Abstract
AIM: To assess the vitamin A status of patients with Crohn’s disease (CD) by evaluating serum retinol levels and the relative dose response (RDR) test (liver retinol stores).
METHODS: Vitamin A nutritional status was measured by serum retinol obtained by high performance liquid chromatography and the RDR test for evaluation of the hepatic stores. Body composition was performed by densitometry by dual-energy X-ray absorptiometry. Vitamin A dietary intake was assessed from a semi-quantitative food frequency questionnaire.
RESULTS: This study included 38 CD patients and 33 controls. Low serum retinol concentrations were detected in 29% of CD patients vs 15% in controls (P < 0.005). The RDR test was positive in 37% of CD patients vs 12% in controls, which indicated inadequate hepatic vitamin A stores (P < 0.005). Individuals with hypovitaminosis A had lower BMI and body fat compared with those without this deficiency. There was no association between vitamin A deficiency and its dietary intake, ileal location, presence of disease activity and prior bowel resections.
CONCLUSION: Patients with CD have higher prevalence of vitamin A deficiency, as assessed by two independent methods.
Keywords: Crohn’s disease, Vitamin A, Serum retinol, Relative dose response test, Body composition
Core tip: In this study, a higher prevalence of vitamin A deficiency was detected in Crohn’s disease (CD) patients compared with healthy controls by measuring serum retinol levels and the relative dose-response test. According to the relative dose-response test, almost 40% of the CD patients had inadequate hepatic vitamin A stores, which was three times the value found in healthy controls. CD Patients with hypovitaminosis A had lower BMI and body fat compared with those without this deficiency. There was no association between vitamin A deficiency and its dietary intake, ileal location, presence of disease activity and prior bowel resections.
INTRODUCTION
Crohn's disease (CD) is characterized by chronic and recurring intestinal inflammation, with periods of remission and activity[1]. Owing to long-term gut inflammation, CD patients can develop continuous increase in oxidative stress in the affected tissue[2,3]. As a result, the reactive oxygen species (ROS) originating from this process can be an important factor in the pathogenesis of the disease[4]. In ideal conditions, a complex of antioxidant substances, including vitamin A, inhibits the oxidative stress, limiting tissue damage[5]. In CD, however, an imbalance between the formation and the destruction of free radicals seems to be present[6]. This imbalance can be further aggravated by a low intake of micronutrients, which could reduce the antioxidant micronutrient concentration and increase the levels of lipid peroxidation[6].
In the past, important nutritional disorders, including protein-energy malnutrition, were a dominant feature of CD[7]. Although the calorie and macronutrient consumption is currently considered adequate in most CD patients in remission, previous studies demonstrated that the same does not occur for the intake of micronutrients, which tends to be reduced[8-10]. This deficiency in micronutrients with antioxidant function, even when subclinical, can facilitate lipid peroxidation, leading to an increase in oxidative stress, regardless of disease activity[11,12].
Vitamin A is one of these important micronutrients with antioxidant capabilities. This vitamin has a protective role against free radicals and oxidative stress, and participates in several primary functions of the human body[13-15]. In spite of the potential protective role of vitamin A in inflammatory states, very few studies have investigated whether a deficiency in this micronutrient is present in CD patients[5,8,11]. In all previous studies, the serum retinol level (SRL) was used as the only measurement to assess the nutritional status of vitamin A. However, the SRL might be a poor reflection of the vitamin A status: previous studies observed a normal SRL despite low concentrations of retinol in liver biopsies[16]. In this study, for the first time, we aimed to assess the vitamin A status in CD patients, taking into consideration not only the SRL, but also the relative dose response (RDR) test, an accurate, indirect indicator of the hepatic retinol stores[17].
MATERIALS AND METHODS
Study design and patient inclusion
This cross-sectional study included 38 individuals with CD and 33 healthy controls. Patients were recruited at the inflammatory bowel disease (IBD) outpatient clinic of the Federal University of Rio de Janeiro (UFRJ) Hospital (HUCFF), Brazil. Healthy controls were enrolled among university staff and medical students with no history of intestinal disorders and chronic or acute diseases. The Ethics Committee of the Institute of Public Health Studies/UFRJ approved this study. All patients and control subjects gave their written informed consent before enrolment. Data was analyzed anonymously to preserve the patient’s confidentiality.
Patient selection and data collection
All included patients fulfilled the following inclusion criteria: active follow up at the outpatient clinic and established diagnosis of CD by clinical, radiological, endoscopic and histological parameters[18].
The following exclusion criteria were considered for both patients and controls: use of supplementation of vitamin A, drugs that might interfere with the absorption of fat-soluble vitamins, a BMI > 40 kg/m2, autoimmune diseases, liver diseases, previous bowel resection with residual small intestine < 180 cm[19], restricted diet and recent hospitalization (less than 6 mo). In addition, pregnancy, nursing, active smokers, chronic consumption of alcoholic beverages and any intestinal disorder (in the controls) were also considered exclusion criteria. Only severe and symptomatic impairment of pancreatic and biliary function can cause fat-soluble vitamin deficiencies and no patient included had symptoms or a metabolic blood panel suggestive of these conditions.
Vitamin A status in CD patients and controls
Fasting serum retinol measurement: In patients and controls, quantification of SRL was determined by high performance liquid chromatography (HPLC), (Merck Hitachi LaChrom Elite Model equipped with Diode Array Detector-DAD) after a 12-h fast. The resulting SRLs were compared with the normal cut-off points proposed by the World Health Organization (WHO) and were presented in interval classes of 0.35 μmol/L[20]. Thus, vitamin A deficiency according to SRL was classified as severe (< 0.35 μmol/L), moderate (≥ 0.35 μmol/L, < 0.70 μmol/L) and mild (≥ 0.70 μmol/L, < 1.05 μmol/L)[20].
Vitamin A liver stores-the RDR test: The RDR test is a noninvasive, functional test that allows an indirect, accurate estimation of the total hepatic stores of vitamin A[21]. In all subjects, the RDR test was performed according to the following instructions. After a baseline blood collection (T0), 2500 IU of retinyl palmitate (UNICEF, Batch, SchemPTY. Co, Melbourne, Australia) was diluted in 1mL of oil solution and orally administered. All subjects then received a standard breakfast, with estimated total content of 16.2 g of lipids and 111.5 μg retinol activity equivalents (RAE) of vitamin A. After a 5-h interval with no food intake, a second blood collection was carried out. The therapeutic response was evaluated by assessing circulating values of serum retinol 5 h after vitamin A administration. RDR was calculated by the formula described by Loerch et al[22], and an RDR ≥ 20% was used as the cut-off point, above which is considered a positive RDR and an indirect indication of inadequate hepatic stores[23].
Assessment of variables associated with low SRL in CD patients
Clinical variables: Clinical and demographic data on CD characteristics (i.e., disease history, disease activity and previous surgeries) were collected from medical records. The Montreal classification was used to classify patients regarding disease behavior and location[24]. At enrollment, disease activity was assessed by the Harvey-Bradshaw index (HB)[25].
Dietary intake of vitamin A, nutritional status and other laboratory parameters: The dietary intake of vitamin A was calculated using a semi-quantitative food frequency method. The cut-off point for adequate dietary intake was 900 μg/d for men and 700 μg/d for women[26]. CD patients were also evaluated by anthropometric measurements, which included weight, height and body mass index (BMI), classified according to the cut-off points proposed by the WHO[27]. Densitometry by dual X-ray emission (DEXA) was conducted to allow body composition assessment based on the following parameters: body fat percentage, lean body mass, bone mineral content and fat mass. Average values of 9%-16% for men and 15%-22% for women were used to categorize body fat percentages[28]. In addition, C-reactive protein (CRP) and hemoglobin values were evaluated. CRP was measured by nephelometry, and values greater than 3.0 mg/L were used as the reference value for high CRP. The normal values of hemoglobin obtained by the colorimetric method were in the range of 14-18 g/dL for men and 12-16 g/dL for women.
Statistical analysis
Qualitative variables were described according to their absolute and relative frequencies. The quantitative variables of symmetric and asymmetric distribution were described by the mean and standard deviation, and the median was used to present the data concerning the intake of vitamin A. The t-student test and the Mann-Whitney U test were used for comparison of parametric and non-parametric variables, respectively. To analyze the association between the categorical variables, the χ2 test and Fisher's exact test were used. To analyze the correlation between the variables, the Pearson test was used. The significance level adopted was 5% and data analysis was performed with the statistical program SPSS (Statistical Package for Social Sciences) for Windows version 16.0.
RESULTS
Clinical and demographic characteristics
Of the 38 included CD patients, 53% (n = 20) were female, and the mean age was 39.2 ± 10.3 years. The mean disease duration was 3.76 ± 5.21 years. An overall summary of patients’ characteristics is presented in Table 1. In the control group, 33 healthy subjects were included, 58% were female and the mean age was 34.7 ± 13.05 years. There were no differences between the CD group and controls with respect to age and gender.
Table 1.
Clinical characteristics of the Crohn's disease population
| Parameters | n (%) | mean ± SD |
| Disease activity (n = 38) | ||
| In remission | 34 (89.4) | |
| Moderate activity | 4 (10.52) | |
| Severe activity | 0 (0) | |
| Disease Location (n = 35) | ||
| Ileum (L1) | 12 (34.3) | |
| Colon (L2) | 8 (22.9) | |
| Ileocolonic (L3) | 15 (42.9) | |
| Upper digestive tract (L4) | 0 (0) | |
| Phenotype (n = 31) | ||
| Non-stricturing and non-penetrating (B1) | 6 (19.4) | |
| Stricturing (B2) | 9 (29.0) | |
| Penetrating (B3) | 16 (51.6) | |
| Surgery (n = 38) | ||
| None | 18 (47.4) | |
| Small bowel resection | 5 (13.1) | |
| Colonic resection | 3 (7.9) | |
| Other procedures | 12 (31.6) | |
| Medical treatment (n = 38) | ||
| 5-ASA | 25 (65.8) | |
| Immunosuppressants | 28 (73.7) | |
| Corticosteroids | 3 (7.9) | |
| Anti-TNF therapy | 3 (7.9) | |
| Antibiotics | 1 (2.6) | |
| Body composition parameters (n = 34) | ||
| LBM (kg) | 42.01 ± 11.63 | |
| BMC (kg) | 2.39 ± 9.73 | |
| Body fat (kg) | 20.34 ± 0.51 | |
| Body fat (%) | 31.59 ± 10.89 |
LBM: Lean body mass; BMC: Bone mineral content.
Prevalence of vitamin A deficiency according to SRL and the RDR test
SRL with different cut-off categories and the results for the RDR test are shown in Table 2. Considering an SRL below 1.05 μmol/L, which is traditionally considered vitamin A deficiency, 29% in the CD group and 15% in the control group had hypovitaminosis A (P < 0.005). Notably, 24% of CD subjects had moderate to severe depletion compared with 6 % of the controls (P < 0.005). With respect to the hepatic retinol stores, 37% of the subjects in the CD group and 12% in the control group had decreased retinol stores in the liver, as suggested by a positive RDR test (P < 0.005).
Table 2.
Serum retinol levels and relative dose response test results with mean and standard deviation
| Serum retinol categories1 |
CD Patients |
Controls |
||
| n (%) | Serum retinol values | n (%) | Serum retinol values | |
| Normal levels (≥ 1.05 μmol/L) | 27 (71) | 2.69 ± 1.47 | 28 (85) | 2.63 ± 1.29 |
| Deficiency (< 1.05 μmol/L) | 11 (29) | 0.49 ± 0.26 | 5 (15) | 0.74 ± 0.32 |
| Mild deficiency (1.05-0.70 μmol/L) | 2 (5) | 1.00 ± 0.00 | 3 (9) | 0.96 ± 0.05 |
| Moderate deficiency (0.70-0.35 μmol/L) | 5 (13) | 0.46 ± 0.05 | 1 (3) | 0.50 ± 0.00 |
| Severe deficiency (< 0.35 μmol/L) | 4 (11) | 0.28 ± 0.02 | 1 (3) | 0.3 ± 0.00 |
| RDR test | ||||
| Positive (≥ 20%) | 14 (37) | 0.97 ± 0.87 | 4 (12) | 0.67 ± 0.33 |
| Negative (< 20%) | 24 (63) | 2.68 ± 1.59 | 29 (88) | 2.58 ± 1.30 |
WHO, 1996[29]. CD: Crohn's disease; RDR: Relative dose response.
The relationship between serum retinol and RDR test results in the patients can be seen in Table 3. Among subjects with normal SRL, five CD patients and no controls had a positive RDR test. Among individuals with low SRL, two CD patients and one control had a negative RDR test. In the CD group, the mean SRL according to vitamin A deficiency status (serum retinol) and RDR test results is shown in Figure 1. SRL gradually reduced from patients with adequate serum retinol with a positive RDR test to patients with serum hypovitaminosis A and a positive RDR test, compared with patients with normal results in both tests.
Table 3.
Relationship between normal and low serum retinol levels and negative and positive relative dose response test results in Crohn's disease patients and controls
| Serum retinol levels |
RDR test |
|||
|
Negative |
Positive |
|||
| CD | CT | CD | CT | |
| Normal | 22 | 28 | 5 | 0 |
| Low | 2 | 1 | 9 | 4 |
| Total | 24 | 29 | 14 | 4 |
CT: Controls; CD: Crohn's disease; RDR: Relative dose response.
Figure 1.
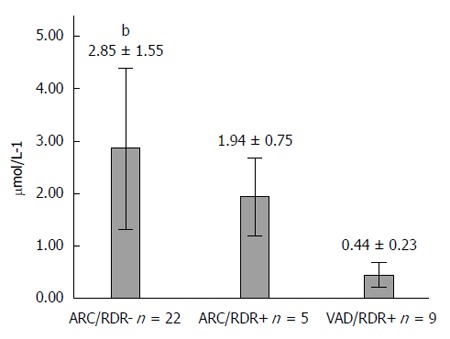
Fasting serum retinol concentration according to relative dose response test results. Values are mean ± standard deviation. ARC (adequate retinol concentration in serum), VAD (vitamin A deficiency in serum), RDR+ (positive relative dose response), RDR- (negative relative dose response test). bP < 0.001 (ARC/RDR- vs VAD/RDR+, one-way ANOVA test).
Assessment of factors associated with low SRL in CD patients
Dietary intake of vitamin A: In the food frequency questionnaire, 11 CD patients (29%) had inadequate intake of vitamin A. There was no clear association between poor consumption of vitamin A and low SRL, because only four patients had low results and inadequate scores in the food questionnaire. Overall, there was no statistically significant difference in the median vitamin A intake between patients with and without low SRL (Table 4). Notably, no difference was observed in the median vitamin A intake according to gender (women, n = 20, 1148.5 μg/d vs men, n = 17, 1112.4 μg/d) in the studied CD population.
Table 4.
Body composition parameters, vitamin A intake, disease duration and clinical characteristics of interest in Crohn's disease patients, according to serum retinol level
| Parameters | < 1.05 μmol/L (n = 11) | ≥ 1.05 μmol/L (n = 27) | P value |
| BMI (kg/m2) | 21.69 ± 2.74 | 25.65 ± 5.48 | 0.029 |
| Weight (kg) | 58.75 ± 9.46 | 68.96 + 15.59 | 0.051 |
| Body fat (%) | 26.13 ± 10.32 | 33.27 ± 11.68 | 0.131 |
| LM (kg) | 38.66 ± 8.35 | 43.04 ± 10.03 | 0.272 |
| BMC (kg) | 2.21 ± 0.55 | 2.44 ± 0.50 | 0.273 |
| Body fat (kg) | 13.22 ± 5.43 | 22.53 ± 11.28 | 0.032 |
| Vitamin A intake (μg/d) | 1125.7 | 1163.9 | 0.561 |
| Disease duration (yr) | 6.73 ± 7.89 | 2.41 ± 2.94 | 0.164 |
| Bowel resection | 7 (63) | 13 (48) | 0.386 |
| Ileal resection | 2 (18) | 3 (11) | 0.956 |
| Ileal involvement | 10 (90) | 20 (74) | 0.430 |
| Active disease (HB index) | 3 (27) | 1 (4) | 0.330 |
| High CRP levels | 2 (18) | 9 (33) | 0.430 |
| Low HB levels | 1 (9) | 5 (18) | 0.345 |
Data are represented as mean ± SD, median or n (%). BMI: Body mass index; LM: Lean body mass; BMC: Bone mineral content.
Nutritional status: According to BMI, overall, 49% (19/38) of the CD patients were eutrophic patients, 24% (9/38) were overweight, 16% (6/38) were obese and only 11% (4/38) were below the ideal level. The mean value of fat percentage was 31.59% (± 10.89%), being 31.6% (± 8.22%) among women and 22.7% (± 8.94%) among men. The body composition of patients evaluated by DEXA is shown in Table 1. The parameters of body composition according to the presence of low SRL can be seen in Table 4. Patients with decreased SRL showed significantly lower BMI and body fat (kg) than those without it (P = 0.029; P = 0.032, respectively).
Clinical characteristics: With respect to clinical characteristics, no association with low SRL was detected when ileal location, bowel resection, ileal resection and disease duration were evaluated (Table 4). In addition, no association between low SRL and anemia or high CRP levels was detected (Table 4).
DISCUSSION
With the current standard multidisciplinary care of IBD and the development of medical therapies that might change the natural history of the disease, the prevalence of malnutrition has markedly decreased[29]. Currently, many IBD patients face a very different nutritional scenario, where obesity and obesity-related diseases affect their quality of life and life expectancy[30]. Even though clinical malnutrition is becoming rarer among IBD outpatients, an important deficiency of micronutrients, including vitamin A, is still frequently found in this population[31-34]. Unfortunately, all studies evaluating the relationship between IBD and vitamin A deficiency used the dosage of fasting SRL as the single parameter to determine vitamin A depletion[5,8,11]. To the best of our knowledge, this is the first study to combine SRL with the more accurate RDR test to assess the status of vitamin A in CD patients.
In the present study, the prevalence of low SRL was 29% (15% in controls) and a positive RDR test was found in 37% (12% in controls), suggesting that vitamin A deficiency is still present in CD patients and might be higher compared with results solely based on retinol measurements in sera. In previous studies using SRL, the prevalence of vitamin A deficiency in CD patients ranged from 0%[35] to 21%[36] in adults, and 16% in children[37]. The dosage of fasting SRL underestimates the actual vitamin A deficiency[17,38-40]; therefore, these studies need to be interpreted carefully. In this regard, SRL can be kept quite constant by release of hepatic retinol until the global body stores are quite depleted, making the plasma retinol concentration a poor reflection of the global body vitamin A status[41].
Low body stores of vitamin A are detected reliably only when plasma retinol concentrations are lower than 100 μg/L[42]. To resolve these inaccuracies, the RDR test has been proposed as a way to calculate the total body store of retinol, based on the principle that the plasma retinol concentration is little affected by oral administration of vitamin A when the hepatic stores of retinol are high. When the liver reserves are low, however, the plasma retinol concentration increases markedly, reaching a peak in 5 h[42]. A positive RDR test in individuals with normal SRL indicates that their hepatic stores of retinol are affected, while still maintaining an adequate serum retinol concentration[42]. In our series, five CD patients had normal SRL with a positive RDR test, which indicated normal retinol levels in their sera, despite low retinol stores in the liver. This active balance between serum levels and the hepatic stores of retinol was also demonstrated by the finding that the mean SRL was lower in patients with a positive RDR test compared with subjects with a negative test. It is important, therefore, to evaluate the SRL along with the RDR test to achieve an accurate global diagnosis of hypovitaminosis A.
Importantly, hypovitaminosis A occurred in parallel with an adequate dietary consumption of this micronutrient, suggesting that the vitamin intake might not be the determining factor for the observed deficiency. In fact, in CD patients, the finding that the dietary intake of vitamin A does not reflect SRL is not novel. Imes et al[35] previously found normal SRL in a CD population in which one third of all subjects had low dietary vitamin A intake. In this regard, the severity of disease activity is a better predictor of low SRL than the nutritional status[37]. It is also important to note that there were no significant differences in serum retinol concentration in the presence of ileal involvement, even though the ileum is the main site of absorption of vitamin A[42]. This finding may be related to the patchy nature of CD, in which the inflammatory process affects non-continuous areas of the gut within areas of non-inflamed mucosa that are fully capable of absorbing micronutrients. In addition, the chronic inflammatory state associated with CD might represent an important cause of vitamin A deficiency, regardless of disease location, because of the elevated oxidative stress that is characteristic of this condition[2,3]. In this regard, one previous study showed that patients with active CD had significantly lower SRL and that these levels became significantly higher after resection of the inflamed area[5].
Given the predominant inclusion of inactive subjects and the limited number of patients when individuals were categorized into groups of normal or low SRL, it is important to stress that the lack of association between clinical characteristics and low SRL might reflect a lack of statistical power and these results should be interpreted carefully. In addition, owing to the small cohort of patients enrolled, further analysis of potential risk factors for vitamin A deficiency related to CD characteristics and treatment was not possible. Future studies should address whether there is a subgroup of CD patients at higher risk of developing vitamin A deficiency. In this specific, high risk population, screening for such deficiency with SRL measurements and the RDR test would be reasonable in clinical practice.
In conclusion, the nutritional profile of individuals with CD has undergone many changes because of major advances in the clinical management of the disease. Those changes have shifted the focus towards overweight and obesity, which is different from the characteristic protein energy malnutrition established in the past. Deficiencies in micronutrients such as vitamin A, however, are still prevalent in this specific population. Importantly, such deficiencies in antioxidant micronutrients can aggravate the imbalance between the formation and destruction of free radicals in the intestinal mucosa of these patients. The present results suggest that there is a deficit of vitamin A in CD patients, and that the exclusive use of SRL as a diagnostic tool might underreport its actual prevalence compared with the more accurate RDR test. In this series, low SRL were not related to vitamin A quantitative intake. More studies are necessary to explain the mechanisms behind the development of vitamin A deficiency in patients with IBD.
COMMENTS
Background
Vitamin A is an important micronutrient, with antioxidant capabilities. This vitamin has a protective role against free radicals and oxidative stress, and participates in several primary functions of the human body. In spite of the potential protective role of vitamin A in inflammatory states, very few studies have addressed whether a deficiency in this micronutrient is present in Crohn’s disease (CD) patients.
Research frontiers
A higher prevalence of vitamin A deficiency among CD patients is still to be established.
Innovations and breakthroughs
In this study, for the first time, the vitamin A statuses of CD patients were evaluated, taking into consideration not only their serum retinol levels, but also the relative dose response (RDR) test, an accurate, indirect indicator of hepatic retinol stores.
Applications
The present results suggest that there is a deficit of vitamin A in CD patients, and that the exclusive use of serum retinol levels as a diagnostic tool might underreport its actual prevalence compared with the more accurate relative dose-response test.
Terminology
The RDR test is a noninvasive functional test permitting an indirect, accurate estimation of the total hepatic stores of vitamin A, using measurements of serum retinol before and after oral administration of retinyl palmitate.
Peer-review
This is an interesting and useful study that accomplishes its stated goals.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 16, 2014
First decision: September 15, 2014
Article in press: January 23, 2015
P- Reviewer: Feuerstadt P, Imaeda H S- Editor: Qi Y L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 3.Pinto MA, Lopes MS, Bastos ST, Reigada CL, Dantas RF, Neto JC, Luna AS, Madi K, Nunes T, Zaltman C. Does active Crohn’s disease have decreased intestinal antioxidant capacity? J Crohns Colitis. 2013;7:e358–e366. doi: 10.1016/j.crohns.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 5.Sampietro GM, Cristaldi M, Cervato G, Maconi G, Danelli P, Cervellione R, Rovati M, Bianchi Porro G, Cestaro B, Taschieri AM. Oxidative stress, vitamin A and vitamin E behaviour in patients submitted to conservative surgery for complicated Crohn’s disease. Dig Liver Dis. 2002;34:696–701. doi: 10.1016/s1590-8658(02)80020-2. [DOI] [PubMed] [Google Scholar]
- 6.Aghdassi E, Wendland BE, Steinhart AH, Wolman SL, Jeejeebhoy K, Allard JP. Antioxidant vitamin supplementation in Crohn’s disease decreases oxidative stress. a randomized controlled trial. Am J Gastroenterol. 2003;98:348–353. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]
- 7.Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315–319. doi: 10.1016/j.ejim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. 2006;12:185–191. doi: 10.1097/01.MIB.0000206541.15963.c3. [DOI] [PubMed] [Google Scholar]
- 9.Aghdassi E, Wendland BE, Stapleton M, Raman M, Allard JP. Adequacy of nutritional intake in a Canadian population of patients with Crohn’s disease. J Am Diet Assoc. 2007;107:1575–1580. doi: 10.1016/j.jada.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311–319. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744–748. [PubMed] [Google Scholar]
- 12.Gassull MA. Review article: the role of nutrition in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:79–83. doi: 10.1111/j.1365-2036.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosca L, Rubenfire M, Mandel C, Rock C, Tarshis T, Tsai A, Pearson T. Antioxidant nutrient supplementation reduces the susceptibility of low density lipoprotein to oxidation in patients with coronary artery disease. J Am Coll Cardiol. 1997;30:392–399. doi: 10.1016/s0735-1097(97)00188-5. [DOI] [PubMed] [Google Scholar]
- 14.Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 1999;26:746–761. doi: 10.1016/s0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 15.Parslow RA, Sachdev P, Salonikas C, Lux O, Jorm AF, Naidoo D. Associations between plasma antioxidants and hypertension in a community-based sample of 415 Australians aged 60-64. J Hum Hypertens. 2005;19:219–226. doi: 10.1038/sj.jhh.1001809. [DOI] [PubMed] [Google Scholar]
- 16.Weinman AR, Jorge SM, Martins AR, de Assis Md, Martinez FE, Camelo JS. Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition. 2007;23:454–460. doi: 10.1016/j.nut.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Arantes Ferreira Peres W, Villaça Chaves G, Saraiva Gonçalves JC, Ramalho A, Moraes Coelho HS. Assessment of the relative dose-response test as indicators of hepatic vitamin A stores in various stages of chronic liver disease. Nutr Clin Pract. 2013;28:95–100. doi: 10.1177/0884533612455827. [DOI] [PubMed] [Google Scholar]
- 18.Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–1532. doi: 10.1053/j.gastro.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JS, Iyer KR, DiBaise JK, Young RL, Brown CR, Langnas AN. Short bowel syndrome and Crohn’s disease. J Gastrointest Surg. 2003;7:1069–1072. doi: 10.1016/j.gassur.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.DeMaeyer EM. The WHO programme of prevention and control of vitamin A deficiency, xerophthalmia and nutritional blindness. Nutr Health. 1986;4:105–112. doi: 10.1177/026010608600400206. [DOI] [PubMed] [Google Scholar]
- 21.Mobarhan S, Russell RM, Underwood BA, Wallingford J, Mathieson RD, Al-Midani H. Evaluation of the relative dose response test for vitamin A nutriture in cirrhotics. Am J Clin Nutr. 1981;34:2264–2270. doi: 10.1093/ajcn/34.10.2264. [DOI] [PubMed] [Google Scholar]
- 22.Loerch JD, Underwood BA, Lewis KC. Response of plasma levels of vitamin A to a dose of vitamin A as an indicator of hepatic vitamin A reserves in rats. J Nutr. 1979;109:778–786. doi: 10.1093/jn/109.5.778. [DOI] [PubMed] [Google Scholar]
- 23.Flores H, Campos F, Araujo RC, Underwood BA. Assessment of marginal vitamin A deficiency in Brazilian children using the relative dose response procedure. Am J Clin Nutr. 1984;40:1281–1289. doi: 10.1093/ajcn/40.6.1281. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 25.Best WR. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12:304–310. doi: 10.1097/01.MIB.0000215091.77492.2a. [DOI] [PubMed] [Google Scholar]
- 26.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 27.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1-253. [PubMed] [Google Scholar]
- 28.Deurenberg P, Yap M. The assessment of obesity: methods for measuring body fat and global prevalence of obesity. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:1–11. doi: 10.1053/beem.1999.0003. [DOI] [PubMed] [Google Scholar]
- 29.Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn’s disease treated with Infliximab. Clin Nutr. 2011;30:86–91. doi: 10.1016/j.clnu.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–e248. doi: 10.1016/j.crohns.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Hartman C, Eliakim R, Shamir R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol. 2009;15:2570–2578. doi: 10.3748/wjg.15.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sousa Guerreiro C, Cravo M, Costa AR, Miranda A, Tavares L, Moura-Santos P, MarquesVidal P, Nobre Leitão C. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: a case-control study. Am J Gastroenterol. 2007;102:2551–2556. doi: 10.1111/j.1572-0241.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 33.Bin CM, Flores C, Alvares-da-Silva MR, Francesconi CF. Comparison between handgrip strength, subjective global assessment, anthropometry, and biochemical markers in assessing nutritional status of patients with Crohn’s disease in clinical remission. Dig Dis Sci. 2010;55:137–144. doi: 10.1007/s10620-008-0692-1. [DOI] [PubMed] [Google Scholar]
- 34.Lanfranchi GA, Brignola C, Campieri M, Bazzocchi G, Pasquali R, Bassein L, Labò G. Assessment of nutritional status in Crohn’s disease in remission or low activity. Hepatogastroenterology. 1984;31:129–132. [PubMed] [Google Scholar]
- 35.Imes S, Pinchbeck B, Dinwoodie A, Walker K, Thomson AB. Vitamin A status in 137 patients with Crohn’s disease. Digestion. 1987;37:166–170. doi: 10.1159/000199495. [DOI] [PubMed] [Google Scholar]
- 36.Main AN, Mills PR, Russell RI, Bronte-Stewart J, Nelson LM, McLelland A, Shenkin A. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169–1175. doi: 10.1136/gut.24.12.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bousvaros A, Zurakowski D, Duggan C, Law T, Rifai N, Goldberg NE, Leichtner AM. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: effect of disease activity. J Pediatr Gastroenterol Nutr. 1998;26:129–135. doi: 10.1097/00005176-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 38.de Paula TP, Ramalho A, Braulio VB. The effectiveness of relative dose response to retinol intake as an evaluation of vitamin A status of cirrhotic patients. J Hum Nutr Diet. 2010;23:583–589. doi: 10.1111/j.1365-277X.2010.01072.x. [DOI] [PubMed] [Google Scholar]
- 39.Fujita M, Brindle E, Rocha A, Shell-Duncan B, Ndemwa P, O’Connor KA. Assessment of the relative dose-response test based on serum retinol-binding protein instead of serum retinol in determining low hepatic vitamin A stores. Am J Clin Nutr. 2009;90:217–224. doi: 10.3945/ajcn.2009.27569. [DOI] [PubMed] [Google Scholar]
- 40.Agne-Djigo A, Idohou-Dossou N, Kwadjode KM, Tanumihardjo SA, Wade S. High prevalence of vitamin A deficiency is detected by the modified relative dose-response test in six-month-old Senegalese breast-fed infants. J Nutr. 2012;142:1991–1996. doi: 10.3945/jn.112.166454. [DOI] [PubMed] [Google Scholar]
- 41.Tanumihardjo SA, Permaesih D, Dahro AM, Rustan E, Muhilal D, Olson JA. Comparison of vitamin A status assessment techniques in children from two Indonesian villages. Am J Clin Nutr. 1994;60:136–141. doi: 10.1093/ajcn/60.1.136. [DOI] [PubMed] [Google Scholar]
- 42.Tanumihardjo SA. Assessing vitamin A status: past, present and future. J Nutr. 2004;134:290S–293S. doi: 10.1093/jn/134.1.290S. [DOI] [PubMed] [Google Scholar]


