Abstract
AIM: To evaluate whether the application of sorafenib during the peri-operative period of liver transplantation improves prognosis in liver cancer patients.
METHODS: We searched PubMed, EMBASE and MEDLINE for eligible articles. A total of 4 studies were found that fulfilled the previously agreed-upon standards. We then performed a systematic review and meta-analysis on the enrolled trials that met the inclusion criteria.
RESULTS: Out of the 104 studies identified in the database, 82 were not clinical experiments, and 18 did not fit the inclusion standards. Among the remaining 4 articles, only 1 was related to the preoperative use of sorafenib, whereas the other 3 were related to its postoperative use. As the heterogeneity among the 4 studies was high, with an I2 of 86%, a randomized effect model was applied to pool the data. The application of sorafenib before liver transplantation had a hazard ratio (HR) of 3.29 with a 95% confidence interval (CI) of 0.33-32.56. The use of sorafenib after liver transplantation had an HR of 1.44 (95%CI: 0.27-7.71). The overall pooled HR was 1.68 (95%CI: 0.41-6.91).
CONCLUSION: The results showed that the use of sorafenib during the peri-operative period of liver transplantation did not improve patient survival significantly. In fact, sorafenib could even lead to a worse prognosis, as its use may increase the hazard of poor survival.
Keywords: Liver transplantation, Sorafenib, Peri-operative period, Kaplan-Meier curve, Hazard ratio
Core tip: The data were extracted from the Kaplan-Meier curves of every study identified and then input into a hazard ratio calculation spreadsheet. The HRs generated from the sheet were combined with RevMan5.0. To the best of our knowledge, this is the first meta-analysis assessing the use of sorafenib in the peri-operative period of liver transplantation.
INTRODUCTION
Liver cancer is the sixth most common cancer in the world and represents the third most common cause of cancer-related death[1]. Surgical resection and liver transplantation have been considered the most potentially curative treatments up to now. For patients with a solitary lesion < 5 cm or three nodules < 3 cm that are not suitable for resection [III, A], liver transplantation is the ultimate best choice. However, sufficient improvements in 5-year disease-free and overall survival rates for patients receiving transplantations have not been obtained, as the post-transplantation recurrence rate of carcinoma is as high as 66.7%[2]. As a result, there is an urgent need for an effective method to decrease the post-transplantation recurrence rate.
Sorafenib is a multi-kinase inhibitor that is able to block the Raf/mitogen-activated protein kinase extracellular signal-regulated kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway[3]. Due to the involvement of this pathway in tumorigenesis, including liver carcinogenesis, sorafenib could be used to restrain the proliferation and survival of tumor cells. Consequently, sorafenib has been introduced for the treatment of liver cancer.
Up to now, there have been several clinical experiments focusing on the peri-operational utility of sorafenib in liver transplantation, rating its validity as an adjuvant therapy for cancer patients. However, sufficiently large multi-center studies to provide an overall evaluation of sorafenib in the peri-operative period of liver transplantation are still lacking. The present meta-analysis was intended to combine all of the relevant studies to assess the curative effect of sorafenib as an adjuvant therapy.
MATERIALS AND METHODS
Literature search
Articles were identified by an electronic search of PubMed, EMBASE, and MEDLINE using the keywords “liver transplantation” and “sorafenib”, and the personal bibliographies of two of the authors were also included. The bibliographies reported in any of the studies identified were used for further trial identification.
The articles are limited to published trials with at least an abstract given in English. No contact was made with the authors to obtain unpublished data.
Selection of trials
A total of 104 articles was obtained, spanning November 2008 to September 2013.
Before determining the targets, several standards were decided. The potential literature to be included had to fulfill the following criteria: (1) the experiment was carried out on humans who were going to or had received a liver transplantation; (2) sorafenib was compared with a placebo or other non-sorafenib treatment during the peri-operative period of liver transplantation; (3) randomized controlled trails were the first choice, followed by cohort and then case-control studies; (4) all of the studies had to have a common end point, which was defined as the time of patient death or the last time of follow-up; and (5) all potentially included studies should provide survival curves or hazard ratios (HRs) with corresponding 95% confidence intervals (CIs).
Out of the 104 studies identified, none were randomized controlled trials; 82 were not clinical experiments; and 18 did not fulfill the inclusion standards. Among the remaining 4 articles, only 1 was related to the preoperative use of sorafenib, whereas the other 3 were related to its postoperative use. As a result, only 4 retrospective cohort trials[4-7] were included in this meta-analysis (Figure 1).
Figure 1.
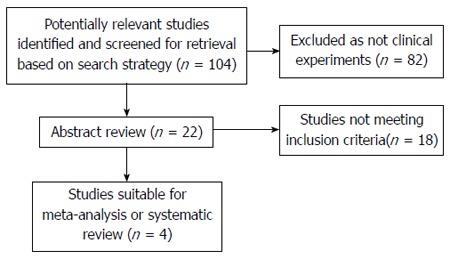
Study flow.
Data extraction
Except for one article, the remaining three ones did not directly provide the HRs and corresponding 95%CIs, although the survival curves were available. Using widely proven, accepted scientific methods[8,9], the data were extracted from the survival curves with Engauge 4.0. Then, the data were input into the HR calculation spreadsheet, which was created by Tierney et al[10]. Using the methodology stated above, the HRs, standard errors (SEs) and their corresponding 95%CIs were estimated from the curves. The detailed process is shown in Figures 2 and 3.
Figure 2.
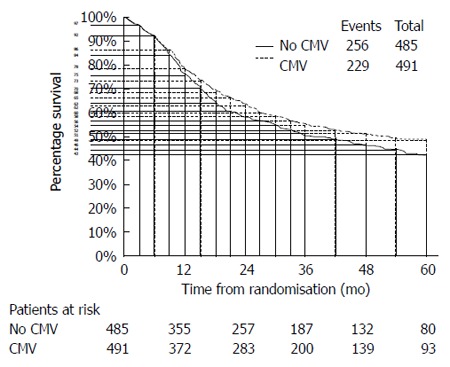
An example of how to extract the data from the Kaplan-Meier curves[10].
Figure 3.
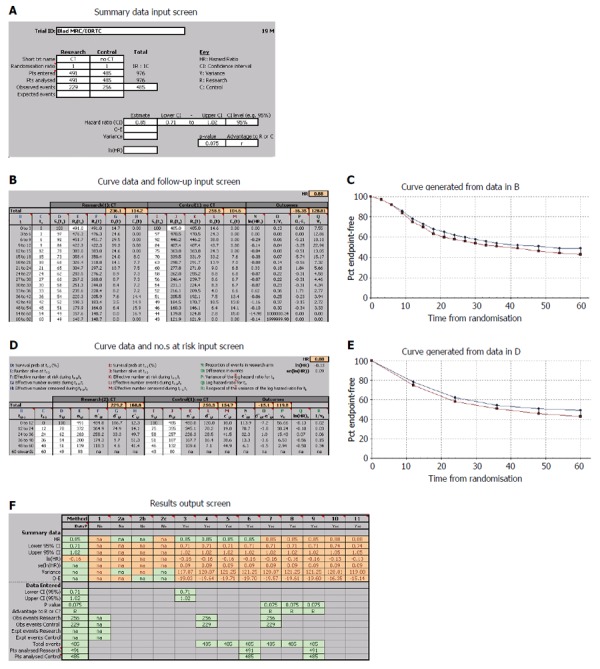
An example depicting the process by which the data extracted from the K-M curves are input into the HR calculation spreadsheet, step by step[10]. Data input screens (A, B and D), generated curves (C and E) and output screen (F) from the calculations spreadsheet.
Statistical analysis
HRs and their SEs were analyzed as a whole using Review Manager 5.0, and statistical heterogeneity was defined as P < 0.10 or I2 > 50%. As the potential heterogeneity was determined using the standard above, a randomized effect model was used to measure the outcomes.
RESULTS
As shown in the forest figure, the HRs, as extracted from the Kaplan-Meier curves using the formula recommended by Parmar, Tierney et al[9,10], were transformed to In[HR] to make the data fulfill a normal distribution (Figure 4).
Figure 4.
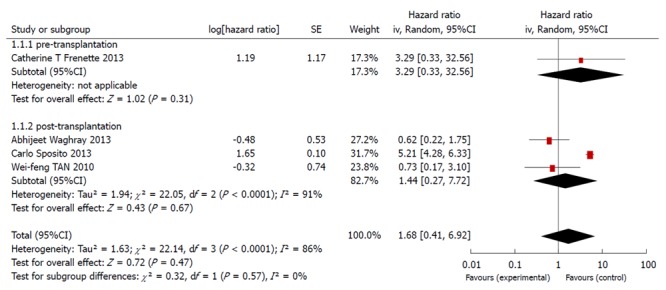
Meta-analysis of the cohort trials comparing the effects of sorafenib in improving survival time during liver transplantation.
Among the four studies identified, one was related to sorafenib use before liver transplantation, whereas the other three investigated the use of sorafenib after liver transplantation.
The present meta-analysis showed that the use of sorafenib during liver transplantation did not significantly improve the overall survival. The use of sorafenib before liver transplantation had an HR of 3.29 (95%CI: 0.33-32.56), and the therapy used after liver transplantation had an HR of 1.44 (95%CI: 0.27-7.71). The overall HR was 1.68 (95%CI: 0.41-6.91). Based on the funnel plot, publication bias might have been detected (Figure 5).
Figure 5.
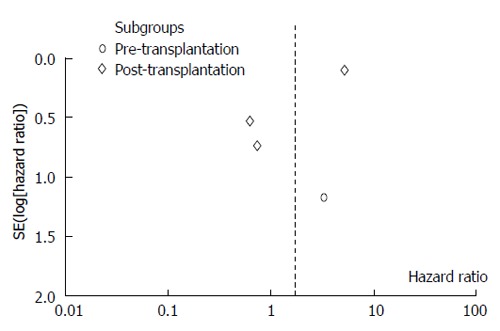
Funnel plot of the included studies.
DISCUSSION
To the best of our knowledge, this is the first meta-analysis to examine the use of sorafenib in the peri-operative period of liver transplantation. As a targeted drug, sorafenib was first used to treat renal cell carcinoma. Then, it only took a few years before sorafenib was first applied as a novel adjuvant treatment for hepatocellular carcinoma, especially for patients requiring liver transplantation[11].
Although sorafenib has anti-tumor potentiality in theory, the outcomes of multi-center cohort or case-control trials indicate that sorafenib does not have any apparent effect in improving overall survival. Moreover, sorafenib therapy may lead to a poor prognosis, as it can increase the hazard of poor survival.
Considerable side effects have been observed among patients receiving sorafenib[12]. Based on the published literature[13], high grade toxicities were reported in 25%-30% (Yoon et al, 4/13 patients; Pfiffer et al, 2/8 patients) of patients under sorafenib/calcineurin inhibitor (CNI) combination therapy and in 55% (Kim et al, 5/9 patients) in another series using sorafenib in combination with mTORi.
From the authors’ perspective, the following reasons may account for the present discouraging conclusion. Sorafenib, as a newly developed targeted drug, has been used for too short a time for true analysis, and its popularization and application have been constricted due to its costs, which are too high for patients. Furthermore, liver transplantation, as the final treatment for liver cancer, is not available for all cancer patients. As a result, the number of participants that could have been included in the experiments is small, and we here may have underestimated the potential of sorafenib.
In conclusion, sorafenib should not be recommended for patients suffering from liver cancer or those waiting for or having received liver transplantation.
Unfortunately, only 4 eligible articles were included in the present study; sorafenib has not yet been applied in liver transplantation for a very long period. Because of the limited data, we could consider only the overall survival rates and the estimated HRs in our analysis, making the results of this study not very persuasive. However, the present study represents the first effort in this new area, and our work could provide some suggestive evidence.
More cohort trials and, optimally, RCTs are needed to verify our conclusion. Research on sorafenib and any other targeted drugs should be encouraged, as such drugs may have as-yet-underestimated anti-tumor abilities.
COMMENTS
Background
Liver cancer is the sixth most common cancer in the world, and liver transplantation is the ultimate best option. However, there is a low overall survival rate for patients receiving transplantations, as the post-transplantation recurrence rate of carcinoma can be as high as 66.7%. As a multi-kinase inhibitor, sorafenib has the potential to take part in the treatment of liver cancer.
Research frontiers
Sorafenib is a multi-kinase inhibitor that has the potential to restrain the proliferation and survival of tumor cells. This research investigates whether sorafenib can improve the prognosis of patients who are going to receive or already have received liver transplantation due to liver cancer.
Innovations and breakthroughs
The data were extracted from the survival curves using Engauge 4.0 software. Next, the data were input into the HR calculation spreadsheet to generate the hazard ratios, standard errors and 95% confidential intervals. This approach represents the first effort in this new area of research, and our data may provide some useful evidence.
Applications
Our research has indicated that sorafenib does not have any apparent effect on overall survival. Moreover, sorafenib therapy might lead to a worse prognosis.
Terminology
Sorafenib is a multi-kinase inhibitor that can block the Raf/mitogen-activated protein kinase-extracellular signal-regulated kinase/extracellular signal-regulated kinase pathway.
Peer-review
The concept and methodology used are appropriate and interesting. This is a very important issue because in the literature some researchers have wondered whether sorafenib can improve patient survival during the perioperational period of liver transplantation. Thus, this meta-analysis provides us with a clear answer.
Footnotes
Supported by National Natural Science Foundation of China, No. 81172349 and No. 30872491.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 28, 2014
First decision: July 21, 2014
Article in press: September 19, 2014
P- Reviewer: Fisher RA, Ramsay M, Yan LN S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii41–vii48. doi: 10.1093/annonc/mds225. [DOI] [PubMed] [Google Scholar]
- 2.Jia N, Liou I, Halldorson J, Carithers R, Perkins J, Reyes J, Yeh M, Stohr E, Rao S, Lin EH. Phase I adjuvant trial of sorafenib in patients with hepatocellular carcinoma after orthotopic liver transplantation. Anticancer Res. 2013;33:2797–2800. [PubMed] [Google Scholar]
- 3.Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25:1158–1164. doi: 10.1111/j.1432-2277.2012.01540.x. [DOI] [PubMed] [Google Scholar]
- 4.Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, Estfan B, Romero-Marrero C, Aucejo F. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;27:555–561. doi: 10.1111/ctr.12150. [DOI] [PubMed] [Google Scholar]
- 5.Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59–66. doi: 10.1016/j.jhep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Tan WF, Qiu ZQ, Yu Y, Ran RZ, Yi B, Lau WY, Liu C, Qiu YH, Feng FL, Wang JH, et al. Sorafenib extends the survival time of patients with multiple recurrences of hepatocellular carcinoma after liver transplantation. Acta Pharmacol Sin. 2010;31:1643–1648. doi: 10.1038/aps.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frenette CT, Boktour M, Burroughs SG, Kaseb A, Aloia TA, Galati J, Gaber AO, Monsour H, Ghobrial RM. Pre-transplant utilization of sorafenib is not associated with increased complications after liver transplantation. Transpl Int. 2013;26:734–739. doi: 10.1111/tri.12117. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira IR, Santos-Jesus R, Po AL, Poolsup N. Extracting numerical data from published reports of pharmacokinetics investigations: method description and validation. Fundam Clin Pharmacol. 2003;17:471–472. doi: 10.1046/j.1472-8206.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 9.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, Otto G, Galle PR, Wörns MA. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44:432–437. doi: 10.1016/j.dld.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180–186. doi: 10.1097/MEG.0b013e328359e550. [DOI] [PubMed] [Google Scholar]
- 13.Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768–773. doi: 10.1093/jjco/hyq055. [DOI] [PubMed] [Google Scholar]


