Abstract
Quantification of the association between the intake of cholesterol and risk of pancreatic cancer is still conflicting. We therefore conducted a meta-analysis to summarize the evidence from epidemiological studies of cholesterol intake and the risk of pancreatic cancer. Pertinent studies were delivered by PubMed and Web of Knowledge issued through April of 2014. A random effects model was used to process the data for analysis. Sensitivity analysis and publication bias were conducted. Dose-response relationship was assessed by restricted cubic spline and variance-weighted least squares regression analysis. With 4513 pancreatic cases exemplified, 16 articles were applied in the meta-analysis. Pooled results suggest that cholesterol intake level was significantly associated with the risk of pancreatic cancer [summary relative risk (RR) = 1.371, 95%CI = 1.155–1.627, I2 = 58.2%], especially in America [summary RR = 1.302, 95%CI = 1.090–1.556]. A linear dose-response relation was attested that the risk of pancreatic cancer rises by 8% with 100 mg/day of cholesterol intake. [summary RR = 1.08, 95% CI = 1.04–1.13]. In conclusion, our analysis suggests that a high intake of cholesterol might increase the risk of pancreatic cancer, especially in America.
Pancreatic cancer is one of the most dismal malignancies. Lacking highly sensitive and specific test methods and early symptoms, early diagnosis and treatment are rarely satisfactory, much less than discovery. Pancreatic cancer as an aggressive malignancy takes the eighth place in cancer-related mortality worldwide, the estimated deaths from pancreatic cancer are 39,590 in United States1. However, the only option for cure is surgery and only 20% patients have such chance due to late detection and diagnosis2. Thus, primary prevention is a priority. The recent genome-wide association studies (GWAS) showed that pancreatic cancer is associated with genetic factors3,4. Furthermore, several other modifiable risk factors have been confirmed the risk of pancreatic cancer, including cigarette smoking, diabetes, alcohol intake, obesity, chronic pancreatitis and diet5,6,7,8,9.
It has been hypothesized that higher intake of cholesterol may be associated with an elevated risk of pancreatic cancer10. Up to date, a number of epidemiologic studies have been published to explore the relationship between cholesterol intake and pancreatic cancer risk. However, the results are not consistent. Therefore, we conducted a meta-analysis to assess the pancreatic cancer risk for the highest vs. lowest categories of cholesterol intake and assess the dose-response association of pancreatic cancer for every 100 mg/day increment in cholesterol intake. Furthermore, we also assess the heterogeneity among studies and publication bias.
Methods
Search Strategy
Studies were identified by using a literature search of PubMed and Web of Knowledge through April of 2014 and by hand-searching the reference lists of the retrieved articles. The following search terms were used: ‘pancreatic cancer’ or ‘pancreatic carcinoma’ combined with ‘nutrition’, ‘diet’, ‘lifestyle’ or ‘cholesterol’. Two investigators searched articles and reviewed all the retrieved studies independently.
Study Selection
For inclusion, studies had to fulfill the following criteria: (1) prospective or case-control study design; (2) cholesterol intake was the independent variable of interest; (3) the dependent variable of interest was pancreatic cancer; (4) relative risk (RR) or odds ratio (OR) with 95% confidence interval (CI) was provided; and (5) for dose-response analysis, the intake of cholesterol for each response category must also have been provided (or data available to calculate them).
Data extraction
Two researchers independently extracted the following data from the included studies: the first author's last name, year of publication, geographic locations, study design, sample source, the age range of study participants, duration of follow-up, the number of cases and participants (person-years), and RR (95%CI) for each category of cholesterol. From each study, we extracted the RR that reflected the greatest degree of control for potential confounders.
Statistical analysis
We carried out a random-effect dose–response meta-analysis with the method proposed by Greenland and Longnecker11 and Orsini et al.12, which takes into account the correlation between the log RR estimates across categories of cholesterol intake. We also explored the possibility of nonlinear relationships by modeling cholesterol intake by using restricted cubic splines with three knots (i.e. two spline transformations) at fixed percentiles (25%, 50% and 75%) of cholesterol intake distribution. A P-value for nonlinearity was calculated by testing against the null hypothesis that the coefficient of the second spline transformation was equal to zero13. The preconditions for the methods are that the distribution of cases and person-years or noncases and the RR with the variance estimates for at least three quantitative exposure categories are known. When this information was not available, we estimated the slopes (linear trends) by using variance-weighted least squares regression analysis14,15. The median cholesterol intake for each specific category was assigned to each corresponding log RR estimate. If the median intake was not reported in the article, we used the midpoint between the upper and lower boundary. If the lowest category was open-ended, its lower boundary was set to zero. If the upper boundary of the highest category was left unspecified, we assumed the category to be of the same amplitude as the preceding one. Statistical heterogeneity across studies was assessed using the Q and I2 statistics16. An I2 statistic <30% indicated no or marginal between-study heterogeneity, 30%–75% considerable moderate heterogeneity and >75% considerable heterogeneity. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariates that might exert substantial impact on between-study heterogeneity17. A study of influence analysis18 was conducted to describe how robust the pooled estimator was to removal of individual studies. Publication bias was evaluated by means of Egger's regression test19.
All statistical analyses were conducted with STATA version 11.0 (StataCorp LP, College Station, Texas, USA). Two-tailed P ≤ 0.05 was accepted as statistically significant.
Results
Search results and study characteristics
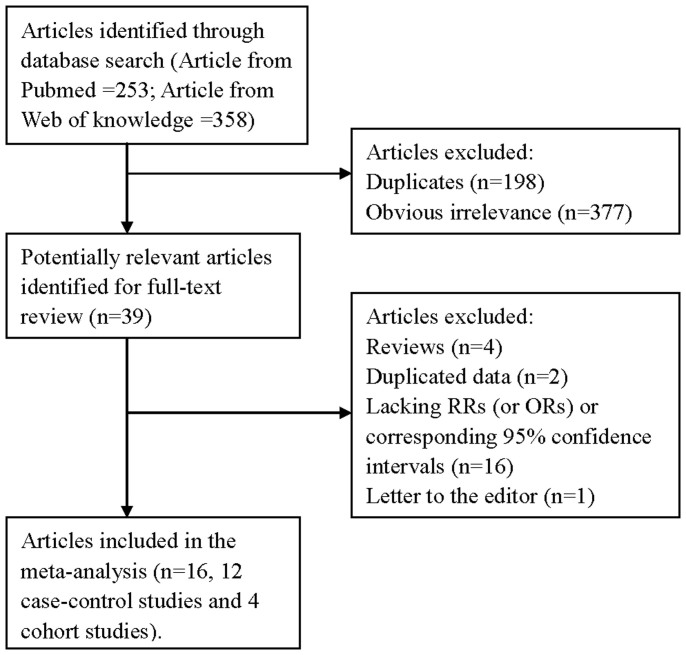
The research strategy helped the researchers to collect 253 articles from PubMed and 358 from the Web of Knowledge, with 36 articles reviewed fully after reading the titles and the abstracts. By studying reference lists, we identified 3 additional articles. Twenty-three of these 39 articles were subsequently excluded from the meta-analysis for various reasons. In total, 16 articles20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 (4 cohort studies and 12 case-control studies) involving 4513 pancreatic cancer cases were used in this meta-analysis. The detailed steps of our literature search are shown in Figure 1. The characteristics of these studies are presented in Table 1. Four studies were conducted in the United States, 3 in the Canada, 2 in the Netherlands, 1 in the Australia, 1 in the Poland, 1 in the Greece, 1 in the Finland, 1 in the Japan and 1 in the Italy.
Figure 1. The flow diagram of screened, excluded, and analyzed publications.
Table 1. Characteristics of studies on cholesterol intake and pancreatic cancer risk.
| Study, Year (Ref.) | Country | Study design | Participants (cases) | Age (years) | RR (95%CI) for highest versus lowest category | Adjustment for covariates |
|---|---|---|---|---|---|---|
| Howe et al. 1990 [20] | Canada | Case-control | 754 (249) | 35–79 | 0.95(0.51–1.75) | Adjust for caloric and fibre intake, lifetime cigarette consumption. |
| Baghurst et al. 1991 [21] | Australia | Case-control | 357 (104) | <50–≥80 | 3.19(1.58–6.47) | Adjust for age; pack-years of smoking, tobacco consumption and vice versa. |
| Bueno de Mesquita et al. 1991 [22] | Netherlands | Case-control | 644 (164) | 35–79 | 1.33(0.72–2.45) | Adjust for age, sex, response status, total smoking and dietary intake of energy. |
| Zatonski et al. 1991 [23] | Poland | Case-control | 305 (110) | 62.2 | 4.31(1.60–11.59) | Adjust for cigarette lifetime consumption and calories. |
| Olsen et al. 1991 [24] | United States | Case-control | 432 (212) | 40–84 | 1.5(0.8–2.6) | Adjusted for total energy, age, cigarette usage, alcohol consumption, respondent-reported history of diabetes mellitus, and educational level. |
| Howe et al. 1992 [25] | Europe | Case-control | 2471 (802) | 28–87 | 2.13(1.42–3.20) | Adjusted for age, sex, nutrient variables (categorical), and lifetime cigarette consumption (continuous). |
| Kalapothaki et al. 1993 [26] | Greece | Case-control | 362 (181) | Na | 1.19(0.96–1.47) | Adjust for age, gender, hospital, past residence, years of schooling, cigarette smoking, diabetes mellitus and energy intake. |
| Ghadirian et al. 1995 [27] | Canada | Case-control | 418 (179) | 35–79 | 2.24(0.83–6.05) | Adjust for age, sex, lifetime cigarette consumption, response status, and total energy intake. |
| Stolzenberg-Solomon et al. 2002 [28] | Finland | Cohort | 27111 (163) | 50–69 | 0.92(0.53–1.59) | Adjust for by the residual method and for age and years of smoking, energy-adjusted folate intake and energy-adjusted saturated fat intake. |
| Michaud et al. 2003 [29] | United States | Cohort | 88802 (178) | 30–55 | 1.11(0.67–1.83) | Adjust for age, pack-years of smoking, body mass index, history of diabetes mellitus, caloric intake, height, physical activity, menopausal status, and glycemic load intake. |
| Nothlings et al. 2005 [30] | United States | Cohort | 190545 (482) | 45–75 | 1.09(0.89–1.32) | Adjust for age at cohort entry, ethnicity, history of diabetes mellitus, and familial history of pancreatic cancer, smoking status, and energy intake. |
| Lin et al. 2005 [31] | Japan | Case-control | 327 (109) | 40–79 | 2.06(1.11–3.85) | Adjust for age, pack-years of smoking and energy intake. |
| Chan et al. 2007 [32] | United States | Case-control | 2233 (532) | 21–85 | 1.5(1.1–2.0) | Adjust for age, sex using energy-adjusted residual model, body mass index, race, education, smoking and history of diabetes using energy-adjusted residual model. |
| Heinen et al. 2009 [33] | Netherlands | Cohort | 120852 (350) | 55–69 | 0.78(0.52–1.18) | Adjust for gender, age, energy, smoking, alcohol, history of diabetes mellitus, history of hypertension, body mass index, vegetables and fruit. |
| Lucenteforte et al. 2010 [34] | Italy | Case-control | 978 (326) | 34–80 | 1.10(0.68–1.77) | Adjust for age, sex, centre year of interview, education, tobacco smoking, history of diabetes and total energy intake. |
| Hu et al. 2012 [35] | Canada | Case-control | 5667 (628) | 20–76 | 1.57(1.09–2.26) | Adjust for sex, age, province, education, body mass index, alcohol drinking, pack-year smoking, total of vegetable and fruit intake, saturated fat and total energy intake. |
Abbreviations: Ref. = references; CI = confidence interval; RR = relative risk; Na = not available.
High versus low analyses
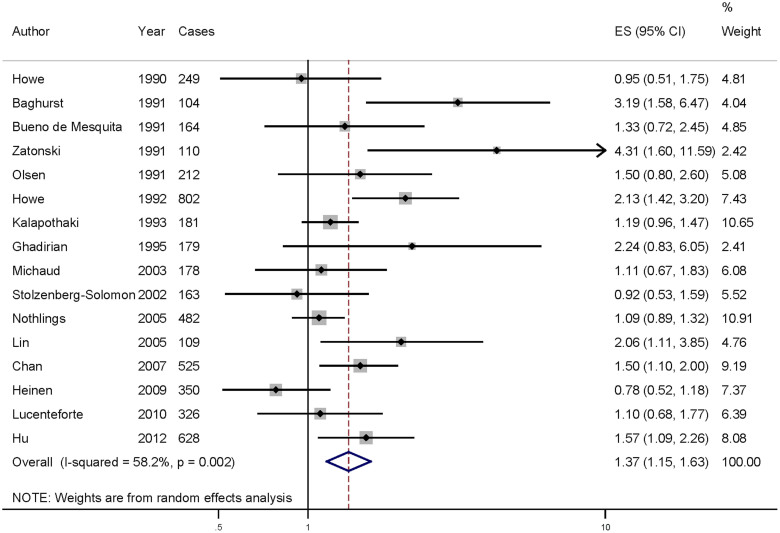
Data from 16 articles including 4513 pancreatic cancer cases were used in this meta-analysis. Six of the studies included in our analysis report that cholesterol intake could increase the risk of pancreatic cancer, while no significant association was reported in 10 studies. Our pooled results suggested that the highest cholesterol intake level compared to the lowest level was significantly associated with the risk of pancreatic cancer [summary RR = 1.371, 95%CI = 1.155–1.627, I2 = 58.2%] (Figure 2).
Figure 2. The forest plot between highest versus lowest categories of cholesterol intake and pancreatic cancer risk.
When the studies were stratified by design, the association was also found in the case-control studies [summary RR = 1.577, 95%CI = 1.298–1.915] but not in the cohort studies. In subgroup analyses for geographic locations, highest cholesterol intake level versus lowest level was significantly associated with the risk of pancreatic cancer in America [summary RR = 1.302, 95%CI = 1.090–1.556], but not in the Europe or others. The details results are summarized in Table 2.
Table 2. Summary risk estimates of the association between cholesterol intake and pancreatic cancer risk.
| No. | No. | Heterogeneity test | |||
|---|---|---|---|---|---|
| Subgroups | (cases) | studies | Risk estimate (95% CI) | I2 (%) | P-value |
| All studies | 4513 | 16 | 1.371(1.155–1.627) | 58.2 | 0.002 |
| Study design | |||||
| Prospective | 1173 | 4 | 1.023(0.871–1.200) | 0.0 | 0.508 |
| Case-control | 3340 | 12 | 1.577(1.298–1.915) | 49.3 | 0.022 |
| Geographic locations | |||||
| America | 2453 | 7 | 1.302(1.090–1.556) | 26.5 | 0.217 |
| Europe | 2096 | 7 | 1.291(0.949–1.756) | 69.0 | 0.004 |
Dose-response analysis
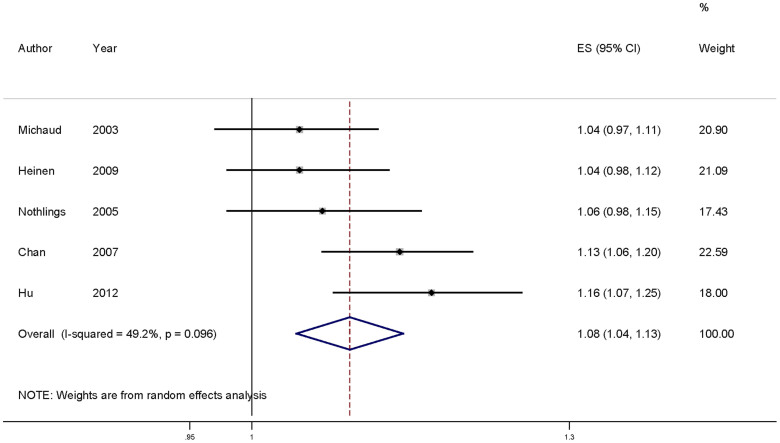
For dose-response analysis, data from 5 studies29,30,32,33,35 comprising 2163 pancreatic cancer cases were used for cholesterol intake and pancreatic cancer risk. We found no evidence of statistically significant departure from linearity (P for nonlinearity = 0.24). Our dose-response analysis indicates that an increase in cholesterol intake of 100 mg/day is statistically significantly associated with an 8% increase in the risk of developing pancreatic cancer (summary RR = 1.08, 95%CI = 1.04–1.13) (Figure 3).
Figure 3. Dose-response meta-analyses of every 100 mg/day increased intake of cholesterol and the risk of pancreatic cancer.
Squares represent study-specific RR, horizontal lines represent 95%CI and diamonds represent summary relative risks.
Sources of heterogeneity and meta-regression
As shown in Figure 2, evidence of heterogeneity (I2 = 58.2%, Pheterogeneity = 0.002) was found in the pooled results. In order to explore the moderate to high between-study heterogeneity founded in several analysis, univariate meta-regression with the covariates of publication year, location where the study was conducted, study design (case-control or cohort), number of cases and source of controls was performed. No significant findings were found in the above-mentioned analysis.
Influence analysis and publication bias
Influence analysis shows that no individual study exerted excessive influence on the association of cholesterol intake and pancreatic cancer risk. Egger's test (P = 0.164) showed no evidence of significant publication bias related to the association between cholesterol intake and pancreatic cancer risk.
Discussion
Finding from this meta-analysis suggests that the higher intake of cholesterol could increase the risk of pancreatic cancer. The associations were also found in subgroups of America and case-control studies of cholesterol intake and pancreatic cancer risk.
Several mechanisms have been proposed to explain the possible role of cholesterol in cancer development. Alterations in lipid and apolipoprotein levels could contribute to cellular inflammation36. Decreased levels of HDL-C and increased low-density lipoprotein cholesterol (LDL-C) and total cholesterol levels have been related to increased levels of proinflammatory cytokines, including tumor necrosis factor-a and interleukin-637. The Framingham Offspring Cohort study suggests that elevated serum iron levels coupled with either high very low density lipoprotein cholesterol or low HDL-C appeared to interact to increase cancer risk38. Another cohort study indicated that independent elevations of either iron or total cholesterol were not significantly related to the development of cancer, but a combination of iron and total cholesterol above the 75th percentile was associated with significant increases in the risk of all cancers and supported the theory that the iron-induced oxidation of serum lipids is important in the pathogenesis of cancer39.
As a meta-analysis of published studies, our findings showed some advantages. First, we report here the first comprehensive dose-response meta-analysis of cholesterol intake and pancreatic cancer risk based on high versus low analysis and dose-response meta-analysis. Second, our study employed a large number of participants, allowing a much greater possibility of reaching reasonable conclusions between cholesterol intake and pancreatic cancer risk. Third, no significant publication bias was found, indicating that our results are stable. However, there were some limitations in this meta-analysis. First of all, a meta-analysis of observational studies is susceptible to potential bias inherent in the original studies, especially for case-control studies. Overstated association may be expected from the case-control studies because of recall or selection bias, and early symptoms in patients may have resulted in a change in dietary habits. In our meta-analysis, the significant association was found in case-control studies, but not in the cohort studies, while only 4 studies included were prospective design. More studies with prospective design are recommended in the future studies. Therefore, this meta-analysis only discovers “an association” between the cause “cholesterol” and the effect “pancreatic cancer”. The increasing of cholesterol intake may result in the risk of pancreatic cancer. Second, measurement errors tend to influence the assessment of dietary intake, which can lead to overestimation of the range of intake and underestimation of the magnitude of the relationship between dietary intake and cancer risk40,41. Third, for the subgroups of geographic locations, the association was only significant in the America, but not in the Europe. And only one study comes from Japan and one from Austrilia. Due to the limitation, the results are applicable to the America, but not referential populations elsewhere. More studies conducted in other countries are required to investigate the association between cholesterol intake and pancreatic cancer risk. Fourth, between-study heterogeneity was found in some analysis in this meta-analysis, but not fully explained by the subgroup analysis and meta-regression. However, other genetic and environment variables, as well as their possible interaction may be potential contributors to this disease-effect unconformity.
In summary, results from this meta-analysis suggest that a high intake of cholesterol might increase the risk of pancreatic cancer, especially in America. Dose-response analysis indicates that the risk of pancreatic cancer estimatedly increases by 8% with every 100 mg/day intake of cholesterol.
Author Contributions
Hongqiang Chen, Shiyong Qin, Minghai Wang, Tao Zhang, Shuguang Zhang, HQC and SYQ designed the experiments; SYC, MHW and TZ collected the data; HQC and SGZ wrote the main manuscript text and all authors reviewed the manuscript.
References
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- Ahrendt S. A. & Pitt H. A. Surgical management of pancreatic cancer. Oncology (Williston Park) 16, 725–34; discussion 34, 36–8, 40, 43 (2002). [PubMed] [Google Scholar]
- Wu C. et al. Genome-wide association study of survival in patients with pancreatic adenocarcinoma. Gut 63, 152–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. et al. Genes-environment interactions in obesity- and diabetes-associated pancreatic cancer: a GWAS data analysis. Cancer Epidemiol Biomarkers Prev 23, 98–106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve P. & Lowenfels A. B. Epidemiology of pancreatic cancer: an update. Dig Dis 28, 645–56 (2010). [DOI] [PubMed] [Google Scholar]
- Aune D. et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 23, 843–52 (2012). [DOI] [PubMed] [Google Scholar]
- Lucenteforte E. et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 23, 374–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Q. et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 47, 1928–37 (2011). [DOI] [PubMed] [Google Scholar]
- Magruder J. T., Elahi D. & Andersen D. K. Diabetes and pancreatic cancer: chicken or egg? Pancreas 40, 339–51 (2011). [DOI] [PubMed] [Google Scholar]
- Johansen D. et al. Metabolic factors and the risk of pancreatic cancer: a prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol Biomarkers Prev 19, 2307–17 (2010). [DOI] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–9 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N. & Bellocco R. Generalized least squares for trend estimation of summarized dose-response data. Stata J 6, 40–57 (2006). [Google Scholar]
- Orsini N. et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S. C., Giovannucci E. & Wolk A. Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 99, 64–76 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Z. M. et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr 93, 506–15 (2011). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Controlling the risk of spurious findings from meta-regression. Stat Med 23, 1663–82 (2004). [DOI] [PubMed] [Google Scholar]
- Tobias A. Assessing the in fluence of a single study in the meta-analysis estimate. Stata Tech Bull 47, 15–7 (1999). [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G. R., Jain M. & Miller A. B. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int J Cancer 45, 604–8 (1990). [DOI] [PubMed] [Google Scholar]
- Baghurst P. A. et al. A case-control study of diet and cancer of the pancreas. Am J Epidemiol 134, 167–79 (1991). [DOI] [PubMed] [Google Scholar]
- Bueno de Mesquita H. B., Maisonneuve P., Runia S. & Moerman C. J. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int J Cancer 48, 540–9 (1991). [DOI] [PubMed] [Google Scholar]
- Zatonski W. et al. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. Int J Cancer 48, 390–4 (1991). [DOI] [PubMed] [Google Scholar]
- Olsen G. W. et al. Nutrients and pancreatic cancer: a population-based case-control study. Cancer Causes Control 2, 291–7 (1991). [DOI] [PubMed] [Google Scholar]
- Howe G. R. et al. A collaborative case-control study of nutrient intake and pancreatic cancer within the search programme. Int J Cancer 51, 365–72 (1992). [DOI] [PubMed] [Google Scholar]
- Kalapothaki V. et al. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer Causes Control 4, 383–9 (1993). [DOI] [PubMed] [Google Scholar]
- Ghadirian P., Baillargeon J., Simard A. & Perret C. Food habits and pancreatic cancer: a case-control study of the Francophone community in Montreal, Canada. Cancer Epidemiol Biomarkers Prev 4, 895–9 (1995). [PubMed] [Google Scholar]
- Stolzenberg-Solomon R. Z. et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol 155, 783–92 (2002). [DOI] [PubMed] [Google Scholar]
- Michaud D. S. et al. Dietary meat, dairy products, fat, and cholesterol and pancreatic cancer risk in a prospective study. Am J Epidemiol 157, 1115–25 (2003). [DOI] [PubMed] [Google Scholar]
- Nothlings U. et al. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst 97, 1458–65 (2005). [DOI] [PubMed] [Google Scholar]
- Lin Y. et al. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. J Gastroenterol 40, 297–301 (2005). [DOI] [PubMed] [Google Scholar]
- Chan J. M., Wang F. & Holly E. A. Pancreatic cancer, animal protein and dietary fat in a population-based study, San Francisco Bay Area, California. Cancer Causes Control 18, 1153–67 (2007). [DOI] [PubMed] [Google Scholar]
- Heinen M. M., Verhage B. A., Goldbohm R. A. & van den Brandt P. A. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int J Cancer 125, 1118–26 (2009). [DOI] [PubMed] [Google Scholar]
- Lucenteforte E. et al. Macronutrients, fatty acids, cholesterol and pancreatic cancer. Eur J Cancer 46, 581–7 (2010). [DOI] [PubMed] [Google Scholar]
- Hu J. et al. Dietary cholesterol intake and cancer. Ann Oncol 23, 491–500 (2012). [DOI] [PubMed] [Google Scholar]
- Ferretti G. et al. Structural modifications of HDL and functional consequences. Atherosclerosis 184, 1–7 (2006). [DOI] [PubMed] [Google Scholar]
- Haddy N. et al. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis 170, 277–83 (2003). [DOI] [PubMed] [Google Scholar]
- Mainous A. G. 3rd et al. Iron, lipids, and risk of cancer in the Framingham Offspring cohort. Am J Epidemiol 161, 1115–22 (2005). [DOI] [PubMed] [Google Scholar]
- Wells B. J., Mainous A. G. 3rd, Everett C. J. & Gill J. M. Iron, cholesterol, and the risk of cancer in an 18-year cohort. Asian Pac J Cancer Prev 6, 505–9 (2005). [PubMed] [Google Scholar]
- Prentice R. L. Dietary assessment and the reliability of nutritional epidemiology reports. Lancet 362, 182–3 (2003). [DOI] [PubMed] [Google Scholar]
- Willett W. C. et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122, 51–65 (1985). [DOI] [PubMed] [Google Scholar]