Abstract
AIM: To investigate the diagnostic capability of breath-hold diffusion-weighted imaging (DWI) for differentiation between malignant and benign hepatic lesions.
METHODS: A total of 614 malignant liver lesions (132 hepatocellular carcinomas, 468 metastases and 14 intrahepatic cholangiocarcinomas) and 291 benign liver lesions (102 hemangiomas, 158 cysts, 24 focal nodular hyperplasia, 1 angiomyolipoma and 6 hepatic adenomas) were included from seven studies (eight sets of data).
RESULTS: The pooled sensitivity and specificity of breath-hold DWI were 0.93 [95% confidence interval (CI): 0.91-0.95] and 0.87 (95%CI: 0.83-0.91), respectively. The positive likelihood ratio and negative likelihood ratio were 7.28 (95%CI: 4.51-11.76) and 0.09 (95%CI: 0.05-0.17), respectively. The P value for χ2 heterogeneity for all pooled estimates was < 0.05. From the fitted summary receiver operating characteristic curve, the area under the curve and Q* index were 0.96 and 0.91, respectively. Publication bias was not present (t = 0.49, P = 0.64). The meta-regression analysis indicated that evaluated covariates including magnetic resonance imaging modality, echo time, mean age, maximum b factor, and number of b factors were not sources of heterogeneity (all P > 0.05).
CONCLUSION: Breath-hold DWI is useful for differentiating between malignant and benign hepatic lesions. The diffusion characteristics of benign lesions that mimic malignant ones have rarely been investigated.
Keywords: Breath-hold imaging, Diffusion-weighted imaging, Hepatic tumor, Meta-analysis
Core tip: We investigated the diagnostic capability of breath-hold diffusion-weighted imaging (DWI) and found that it is useful for differentiating between malignant and benign hepatic focal lesions. The diffusion characteristics of the benign liver lesions that mimic malignant lesions have rarely been investigated and further studies are needed. Standardization of the acquisition protocol for breath-hold DWI across multicenter trials is recommended.
INTRODUCTION
Cancer is a leading cause of death worldwide, accounting for 8.2 million deaths in 2012 (Globocan 2012, International Agency for Research on Cancer). It is expected that annual cancer cases will rise from 14 million in 2012 to 22 million within the next two decades. Liver cancer killed 700000 people in 2008. Cancer mortality can be reduced if cases are detected and treated early through diagnosis and screening programs (http://www.who.int/cancer/events). Accurate diagnosis of focal hepatic lesions is essential for adequate treatment planning; in particular, to select patients who are candidates for hepatic resection, local ablation, or systemic chemotherapy[1-4].
Diffusion-weighted imaging (DWI) provides tissue contrast based on the diffusion properties of water molecules in tissue, without using any contrast agents. The inherent sensitivity of DWI sequences to motion remains a source of problems for liver imaging[5-7]. Respiratory motion degrades images through both temporal blurring and generation of discrete artifacts. Several techniques can be used to reduce the artifacts of respiratory motion: respiratory gating, respiratory ordered phase encoding, navigator gating, and signal averaging. None of these methods entirely eliminate the motion-associated degradation of image quality. Breath-hold imaging has proved to be far more satisfactory[8-10].
A review of the literature reveals that DWI is able to differentiate lesions with high water content (cysts and hemangiomas) from solid lesions. Differences in apparent diffusion coefficients have been reported between benign and malignant focal liver lesions[7,11-14]. Preliminary data are promising. The breath-hold technique is useful and considerably enhances magnetic resonance imaging (MRI). The present systematic review and meta-analysis aimed to investigate the diagnostic capability of breath-hold DWI for differentiating malignant and benign hepatic focal lesions.
MATERIALS AND METHODS
Search strategy
A computerized search was performed using PubMed (www.ncbi.nlm.nih.gov/pubmed/) including articles listed through April 2014. The following search terms were used: “liver and apparent diffusion coefficient (ADC)”, “liver and ADC”, “hepatic and ADC”, “hepatic and apparent diffusion coefficient”, “hepatic and DWI”, “liver and diffusion weighted imaging”, “liver and DWI”, “hepatic and diffusion weighted imaging”, and “hepatic and DWI”. The search was limited to English-language studies only. The reference lists of all included studies were examined for relevant publications.
Eligibility criteria for study selection
Studies were included in this analysis if: (1) breath-hold DWI was performed using either a 1.5T or 3.0T magnetic resonance (MR) scanner; (2) the diagnostic criteria of the malignant and benign hepatic focal lesions were clearly stated; (3) method of DWI analysis was reported; and (4) data were available to fill out cross-tabs in order to assess true-positive (TP), true-negative (TN), false-positive (FP) and false-negative (FN) cases.
Data collection
The characteristics of each study including study name, year of publication, MR modalities used, strength of field, pulse, repetition time (TR), echo time (TE), number of b factors, mean age, maximum b factor, mean size of malignant lesions, number of benign lesions [total, hemangiomas, cysts, focal nodular hyperplasia (FNH), angiomyolipoma and hepatic adenomas] and malignant lesions (total, hepatocellular carcinomas, metastases, and intrahepatic cholangiocarcinomas), TP, TN, FP, and FN, are shown in Tables 1 and 2.
Table 1.
Liver breath-hold diffusion-weighted imaging studies and result
| No. | Ref. | MRI unit | Field (T) | Pulse | TR (ms) | TE (ms) | b factors (n) | b factor (Max) | PAT | Acceleration factor | Mean age (yr) | FS | Cutoff (ADC) | Lesion size (mal) |
| 1 | Erturk et al[15] | Philips | 1.5 | SS-SE-EPI | NA | 120-125 | 2 | 1000 | SENSE | 2 | 60.4 | Yes | 1.63 | 2.3 |
| 2 | Ichikawa T et al[16] | Siemens | 1.5 | SS-SE-EPI | NA | 54 | 3 | 55 | NA | NA | 58.0 | Yes | 5.5 | NA |
| 3 | Koh et al[18] | Phillips | 1.5 | SSEPI | 1850 | 56 | 3 | 500 | SENSE | 2 | 57.0 | NA | NA | 1.96 |
| 4 | Löwenthal et al[19] | Phillips | 1.5 | SSEPI | 1850 | 68 | 2 | 500 | SENSE | 2 | 61.6 | NA | Mal < 2.5 benign > 3 | 3 |
| 5 | Taouli et al[20] | Phillips | 1.5 | SSEPI | 2400 | 104 | 2 | 500 | NA | NA | 52.0 | NA | 1.5 | 5 |
| 6 | Phillips | 1.5 | SSEPI | 3106 | 104 | 4 | 400 | NA | NA | 52.0 | NA | 1.5 | 5 | |
| 7 | Yang et al[1] | Phillips | 1.5 | SSEPI | 1338 | 66 | 3 | 800 | SENSE | 2 | 56.0 | Yes | NA | 1.76 |
| 8 | Kim et al[17] | GE | 1.5 | SS-SE-EPI | NA | 70 | 7 | 846 | NA | NA | 60.0 | Yes | 1.6 | NA |
ADC: Apparent diffusion coefficient; Mal: malignant; NA: Not available; PAT: Parallel acquisition technique; SENSE: Sensitivity encoding; SSEPI: Single-shot echo-planar imaging sequence; SS-SE-EPI: Single-shot spin-echo echo-planar imaging; T: Tesla.
Table 2.
Liver breath-hold diffusion-weighted imaging studies and result, n
| No. | Ref. |
Malignant |
Benign |
TP | FP | FN | TN | ||||||||
| Total | HCC | Met | Chol | Total | Hem | Cysts | FNH | Ang | Hep | ||||||
| 1 | Erturk et al[15] | 42 | 21 | 21 | 0 | 44 | 16 | 28 | 0 | 0 | 0 | 40 | 4 | 2 | 40 |
| 2 | Ichikawa et al[16] | 63 | 48 | 15 | 0 | 11 | 11 | 0 | 0 | 0 | 0 | 59 | 0 | 4 | 11 |
| 3 | Koh et al[18] | 83 | 0 | 83 | 0 | 50 | 1 | 49 | 0 | 0 | 0 | 65 | 2.5 | 18 | 47.5 |
| 4 | Löwenthal et al[19] | 278 | 0 | 278 | 0 | 54 | 24 | 30 | 0 | 0 | 0 | 271 | 15 | 7 | 39 |
| 5 | Taouli et al[20] | 24 | 9 | 15 | 0 | 28 | 7 | 6 | 12 | 0 | 3 | 21 | 3 | 4 | 24 |
| 6 | 24 | 9 | 15 | 0 | 28 | 7 | 6 | 12 | 0 | 3 | 23 | 1 | 6 | 22 | |
| 7 | Yang et al[1] | 51 | 12 | 26 | 13 | 46 | 19 | 27 | 0 | 0 | 0 | 49 | 5 | 2 | 41 |
| 8 | Kim et al[17] | 49 | 33 | 15 | 1 | 30 | 17 | 12 | 0 | 1 | 0 | 48 | 6 | 1 | 24 |
Ang: Angiomyolipoma; Chol: Cholangiocarcinoma; FN: False negative; FNH: Focal nodular hyperplasia; FP: False positive; HCC: Hepatocellular carcinoma; Hem: Hemangioma; Hep: Hepatic adenoma; Met: Metastases; TN: True negative; TP: True positive.
Statistical analysis
Statistical analyses were performed using Meta-DiSc version 1.4 or Stata 12.0 (StataCorp, College Station, TX, United States). Potential threshold effects were investigated using Spearman’s correlation coefficient. We assessed heterogeneity through visual inspection of the forest plots and with the I2 statistic quantifying inconsistency across studies. For each study, the sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR) was calculated (DerSimonian-Laird random effects model). A symmetric summary receiver operating characteristics (SROC) curve was fitted. Publication bias was evaluated by Deeks’ asymmetry test. To explore the sources of heterogeneity in the studies, we performed meta-regression analyses using the Moses-Shapiro-Littenberg method. P < 0.05 was considered to be statistically significant.
RESULTS
Study selection and data extraction
The initial database search identified 827 relevant articles that were published through April 2014. The initial screening by one reviewer reduced the total to 28. Finally, we selected eight sets of data in seven articles that met all the inclusion criteria for meta-analysis (Figure 1).
Figure 1.
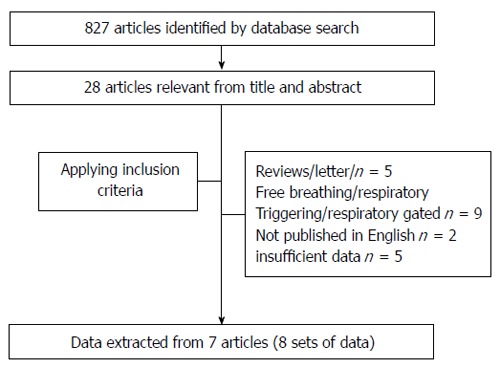
Flow chart for articles identified and included in this meta-analysis.
Description of studies
This meta-analysis was performed on a per-lesion basis. A total of 614 malignant liver lesions (132 hepatocellular carcinomas, 468 metastases and 14 intrahepatic cholangiocarcinomas) and 291 benign liver lesions (102 hemangiomas, 158 cysts, 24 FNH, one angiomyolipoma and six hepatic adenomas) were included (No.1-8; Table 1)[1,15-20]. The mean age of patients was 57.1 years.
All studies used a 1.5T MR scanner with single-shot echo-planar imaging sequence (No.1-8). Seven studies (No.1, 3-8) used a sequence with maximum b factor in the range of 400-1000 ms, while one study used a sequence with maximum b factor of 55 (No.2). Typical acquisition parameters include TE (No.1-8) of ≥ 54 ms (range: 56-125 ms) and TR of ≥ 1338 ms (range: 1338-3106 ms) (No.3-7). Three studies did not provide information on TR (No.1, 2, 8). Four studies did not provide information on the fat-suppressed technique (No.3-6). The parallel acquisition technique was used in four studies (No.1, 3, 4, 7) and the typical acceleration factor was 2. The results of all analyses are reported in Tables 1 and 2.
Synthesis of general diagnostic parameters
Figure 2 shows the forest plots of sensitivity (Figure 2A), specificity (Figure 2B), PLR (Figure 2C), and NLR (Figure 2D) of breath-hold DWI for differential diagnosis between focal malignant and benign hepatic lesions. The threshold effect was not present (P = 0.058).
Figure 2.

Forest plots. A: Sensitivity; B: Specificity; C: Positive likelihood ratio (LR); and D: Negative LR. CI: Confidence interval.
The pooled sensitivity and specificity of breath-hold DWI were 0.93 [95% confidence interval (CI): 0.91-0.95] and 0.87 (95%CI: 0.83-0.91), respectively. PLR and NLR were 7.28 (95%CI: 4.51-11.76) and 0.09 (95%CI: 0.05-0.17), respectively. The P value for χ2 heterogeneity for all pooled estimates was < 0.05.
The overall accuracy was further explored by drawing SROC curves, and the area under the curve (AUC) and Q* index (Figure 3) were 0.96 and 0.91, respectively, indicating good diagnostic accuracy. Publication bias was not present (t = 0.49, P = 0.64) (Figure 4).
Figure 3.

Summary receiver operating characteristics curve. Sensitivity and specificity are plotted for individual studies. AUC: Area under the curve. SROC: Summary receiver operating characteristics.
Figure 4.
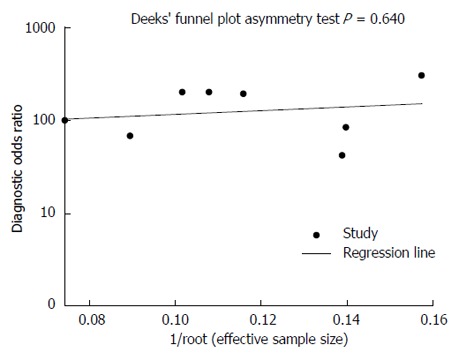
There is no significant publication bias.
The meta-regression analysis indicated that evaluated covariates, including MRI modality, TE, mean age, maximum b factor, and number of b factors, were not sources of heterogeneity (all P > 0.05).
DISCUSSION
DWI has a strongpoint in that it provides excellent lesion-to-liver contrast with the suppression of the background signal of liver parenchyma as well as vessels, which reduces the likelihood of overlooked lesions[7,21,22]. Malignant tumors with hypercellularity, narrowed intercellular spaces, and increased density of cell membranes that hamper water molecule diffusion may well exhibit increased signal intensity on DWI[22]. Breath-hold imaging has proved to be more satisfactory. We used commonly available MRI techniques (e.g., no respiratory triggering) so that our results are applicable to most MRI units and not restricted to major academic centers[15].
Based on calculations of the relevant data available in the current published articles, our systematic review and meta-analysis demonstrated that breath-hold DWI was useful for differentiating between malignant and benign hepatic focal lesions. The results demonstrated that the overall diagnostic performance of the test with DWI to differentiate malignant and benign hepatic focal lesions was high. However, significant heterogeneity among studies was noted in our analysis.
Our meta-regression analysis indicated that evaluated covariates were not sources of heterogeneity. These results are consistent with recent systematic reviews[23], which have reported that neither threshold effect nor evaluated covariates including MR scanner, scanning technique, TR, TE, maximum b factor, number of b factors used for ADC calculation, mean tumor size, and mean patient age, were sources of heterogeneity. It is known that the best acquisition strategies for DWI sequences in focal liver disease are still a matter of debate. There was considerable variation in the results, which may be an indicator that more detailed investigation should be carried out on the presence of heterogeneity.
ADCs tend to decrease in the order of cysts, hemangiomas, HCCs, and metastases[24]. The malignant lesions, including metastases and HCCs, had the lowest ADCs, whereas the benign lesions, including hemangiomas and cysts, had the highest ADCs. Benign hepatocellular lesions had intermediate ADCs[20]. FNH and hepatic adenoma readily mimic malignant hepatic tumors, and these benign lesions often show increased signal intensity on DWI. However, the diffusion characteristics of the benign hepatocellular lesions, including cases of FNH (24/291) and adenoma (6/291), have rarely been reported and need further studies. It is known that DWI is more useful with hepatic metastases than with HCCs, primarily because the T2 relaxation time is long enough with most metastases, and there is no resemblance of histopathologic architecture between metastases and surrounding liver parenchyma[22]. However, the relevant data available for malignant hepatic focal lesions in the current published articles focus on hepatic metastases (468/614). All these data have demonstrated that the diagnostic capability of breath-hold DWI for differentiation of malignant and benign hepatic focal lesions might be overestimated.
Asymmetrical funnel plots are linked to publication bias, although there are other sources of asymmetry that have to be considered, including other dissemination biases, differences in the quality of smaller studies, presence of true heterogeneity, and chance[25-28]. In the present meta-analysis, the funnel plot indicated that there may not have been publication bias.
The present study had several limitations. First, there was notable heterogeneity among the studies. Evaluated covariates were not the sources and this needs further investigation. Second, diagnostic capability might be overestimated due to the possibility of selection bias. The diffusion characteristics of the benign liver lesions (e.g., FNH and adenoma) that mimic malignant lesions have rarely been investigated and require further studies.
In conclusion, breath-hold DWI was useful for differentiation between malignant and benign hepatic focal lesions. However, diagnostic capability might be overestimated due to the possibility of selection bias. Standardization of the acquisition protocol for breath-hold DWI across multicenter trials is recommended.
COMMENTS
Background
Diffusion-weighted imaging (DWI) provides tissue contrast based on the diffusion properties of water molecules in tissue, without using any contrast agents. The inherent sensitivity of DWI sequences to motion remains a problem for liver imaging. Breath-hold DWI has proven to be more satisfactory.
Research frontiers
There is no current consensus on the diagnostic capability of hepatic breath-hold DWI. We conducted a systematic review to investigate the diagnostic capability of breath-hold DWI for differentiating between malignant and benign hepatic focal lesions.
Innovations and breakthroughs
The diffusion characteristics of the benign liver lesions that mimic malignant lesions have rarely been investigated and need further study. Standardization of the acquisition protocol for breath-hold DWI across multicenter trials is recommended.
Applications
Breath-hold DWI was useful for differentiation of malignant and benign hepatic focal lesions.
Terminology
DWI provides tissue contrast based on the diffusion properties of water molecules in tissue. DWI plays a potential role in the differentiation and evaluation of liver tumors on the basis of high contrast between the lesion and normal tissue.
Peer-review
The paper discusses the prognostic value of DWI in differentiation of benign versus malignant hepatic masses. The meta-analysis is comprehensive and carefully done.
Footnotes
Supported by Grants from the Science Foundation of Guangdong Province for Doctorate Startup Project, No. S2012040006618; and the Postdoctoral Fund of Guangzhou University of Traditional Chinese Medicine, No. 20120621.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 27, 2014
First decision: July 21, 2014
Article in press: September 30, 2014
P- Reviewer: He ST, Morales-Gonzalez JA, Pan WS S- Editor: Qi Y L- Editor: AmEditor E- Editor: Wang CH
References
- 1.Yang DM, Jahng GH, Kim HC, Jin W, Ryu CW, Nam DH, Lee YK, Park SY. The detection and discrimination of malignant and benign focal hepatic lesions: T2 weighted vs diffusion-weighted MRI. Br J Radiol. 2011;84:319–326. doi: 10.1259/bjr/50130643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385–2390. doi: 10.1245/s10434-009-0492-7. [DOI] [PubMed] [Google Scholar]
- 3.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 4.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 5.Haradome H, Grazioli L, Morone M, Gambarini S, Kwee TC, Takahara T, Colagrande S. T2-weighted and diffusion-weighted MRI for discriminating benign from malignant focal liver lesions: diagnostic abilities of single versus combined interpretations. J Magn Reson Imaging. 2012;35:1388–1396. doi: 10.1002/jmri.23573. [DOI] [PubMed] [Google Scholar]
- 6.Nishie A, Tajima T, Ishigami K, Ushijima Y, Okamoto D, Hirakawa M, Nishihara Y, Taketomi A, Hatakenaka M, Irie H, et al. Detection of hepatocellular carcinoma (HCC) using super paramagnetic iron oxide (SPIO)-enhanced MRI: Added value of diffusion-weighted imaging (DWI) J Magn Reson Imaging. 2010;31:373–382. doi: 10.1002/jmri.22059. [DOI] [PubMed] [Google Scholar]
- 7.Mannelli L, Bhargava P, Osman SF, Raz E, Moshiri M, Laffi G, Wilson GJ, Maki JH. Diffusion-weighted imaging of the liver: a comprehensive review. Curr Probl Diagn Radiol. 2013;42:77–83. doi: 10.1067/j.cpradiol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D, Ambrosino MM, Weinreb JC. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212:876–884. doi: 10.1148/radiology.212.3.r99se34876. [DOI] [PubMed] [Google Scholar]
- 9.Katz-Brull R, Rofsky NM, Lenkinski RE. Breathhold abdominal and thoracic proton MR spectroscopy at 3T. Magn Reson Med. 2003;50:461–467. doi: 10.1002/mrm.10560. [DOI] [PubMed] [Google Scholar]
- 10.Gaa J, Hatabu H, Jenkins RL, Finn JP, Edelman RR. Liver masses: replacement of conventional T2-weighted spin-echo MR imaging with breath-hold MR imaging. Radiology. 1996;200:459–464. doi: 10.1148/radiology.200.2.8685342. [DOI] [PubMed] [Google Scholar]
- 11.Vossen JA, Buijs M, Liapi E, Eng J, Bluemke DA, Kamel IR. Receiver operating characteristic analysis of diffusion-weighted magnetic resonance imaging in differentiating hepatic hemangioma from other hypervascular liver lesions. J Comput Assist Tomogr. 2008;32:750–756. doi: 10.1097/RCT.0b013e31816a6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, Stemmer A, Ganter C, Rummeny EJ. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 13.Coenegrachts K, Orlent H, ter Beek L, Haspeslagh M, Bipat S, Stoker J, Rigauts H. Improved focal liver lesion detection: comparison of single-shot spin-echo echo-planar and superparamagnetic iron oxide (SPIO)-enhanced MRI. J Magn Reson Imaging. 2008;27:117–124. doi: 10.1002/jmri.21247. [DOI] [PubMed] [Google Scholar]
- 14.d’Assignies G, Fina P, Bruno O, Vullierme MP, Tubach F, Paradis V, Sauvanet A, Ruszniewski P, Vilgrain V. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology. 2013;268:390–399. doi: 10.1148/radiol.13121628. [DOI] [PubMed] [Google Scholar]
- 15.Erturk SM, Ichikawa T, Sano K, Motosugi U, Sou H, Araki T. Diffusion-weighted magnetic resonance imaging for characterization of focal liver masses: impact of parallel imaging (SENSE) and b value. J Comput Assist Tomogr. 2008;32:865–871. doi: 10.1097/RCT.0b013e3181591cf2. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397–402. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 17.Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–398. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 18.Koh DM, Brown G, Riddell AM, Scurr E, Collins DJ, Allen SD, Chau I, Cunningham D, deSouza NM, Leach MO, et al. Detection of colorectal hepatic metastases using MnDPDP MR imaging and diffusion-weighted imaging (DWI) alone and in combination. Eur Radiol. 2008;18:903–910. doi: 10.1007/s00330-007-0847-z. [DOI] [PubMed] [Google Scholar]
- 19.Löwenthal D, Zeile M, Lim WY, Wybranski C, Fischbach F, Wieners G, Pech M, Kropf S, Ricke J, Dudeck O. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011;21:832–840. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 20.Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71–78. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 21.Wu LM, Hu J, Gu HY, Hua J, Xu JR. Can diffusion-weighted magnetic resonance imaging (DW-MRI) alone be used as a reliable sequence for the preoperative detection and characterisation of hepatic metastases? A meta-analysis. Eur J Cancer. 2013;49:572–584. doi: 10.1016/j.ejca.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Kanematsu M, Goshima S, Watanabe H, Kondo H, Kawada H, Noda Y, Aomatsu A, Moriyama N. Detection and characterization of focal hepatic lesions with diffusion-weighted MR imaging: a pictorial review. Abdom Imaging. 2013;38:297–308. doi: 10.1007/s00261-012-9940-0. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Chen Z, Wang J. Differential diagnosis between malignant and benign hepatic tumors using apparent diffusion coefficient on 1.5-T MR imaging: a meta analysis. Eur J Radiol. 2012;81:484–490. doi: 10.1016/j.ejrad.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 24.Goshima S, Kanematsu M, Kondo H, Yokoyama R, Kajita K, Tsuge Y, Watanabe H, Shiratori Y, Onozuka M, Moriyama N. Diffusion-weighted imaging of the liver: optimizing b value for the detection and characterization of benign and malignant hepatic lesions. J Magn Reson Imaging. 2008;28:691–697. doi: 10.1002/jmri.21467. [DOI] [PubMed] [Google Scholar]
- 25.Souza JP, Pileggi C, Cecatti JG. Assessment of funnel plot asymmetry and publication bias in reproductive health meta-analyses: an analytic survey. Reprod Health. 2007;4:3. doi: 10.1186/1742-4755-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365:1308. doi: 10.1016/S0140-6736(05)61026-4. [DOI] [PubMed] [Google Scholar]
- 27.Ho KM. Forest and funnel plots illustrated the calibration of a prognostic model: a descriptive study. J Clin Epidemiol. 2007;60:746–751. doi: 10.1016/j.jclinepi.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Yeh J, D’Amico F. Forest plots: data summaries at a glance. J Fam Pract. 2004;53:1007. [PubMed] [Google Scholar]


