stents | 3 (4.5) | 0 (0.0) | |
| Median time interval to intervention from | 96.5 (3-803) | 118.0 (1-2930) | 0.699 |
| Initial diagnosis (d) | |||
| Chemotherapy after procedure | 11 (28.9) | 5 (17.2) | 0.265 |
| Median duration of follow-up (d) | 71 (8-592) | 60 (9-827) | 0.411 |
Linear by linear association. Values are presented as n (%), mean ± SD, or d (range). ECOG PS: Eastern cooperative oncology group performance status; PTBD: Percutaneous biliary drainage; ERBD: Endoscopic retrograde biliary drainage.
Technical/clinical success rates according to stent type
The technical success rate was not different between the uncovered and covered stents (100% vs 97%; Table 2). Of the 29 patients who received uncovered stents, one patient experienced technical failure consisting of an inability to pass the guidewire through the obstruction of the third portion of the duodenum caused by pancreatic cancer. This patient ultimately underwent covered stent reintervention 3 d after the first intervention.
Table 2.
Main outcomes and complications
|
Stent type |
P value | ||
| Uncovered (n = 38) | Covered (n = 29) | ||
| Technical success | 38 (100) | 28 (96.6) | 0.433 |
| Clinical success | 34 (89.5) | 24 (82.8) | 0.485 |
| GOOSS score | |||
| Pre-stenting (0/1/2/3) | 31/3/4/0 | 25/2/2/0 | 0.5841 |
| Post-stenting (0/1/2/3) | 4/10/18/61 | 5/11/10/32 | 0.1661 |
| Causes of clinical failure | 4 (10.5) | 4 (13.8) | 0.485 |
| Poor expansion | 2 (5.3) | 0 (0.0) | 0.502 |
| Migration | 0 (0.0) | 2 (6.9) | 0.184 |
| Perforation | 0 (0.0) | 1 (3.4) | 0.433 |
| Peritoneal seeding | 1 (2.7) | 0 (0.0) | 1.000 |
| Septic shock | 1 (2.7) | 1 (3.4) | 1.000 |
| Complications | 12 (31.6) | 12 (41.4) | 0.407 |
| Migration | 0 (0.0) | 6 (20.7) | 0.005 |
| Tumor ingrowth | 6 (15.8) | 1 (3.4) | 0.129 |
| Perforation | 0 (0.0) | 2 (6.9) | 0.184 |
| Biliary obstruction | 3 (7.9) | 0 (0.0) | 0.252 |
| Stent collapse | 0 (0.0) | 3 (10.3) | 0.076 |
| Poor expansion | 2 (5.3) | 0 (0.0) | 0.502 |
| Food impact | 1 (2.6) | 0 (0.0) | 1.000 |
| Retreatment rate | 12 (31.6) | 10 (34.5) | 0.802 |
| Stenting | 5 (13.2) | 9 (31.0) | 0.075 |
| Operation | 1 (2.6) | 1 (3.4) | 1.000 |
| Removal of food materials | 1 (2.6) | 0 (0.0) | 1.000 |
| Ballooning | 2 (5.3) | 0 (0.0) | 0.502 |
| PTBD | 3 (7.9) | 0 (0.0) | 0.252 |
| 30-d mortality | 6 (15.8) | 8 (27.6) | 0.239 |
| Median time interval to reintervention from initial intervention (d) | 60 (14-251) | 71 (2-147) | 0.573 |
Linear by linear association;
The GOOSS score improved significantly after stent placement than those before stent placement (P < 0.001, Wilcoxon signed-rank test). Values are presented as number (%) or d (range). GOOSS: Gastric outlet obstruction scoring system = no oral intake, 0; liquid only, 1; soft solids, 2; low-residue or full diet; PTBD: Percutaneous biliary drainage.
Stent placement was clinically successful in 34 (89.5%) of the 38 patients in the uncovered stent group (Table 2). The remaining 4 patients (10.5%) experienced clinical failure of the stent placement due to poor expansion (n = 2), additional obstruction (n = 1), and poor medical condition due to septic shock (n = 1). In contrast, in the covered stent group, stent placement was clinically successful in 24 (82.8%) of the 29 patients. The remaining 4 patients (13.8%) experienced clinical failure of stent placement due to stent migration (n = 2), perforation (n = 1), and septic shock (n = 1). The clinical success rate did not ultimately differ significantly between the uncovered and covered stent groups (89.5% vs 82.8%; Table 2). The GOOSS scores in the patients with technical success improved significantly compared to the scores before stent placement (mean, 1.55 vs 0.25, P < 0.001; Table 2).
Complications
During the follow-up period after stent insertion, 12 (31.6%) of the 38 patients in the uncovered stent group and 12 (41.4%) of the 29 patients in the covered stent group experienced various types of complications, including stent migration (n = 6), tumor ingrowth (n = 7), bowel perforation (n = 2), biliary obstruction (n = 3), stent collapse (n = 3), poor stent expansion (n = 2), and food impact (n = 1; Table 2). The overall complication rate did not differ significantly between the uncovered and covered stent groups (31.6% vs 41.4%; Table 2). Stent migration was more frequent in covered stents than in uncovered stents [20.7% (6/38) vs 0% (0/29); P = 0.005; Table 2], which was managed with restenting. Tumor ingrowth occurred in the uncovered (n = 6) and covered stent groups (n = 1) and was retreated with restenting (n = 5), surgical operation (n = 1), and no retreatment (n = 1). Perforation was found in 2 patients who underwent a covered stent placement, with one patient undergoing surgery and another patient refusing further treatment. Biliary obstruction developed only in the uncovered stent group without previous biliary drainage (n = 3), which was managed with percutaneous transhepatic biliary drainage (n = 3). Moreover, the retreatment rates for the uncovered and covered stents were not significantly different (31.6% vs 34.5%; Table 2).
Stent patency and survival
The median follow-up was 70 d (range, 8 d-827 d). The overall cumulative median duration of stent patency was significantly longer in the uncovered stent group (251 d; 95%CI: 149.8 d-352.2 d) than in the covered stent group (139 d; 95%CI: 45.5 d-232.5 d; P = 0.033; Figure 1). The overall cumulative median survival period was not different between the uncovered stent (70 d; 95%CI: 45.8 d-94.3 d) and covered stent groups (60 d; 95%CI: 32.3 d-87.7 d; P = 0.673).
Figure 1.
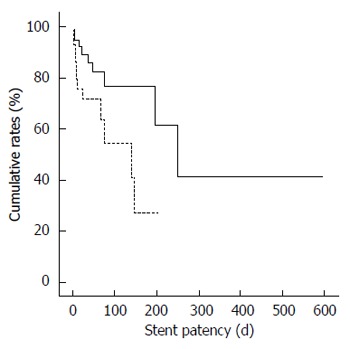
Overall stent patency between the uncovered (solid line) and covered stent groups (dotted line; P = 0.033).
The statistical analyses of the potential factors predisposing to stent patency are summarized in Table 3. Multivariate Cox regression analysis identified that the type of inserted stent (covered) was the only independent prognostic factor for stent patency (HR = 2.586; 95%CI: 1.046-6.388; P = 0.040).
Table 3.
Prognostic factors for stent patency
|
Univariate analysis |
Multivariate analysis |
|||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (> 70 yr) | 1.792 (0.731-4.391) | 0.202 | ||
| Sex (M) | 0.998 (0.418-2.384) | 0.996 | ||
| ECOG PS (> 2) | 1.436 (0.590-3.492) | 0.425 | ||
| Underlying malignancy (pancreatic cancer) | 0.512 (0.205-1.276) | 0.151 | ||
| Stage (> III) | 0.955 (0.345-2.648) | 0.930 | ||
| Obstruction site | 0.414 | |||
| Proximal of 2nd portion | 1.000 | |||
| 2nd portion | 0.552 (0.211-1.441) | 0.225 | ||
| Distal of 2nd portion | 0.486 (0.116-2.042) | 0.325 | ||
| Stent | ||||
| Covered | 2.586 (1.046-6.388) | 0.040 | 2.586 (1.046-6.388) | 0.040 |
| Length (> 100 mm) | 1.261 (0.480-3.307) | 0.638 | ||
| Diameter (> 18 mm) | 0.664 (0.221-1.997) | 0.466 | ||
| Chemotherapy after stent insertion | 0.425 (0.125-1.449) | 0.172 | ||
| Chemotherapy before stent insertion | 0.261 (0.059-1.158) | 0.077 | ||
| Biliary drainage before stent insertion | 0.799 (0.306-2.086) | 0.646 | ||
ECOG PS: Eastern cooperative oncology group performance status.
DISCUSSION
Endoscopic stent placement has been widely used for inoperable malignant gastric outlet obstruction as an alternative treatment to surgery because of the high technical and clinical success rate, and high efficacy[6,11,21-25]. However, most previous studies have included patients with gastric cancer or have not focused on the duodenal stent results[11,21]; thus, previously reported results may not be applicable to malignant duodenal obstruction because gastric cancer and pancreaticobiliary cancer have different mechanisms of gastric outlet obstruction. Gastric cancer is the primary cause of gastric outlet obstruction, with the obstruction site usually being the peripyloric area. In contrast, pancreaticobiliary cancer arises because of secondary involvement of the duodenum and is usually combined with biliary obstruction for anatomical reasons[26].
We found that metal stent placement in patients with malignant duodenal obstruction is technically feasible and clinically effective. Our results (technical and clinical success rates of 97% and 83%, respectively) were consistent with a previous systematic review of enteral stenting[21]. Although duodenal stent placement was technically feasible in most of our patients, endoscopic stent placement is technically more difficult in duodenal obstruction than in distal gastric obstruction, not only because of the loop formation by the endoscope in the distended stomach during the stent placement but also because of the curved configuration of the duodenal C-loop. In the present study, 11.9% (8/67) of patients experienced clinical failure, which is similar to the rate previously reported[27,28]. Interestingly, of the 8 patients who experienced clinical failure after stent placement, 2 patients could not tolerate oral intake because of their poor medical condition associated with septic shock after stenting. Similarly, patients’ performance status has been reported to affect their clinical improvement[28]. We propose that patient-related factors, such as general medical condition after stenting and performance status, should be considered potential factors for the clinical effectiveness of stenting.
The overall complication rate of 36% observed in this study is higher than that previously reported (17%-28%)[11,29]; the discrepancy may originate from differences in patient ages, clinical conditions, sample sizes, anatomical locations, operator experience, and the definitions used for the complications between the studies[21]. Furthermore, we found that the overall complication rate was not different between the patients who underwent uncovered and covered stent placement, which is in agreement with the results reported in a previous prospective study[30]. However, the previous study[30] has a limitation in that the study enrolled only patients with gastric cancer; thus, the results may not be applicable to the present study, which included only patients with malignant duodenal obstruction. Our findings indicate that the complication rates in patients with malignant duodenal obstruction may not differ according to the type of stent used, as demonstrated in gastric cancer cases[30].
In this study, we found that stent migration was more frequent in covered stents than in uncovered stents in patients with malignant duodenal obstruction, which is consistent with previous studies[27,31]. However, the earlier study by Waidmann et al[31] included a relatively small number of patients with uncovered stents (n = 16) and covered stents (n = 16). The other previous study[27] enrolled only patients with pancreatobiliary cancer. Although malignant duodenal obstruction is caused mainly by pancreatobiliary cancer, other malignancies (hepatocellular carcinoma, duodenal cancer, and metastasis from others cancer) may also cause malignant duodenal obstruction, as shown in our study. We postulate that the high rate of stent migration in the covered stents may be attributed to the imprecise approximation of the covering membrane of the stent to the duodenal wall, even when the stent was completely expanded. Given that the duodenal stents were placed in a severely angulated structure of the duodenal C-loop, they may have been subjected to high levels of stress, especially from the peristaltic movement in the area around the stent, which is more prone to stent migration.
Given that duodenal stent placement over the ampulla of Vater may be complicated by biliary obstruction, mandatory biliary decompression prior to stent placement in the second part of the duodenum has been recommended[32]. In contrast, Yoon et al[33] reported that biliary obstruction is a rare complication and that prophylactic biliary drainage is not required in patients who undergo covered stent placement. However, the stents in the present study were placed over the ampulla of Vater in 7 patients without previous biliary drainage. Jaundice occurred in 3 (42.9%) of the 7 patients during the follow-up period, which was associated with the compression of the inserted stent to the ampulla of Vater, as shown in Figure 2. Therefore, we suggest that when considering the endoscopic management of duodenal obstruction in patients without clinically overt biliary obstruction, prophylactic biliary drainage should be considered.
Figure 2.
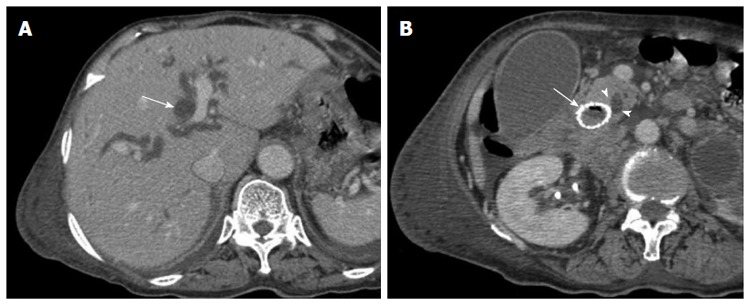
Images from an 81-year-old man with bladder cancer with multiple metastases. A: Computed tomographic (CT) scan obtained 18 d after stent placement showing significant dilatation of the intrahepatic bile duct (long arrow); B: CT scan obtained 18 d after stent placement at the level of the ampulla of Vater (AOV) showing significant dilatation of the common bile duct due to the compression of the inserted stent (long arrow) to the AOV (arrowheads).
We found that the overall stent patency was significantly longer in the patients who received uncovered stents than in those who received covered stents. Similarly, Woo et al[27] reported that stent patency tended to be shorter in covered stents than in uncovered stents. We suggest that the placement of uncovered stents may be more useful in maintaining stent patency compared to covered stents in patients with malignant duodenal obstruction.
Previous studies[29,34] have shown that chemotherapy after stent placement could be independently associated with prolonged stent patency. However, the previous study[29] included a mixed group of patients with not only malignant duodenal obstruction but also peripyloric obstruction. In contrast, Cha et al[35] reported that palliative chemotherapy does not improve stent patency in patients with malignant gastric outlet obstruction. In the current study, the type of inserted stent (covered) was only associated with stent patency (HR = 2.586; 95%CI: 1.046-6.388; P = 0.040). These differences may originate from differences in chemotherapeutic agents, underlying malignancy, anatomical location, and type of inserted stent. Therefore, further prospective randomized trials with control of the aforementioned confounding factors are required to determine the potential factors associated with stent patency and to identify appropriate individual patient groups for the placement of duodenal metal stents.
Our study has several limitations. First, the study was retrospective and conducted at a single center, which could introduce bias. Selection bias in the selection of stent type (uncovered vs covered) may have been involved because the stents were selected based on the preference and experience of the physician. Second, the present study included a relatively small number of patients in the covered stent group compared with the uncovered stent group. Further large-scale randomized prospective studies are necessary to overcome these limitations.
The results of the present study suggest that uncovered stents may be preferable in patients with malignant duodenal obstruction because of their increased resistance to stent migration and longer stent patency compared to covered stents.
COMMENTS
Background
Malignant duodenal obstruction is a terminal and catastrophic complication of advanced cancers. Although the endoscopic placement of a self-expandable metal stent (SEMS) is safe and effective for palliative treatment in patients with gastric outlet obstruction, the clinical outcomes between uncovered and covered SEMS placements have not been well evaluated in malignant duodenal obstruction.
Research frontiers
Most previous studies comparing clinical outcomes between uncovered and covered stent placement have enrolled either patients with malignant gastric outlet obstruction, mainly due to gastric cancer, or patients with malignant duodenal obstruction only as a part of their studies. The authors focused on patients with malignant duodenal obstruction.
Innovations and breakthroughs
A unique finding was that uncovered stents may be more resistant to stent migration and provide longer stent patency than covered stents.
Applications
The authors suggest that uncovered stents may be preferred when SEMS placement is performed in patients with malignant duodenal obstruction.
Terminology
SEMSs are made of nitinol alloy with polytetrafluoroethylene and have a silicone covering or no covering.
Peer-review
This is a very interesting comparative observational study on the clinical outcomes of stent placement (uncovered vs covered) in patients with malignant duodenal obstruction. From the results of this study, practitioners can identify that uncovered stents may be preferable over covered stents.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 24, 2014
First decision: August 15, 2014
Article in press: October 21, 2014
P- Reviewer: Nakase H, Osawa S, Sakata N S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron TH. Management of simultaneous biliary and duodenal obstruction: the endoscopic perspective. Gut Liver. 2010;4 Suppl 1:S50–S56. doi: 10.5009/gnl.2010.4.S1.S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung GS, Song HY, Kang SG, Huh JD, Park SJ, Koo JY, Cho YD. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758–763. doi: 10.1148/radiology.216.3.r00au05758. [DOI] [PubMed] [Google Scholar]
- 5.Bessoud B, de Baere T, Denys A, Kuoch V, Ducreux M, Precetti S, Roche A, Menu Y. Malignant gastroduodenal obstruction: palliation with self-expanding metallic stents. J Vasc Interv Radiol. 2005;16:247–253. doi: 10.1097/01.RVI.0000145227.90754.76. [DOI] [PubMed] [Google Scholar]
- 6.van Hooft JE, Uitdehaag MJ, Bruno MJ, Timmer R, Siersema PD, Dijkgraaf MG, Fockens P. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059–1066. doi: 10.1016/j.gie.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Song HY, Shin JH, Hu HT, Lee SK, Jung HY, Yook JH. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma. AJR Am J Roentgenol. 2009;193:241–247. doi: 10.2214/AJR.08.1760. [DOI] [PubMed] [Google Scholar]
- 8.McLoughlin MT, Byrne MF. Endoscopic stenting: where are we now and where can we go? World J Gastroenterol. 2008;14:3798–3803. doi: 10.3748/wjg.14.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Piano M, Ballarè M, Montino F, Todesco A, Orsello M, Magnani C, Garello E. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005;61:421–426. doi: 10.1016/s0016-5107(04)02757-9. [DOI] [PubMed] [Google Scholar]
- 10.Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283–290. doi: 10.1007/s00535-006-2003-y. [DOI] [PubMed] [Google Scholar]
- 11.Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543–550. doi: 10.1055/s-2004-814434. [DOI] [PubMed] [Google Scholar]
- 12.Kim GH, Kang DH, Lee DH, Heo J, Song GA, Cho M, Yang US. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004;39:1010–1014. doi: 10.1080/00365520410003146. [DOI] [PubMed] [Google Scholar]
- 13.Soetikno RM, Lichtenstein DR, Vandervoort J, Wong RC, Roston AD, Slivka A, Montes H, Carr-Locke DL. Palliation of malignant gastric outlet obstruction using an endoscopically placed Wallstent. Gastrointest Endosc. 1998;47:267–270. doi: 10.1016/s0016-5107(98)70325-6. [DOI] [PubMed] [Google Scholar]
- 14.Solt J, Moizs M, Orovica A, Gárdos A, Battyányi I, Bogneár B. Postoperative ischemic jejunal stenosis treated with balloon catheter dilation and Wallstent implantation. Endoscopy. 1997;29:409–412. doi: 10.1055/s-2007-1004225. [DOI] [PubMed] [Google Scholar]
- 15.Yates MR, Morgan DE, Baron TH. Palliation of malignant gastric and small intestinal strictures with self-expandable metal stents. Endoscopy. 1998;30:266–272. doi: 10.1055/s-2007-1001253. [DOI] [PubMed] [Google Scholar]
- 16.Mauro MA, Koehler RE, Baron TH. Advances in gastrointestinal intervention: the treatment of gastroduodenal and colorectal obstructions with metallic stents. Radiology. 2000;215:659–669. doi: 10.1148/radiology.215.3.r00jn30659. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Yoo BM, Lee KJ, Hahm KB, Cho SW, Park JJ, Kim SS, Park HC, Kim JH. Self-expanding coil stent with a long delivery system for palliation of unresectable malignant gastric outlet obstruction: a prospective study. Endoscopy. 2001;33:838–842. doi: 10.1055/s-2001-17340. [DOI] [PubMed] [Google Scholar]
- 18.Kozarek RA. Malignant gastric outlet obstruction: is stenting the standard? Endoscopy. 2001;33:876–877. doi: 10.1055/s-2001-17343. [DOI] [PubMed] [Google Scholar]
- 19.Park KB, Do YS, Kang WK, Choo SW, Han YH, Suh SW, Lee SJ, Park KS, Choo IW. Malignant obstruction of gastric outlet and duodenum: palliation with flexible covered metallic stents. Radiology. 2001;219:679–683. doi: 10.1148/radiology.219.3.r01jn21679. [DOI] [PubMed] [Google Scholar]
- 20.Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–78. doi: 10.1111/j.1572-0241.2002.05423.x. [DOI] [PubMed] [Google Scholar]
- 21.Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc. 2004;60:1010–1017. doi: 10.1016/s0016-5107(04)02276-x. [DOI] [PubMed] [Google Scholar]
- 23.Kaw M, Singh S, Gagneja H, Azad P. Role of self-expandable metal stents in the palliation of malignant duodenal obstruction. Surg Endosc. 2003;17:646–650. doi: 10.1007/s00464-002-8527-1. [DOI] [PubMed] [Google Scholar]
- 24.Masci E, Viale E, Mangiavillano B, Contin G, Lomazzi A, Buffoli F, Gatti M, Repaci G, Teruzzi V, Fasoli R, et al. Enteral self-expandable metal stent for malignant luminal obstruction of the upper and lower gastrointestinal tract: a prospective multicentric study. J Clin Gastroenterol. 2008;42:389–394. doi: 10.1097/MCG.0b013e318033d30a. [DOI] [PubMed] [Google Scholar]
- 25.Piesman M, Kozarek RA, Brandabur JJ, Pleskow DK, Chuttani R, Eysselein VE, Silverman WB, Vargo JJ, Waxman I, Catalano MF, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol. 2009;104:2404–2411. doi: 10.1038/ajg.2009.409. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Song HY, Shin JH, Hu HT, Lee SK, Jung HY, Yook JH. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma. AJR Am J Roentgenol. 2009;193:241–247. doi: 10.2214/AJR.08.1760. [DOI] [PubMed] [Google Scholar]
- 27.Woo SM, Kim DH, Lee WJ, Park KW, Park SJ, Han SS, Kim TH, Koh YH, Kim HB, Hong EK. Comparison of uncovered and covered stents for the treatment of malignant duodenal obstruction caused by pancreaticobiliary cancer. Surg Endosc. 2013;27:2031–2039. doi: 10.1007/s00464-012-2705-6. [DOI] [PubMed] [Google Scholar]
- 28.Ahn HS, Hong SJ, Moon JH, Ko BM, Choi HJ, Han JP, Park JS, Kang MS, Cho JY, Lee JS, et al. Uncovered self-expandable metallic stent placement as a first-line palliative therapy in unresectable malignant duodenal obstruction. J Dig Dis. 2012;13:628–633. doi: 10.1111/j.1751-2980.2012.00644.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Song HY, Shin JH, Choi E, Kim TW, Jung HY, Lee GH, Lee SK, Kim MH, Ryu MH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256–264. doi: 10.1016/j.gie.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Kim CG, Choi IJ, Lee JY, Cho SJ, Park SR, Lee JH, Ryu KW, Kim YW, Park YI. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010;72:25–32. doi: 10.1016/j.gie.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Waidmann O, Trojan J, Friedrich-Rust M, Sarrazin C, Bechstein WO, Ulrich F, Zeuzem S, Albert JG. SEMS vs cSEMS in duodenal and small bowel obstruction: high risk of migration in the covered stent group. World J Gastroenterol. 2013;19:6199–6206. doi: 10.3748/wjg.v19.i37.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681–1687. doi: 10.1056/NEJM200105313442206. [DOI] [PubMed] [Google Scholar]
- 33.Yoon CJ, Song HY, Shin JH, Bae JI, Jung GS, Kichikawa K, Lopera JE, Castaneda-Zuniga W. Malignant duodenal obstructions: palliative treatment using self-expandable nitinol stents. J Vasc Interv Radiol. 2006;17:319–326. doi: 10.1097/01.RVI.0000194872.42325.A6. [DOI] [PubMed] [Google Scholar]
- 34.Telford JJ, Carr-Locke DL, Baron TH, Tringali A, Parsons WG, Gabbrielli A, Costamagna G. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004;60:916–920. doi: 10.1016/s0016-5107(04)02228-x. [DOI] [PubMed] [Google Scholar]
- 35.Cha BH, Lee SH, Kim JE, Yoo JY, Park YS, Kim JW, Jeong SH, Kim N, Lee DH, Hwang JH. Endoscopic self-expandable metallic stent placement in malignant pyloric or duodenal obstruction: does chemotherapy affect stent patency? Asia Pac J Clin Oncol. 2013;9:162–168. doi: 10.1111/j.1743-7563.2012.01590.x. [DOI] [PubMed] [Google Scholar]


