Abstract
Camptothecin (CPT) belongs to a group of monoterpenoidindole alkaloids (TIAs) and its derivatives such as irinothecan and topothecan have been widely used worldwide for the treatment of cancer, giving rise to rapidly increasing market demands. Genes from Catharanthus roseus encoding strictosidine synthase (STR) and geraniol 10-hydroxylase (G10H), were separately and simultaneously introduced into Ophiorrhiza pumila hairy roots. Overexpression of individual G10H (G lines) significantly improved CPT production with respect to non-transgenic hairy root cultures (NC line) and single STR overexpressing lines (S lines), indicating that G10H plays a more important role in stimulating CPT accumulation than STR in O. pumila. Furthermore, co-overexpression of G10H and STR genes (SG Lines) caused a 56% increase on the yields of CPT compared to NC line and single gene transgenic lines, showed that simultaneous introduction of G10H and STR can produce a synergistic effect on CPT biosynthesis in O. pumila. The MTT assay results indicated that CPT extracted from different lines showed similar anti-tumor activity, suggesting that transgenic O. pumila hairy root lines could be an alternative approach to obtain CPT. To our knowledge, this is the first report on the enhancement of CPT production in O. pumila employing a metabolic engineering strategy.
Camptothecin (CPT), originally isolated from the bark of the Chinese happy tree Camptotheca acuminata1, is a modified terpenoidindole alkaloid (TIA) and exhibits excellent anti-tumor activity, which is due to its ability to inhibit DNA topoisomerase I2. Its two derivatives, irinothecan and topothecan, have been approved by the US Food and Drug Administration (FDA) in 1994 and used extensively for the treatment of metastatic colorectal cancer, ovarian cancer, cervical cancer and small cell lung cancer throughout the world3. Besides their anti-tumor activity, CPT derivatives have also been found to show good activity against viruses such as the human immunodeficiency virus (HIV)4. More derivatives of CPT are now in clinical trials, such as 9-nitrocamptothecin, 9-aminocamptothecin and rubitecan5, which will potentially result in growing demand for these drugs in the future. Due to the excellent pharmacological activity of CPT derivatives, the world market for these CPT analogues has increased rapidly. The combined sales of two CPT analogs (irinotecan and topotecan) in only 2008 had reached 2.2 billion US dollars and is expected to increase further6,7. At present, the annual production of CPT throughout the world is only 600 kg, while approximately 3000 kg of CPT is needed in the international market8. However, all the CPT derivatives which are consumed are synthesized from natural CPT, which is mainly obtained by extraction from the trees C. acuminata and Nothapodytes foetida1,9. Since the limited supply of CPT is from the above two woody plants with slow growth rates and low yields, it is an important and urgent task to develop sustainable and alternative production sources of CPT in order to resolve the worldwide scarcity of natural sources of CPT.
Due to several advantages such as rapid growth rate, unlimited branching, and genetic stability, in vitro hairy root induced by Agrobacterium rhizogenes has been considered as an alternative means to produce high-value secondary metabolites including CPT10,11. To overcome the low yield of active components, the development and application of metabolic engineering strategies provides a promising approach to increase CPT production by introducing multiple CPT biosynthetic genes into CPT-producing plant cells or tissue12,13, followed by culturing transgenic cell lines, hairy roots or regenerated plants on a large scale. Therefore, detailed understanding of the CPT biosynthesis pathway, especially for the committed steps, will be helpful to improve CPT production by genetic manipulation.
Camptothecin belongs to the family of monoterpenoidindole alkaloids (TIA), which are found in some plant species such as Apocynaceae, Loganiaceae, Rubiaceae and Nyssaceae. Although CPT is one of the most promising natural plant-derived anti-tumor drugs, its biosynthetic pathway and regulatory mechanism of production remain unclear14. The biosynthesis of CPT is a complicated process and involves several catalytic steps (Fig. 1). Tryptamine is synthesized via the shikimate pathway, and the secologanin part is synthesized via the MEP pathway15. Strictosidine is then converted to strictosamide, but the remaining details and precise intermediates between strictosamide and CPT are not completely defined6. All TIAs including CPT are derived from the common precursor strictosidine, which is formed via the condensation of the indoletryptamine and the monoterpenoid secologanin7. This important step is catalyzed by strictosidine synthase (STR), which is considered to be a key enzyme in TIA biosynthesis. The STR gene has been successfully isolated from Rauvolfia serpentina16, C. roseus17, O. pumila18 and O. japonica7. Over-expression of STR gene in transgenic C. roseus showed ten-fold higher STR activity than wild-type cultures, which exhibited a great enhancement effect on TIA biosynthesis18. Geraniol 10-hydroxylase (G10H), being a cytochrome P450 monooxygenase, can hydroxylate geraniol at the C-10 position to generate 10-hydroxy-geraniol, which is considered to be a committed step in the biosynthesis of secologanin and even TIAs19. The G10H gene was firstly cloned from C. roseus19 and recently from C. acuminata20. G10H has been reported to be a rate-limiting enzyme in the biosynthesis of terpenoid indole alkaloids in transgenic C. roseus21,22,23.
Figure 1. Camptothecin biosynthetic pathway in Ophiorrhiza pumila.
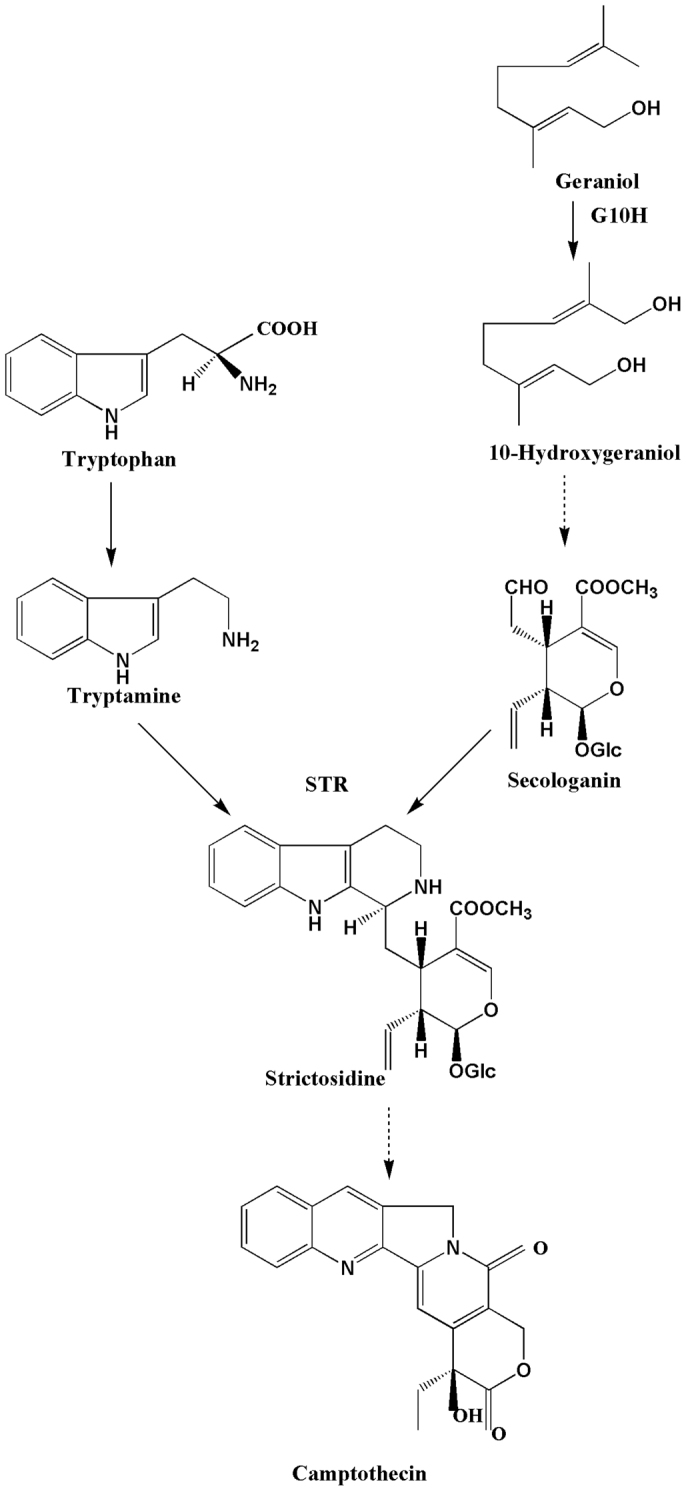
Dotted line arrows indicate multiple steps between intermediates. G10H:geraniol 10-hydroxylase, STR:strictosidine synthase.
Although much is known about biosynthesis of TIA in C. roseus, little is known about regulation of CPT biosynthesis in CPT-producing plants24. The difficulties in establishing a stable transformation system for the CPT-producing woody plant C. acuminata led to few successful reports on introducing a CPT biosynthetic gene into C. acuminata by metabolic engineering in the past two decades25, although much effort was put into optimization of transformation procedures and conditions for C. acuminata. The findings that CPT exists in herbs such as O. mungos26 and the established hairy root culture system of O. pumila provided an alternative experimental model system for CPT biosynthesis and production27. Until now, there has been no report on the introduction of STR and/or G10H genes into any CPT-producing plants including O. pumila. In this present work, we investigated the effects of overexpressing CrG10H and CrSTR individually and simultaneously in hairy root cultures of O. pumila.
Results
Optimization of O. pumila hairy root induction procedure
An efficient sterile plant culture system was established and to induce hairy root formation in O. pumila (Fig. 2). In this study, different explants derived from lamina, petioles and stems from O. pumila sterile plants were co-cultivated with Agrobacterium A4, 15834, and C58C1 on hormone free B5 medium for hairy root induction. Hairy root formation occurred most rapidly with strain C58C1 (induced about 10–15 days after infection, Fig. 2) among the three strains tested, suggesting that the modified strain C58C1 was more competent than the other strains as reported for Anisodus acutangulus10, and was therefore chosen for further experiments. The influence of bacterial strains on transformation frequency has been previously documented in different plant species28. The types of explants also affected hairy root induction frequencies. Hairy roots could be induced from wounded sites on the different explants including lamina, petioles and stems after infection with C58C1. The hairy root induction efficiency of lamina and petioles only reached to about 2% and 8%, respectively. And the explants often went brown, the hairy root derived from lamina and petioles grew slowly. The highest hairy root induction efficiency of 80.4% was achieved using modified strain C58C1 with stem explants, suggesting that stems were more susceptible than lamina and petioles. Out of three tested media (MS, GB5 and White), the hairy root in B5 medium grew much better with normal branching than the other two media (White and MS). After about 14 days, the hairy roots in B5 medium began to branch largely, whereas hairy roots in the other two media didn't branch and grew slowly, became abnormal and aged. The results suggested the liquid B5 medium was the most suitable for O. pumila hairy root growth. Apical tips of hairy roots (1 mg segments) were inoculated in the liquid medium, and the fresh weight of the tissue typically reached about 3 g after 45 days of culture. The above efficient hairy root induction system for the medicinal plant O. pumila was successfully developed and optimized for further genetic transformation.
Figure 2. Flow chart of transgenic O. pumila hairy roots.
(A). soil-grown O. pumila plant; (B). aseptic plant seedling; (C). 2d-pre-cultured O. pumila stems; (D). hairy roots generated from wound sites after infection by Agrobacterium rhizogenes; (E). hairy root on solid B5 medium; (F). hairy root cultures in the liquid B5 medium.
Acquisition of transgenic O. pumila hairy roots with CrSTR and CrG10H
Three plasmids containing the cDNAs encoding CrSTR and/or CrG10H under the control of the CaMV 35S promoter were separately introduced into O. pumila hairy roots by using disarmed A. tumefaciens C58C1 strain after infection of young stems of O. pumila. Hairy root lines generated from transformations with an empty vector that did not contain CrSTR or CrG10H genes was used as a control (NC line). After two weeks, transgenic O. pumila hairy roots were generated with phenotypic characteristics such as being long, thin and golden yellow (Fig. 2).The abbreviations S, G, and SG refer to the transgenic hairy root lines generated from CrSTR single gene, CrG10H single gene, and CrSTR/CrG10H double gene transformations, respectively. In total, 53 STR single gene transformed lines (S line), 34 G10H single gene transformed lines (G line) and 95 STR-G10H double gene transformed lines (SG line) were generated, and 48 S, 31 G, 88 SG hygromycin-resistant (2 mg/L) hairy root lines with normal phenotype were maintained for PCR analysis (Table 1).
Table 1. Gene constructs and derived root cultures.
| Number of root lines | ||||
|---|---|---|---|---|
| Gene constructs | Total | Hgr+ | PCR-positive | Established root lines |
| STR | 53 | 48 | 17 | S8, S14, S16, S26 |
| G10H | 34 | 31 | 5 | G1, G3, G10 |
| G10H + STR | 95 | 88 | 21 | SG1, SG20, SG26, SG28, SG43 |
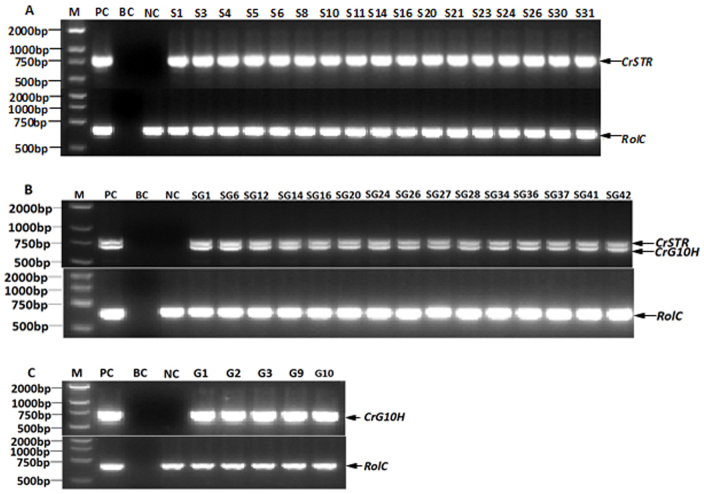
Genomic DNA of all the above independent hairy roots were isolated and used for PCR analysis using primers specially designed to overlap part of the CrG10H, CrSTR and the CaMV 35S promoter sequences. The C58C1 strains harboring plasmids pCAMBIA1304+-CrSTR, pCAMBIA1304+-CrG10H and pCAMBIA1304+-CrSTR-CrG10H were also amplified as positive controls (PC). Control hairy roots generated from empty vector transformations were used as negative controls (NC), and water was used as a blank control (BC). The rolC gene as a marker gene of C58C1 was also detected in all the PCR-positive clones (Fig. 3). No amplicons of CrG10H and CrSTR were detected in the NC and BC lines. In total, the PCR-positive clones amounted to 25.7% (43/167), with 35% (17/48) for S lines, 16% (5/31) for G lines, and 24% (21/88) for SG lines (SG). These results preliminarily suggest that the CrSTR and CrG10H genes were introduced into the genome of S and G lines, while both CrSTR and CrG10H genes were introduced into SG lines. Here we randomly selected 4 S, 3 G and 5 SG of PCR-positive lines with normal growth phenotypes for further establishment of hairy root culture lines for qRT-PCR and HPLC experiments (Table 1).
Figure 3. Representative results of transgenic hairy root by PCR identification.
(A). PCR analysis of CrSTR and RolC for S lines; (B). PCR analysis of CrSTR, CrG10H and RolC gene for SG lines; (C). PCR analysis of CrG10H and RolC gene for G lines; M: marker DL2000; PC: C58C1 strains harboring expression vector as positive control; NC: empty control (hairy root generate from empty-vector transformation) as negative control; BC: ddH2O as blank control.
Transcript analysis of CrG10H and CrSTR gene in hairy roots
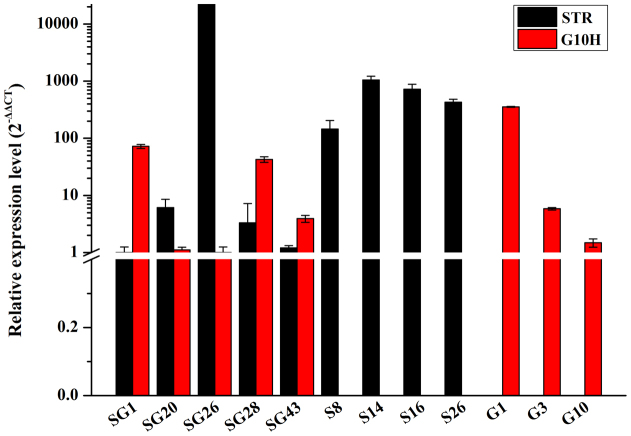
qRT-PCR was determined to further analyze the expression level of the exogenous target gene (CrG10H and CrSTR) and reference gene alpha-tubulin (Tub) was used for the internal control gene. qRT-PCR results indicated that CrG10H and CrSTR effectively expressed with varying levels in the S, G and SG lines, respectively (Fig. 4), while no expression can be detected in the NC line as expected. Among all the transgenic lines, the G1 line had the highest expression level of CrG10H, while SG26 had the highest expression level of CrSTR (Fig. 4). These results indicated that CrG10H and CrSTR genes were expressed in corresponding transgenic lines, but with varied expression levels.
Figure 4. Transcription level of CrSTR and CrG10H in transgenic O. pumila hairy roots.
SG: CrG10H + CrSTR double gene transformed lines; S:CrSTR single gene transformed lines; G: CrG10H single gene transformed lines.
Accumulation patterns of camptothecin
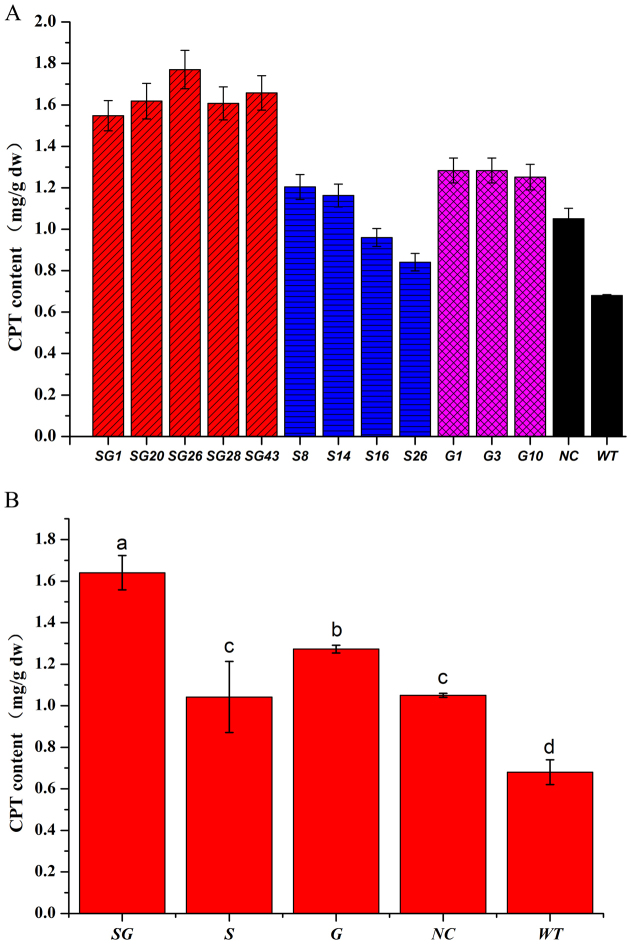
The hairy roots of O. pumila were inoculated into 50 mL liquid B5 medium and cultured for 6 weeks followed by collection for further study. The camptothecin that accumulated in the hairy roots was detected and quantified by HPLC analysis (Fig. 5). Higher levels of CPT ranging from 1.25 to 1.28 mg/g existed in G10H-transformed lines than in NC hairy root (1.05 mg/g) and wild-type plant root (0.68 mg/g dw) (Fig. 5). The average CPT content (1.27 mg/g) of all the four G lines was obviously higher than NC and wild-type root, implying that overexpression of G10H can efficiently promote the accumulation of CPT in O. pumila. The levels of CPT in S lines were variable with a range of 0.83-1.20 mg/g dw, which was higher than wild-type roots but not as good as in G lines, implying that the effect of single STR is much weaker than G10H for CPT biosynthesis in O. pumila. Interestingly, hairy root lines co-overexpressing G10H and STR produced higher levels of CPT with variation from 1.54 to 1.77 mg/g when compared to NC hairy roots and wild-type roots, and even higher than single overexpression of G10H or STR (Fig. 5). These results indicated that co-expression of G10H and STR can produce a synergistic effect for CPT accumulation in O. pumila.
Figure 5. CPT contents in transgenic hairy roots of O. pumila.
SG:CrG10H + CrSTR double gene transformed lines; S:CrSTR single gene transformed lines; G: CrG10H single gene transformed lines; NC: hairy roots induced by C58C1 with empty vector; WT: root of wild-type O. pumila.
One-way analysis of variance (ANOVA) was used to detect the difference of the average total content of CPT among S, G, SG, NC and WT lines to analyze the functional role of STR or/and G10H in CPT biosynthesis. It showed that the average content of CPT was higher in S lines (1.04 mg/g) and NC lines (1.05 mg/g) than WT (0.68 mg/g), while much higher in G (1.27 mg/g) and SG (1.64 mg/g) lines compared with NC and WT lines (P < 0.05) (Fig. 5). Overexpression of G10H in G and SG lines both effectively promote CPT biosynthesis, suggesting that G10H is a key regulation target gene for metabolic engineering of CPT biosynthetic pathway. CPT production is higher in SG lines than G lines, implying that STR also plays an important role in CPT synthesis under some conditions (when G10H was overexpressed). Therefore, compared with a single gene, co-introduction of G10H and STR can produce a coordination effect for camptothecin biosynthesis in O. pumila hairy root.
Inhibitory activity on myelogenous leukemia cells
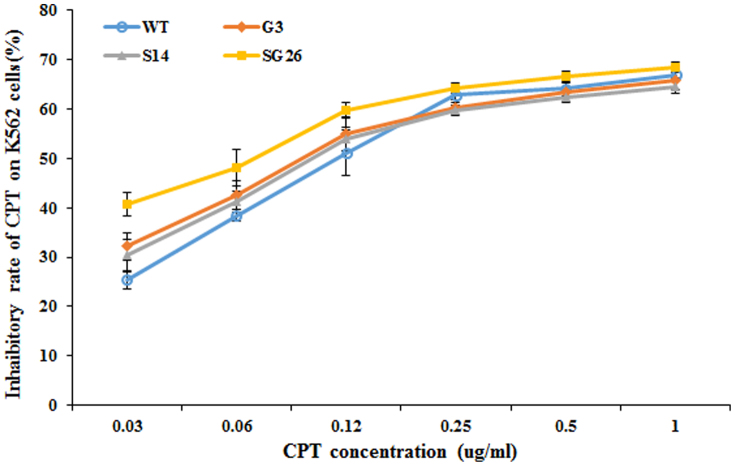
The anti-tumor activity of CPT extracted from different hairy root lines was estimated by the MTT test in this study. The crude CPT extracts from some selected transgenic O. pumila hairy root lines and wild type O. pumila plant root were used for the MTT test, to compare their anti-tumor activities on the human chronic myelogenous leukemia K562 cell line. The MTT test showed that crude CPT extracts from line S14, G1, SG26 and WT at the same concentration with 6 levels showed similar anti-tumor activity (Fig. 6). When CPT from various sources was normalized at concentrations of 100 ug/ml, the inhibiting rate varied from 64.59% to 68.57%. The MTT assay results showed that there are no significant differences of anti-tumor activity among these transgenic lines and WT lines, and it can be deduced that genetic engineering did not change CPT activity.
Figure 6. The inhibitory rate of different transgenic line on K562 cells.
Discussion
Transgenic hairy root cultures have been shown to have the potential to enhance production of secondary metabolites in different plants by metabolic engineering12,13,29,30,31, but an effective transformation system is necessary for successful metabolic engineering of hairy root. The supply of camptothecin mainly relies on extraction from the tree C. acuminata at present32, but it is very hard to obtain transgenic lines of C. acuminata by genetic engineering. Weedy plants such as O. pumila were also found to produce CPT26, which provided an alternative experimental model system for CPT biosynthesis and production. Since CPT mainly existed in the roots of perennial medicinal herb O. pumila, which rarely distributed in limited regions in China and other Asia countries, collection of its roots as drug source may result in its extinction. In vitro hairy root culture is regarded as a promising strategy to obtain CPT without threatening the survival of related resource plants. A hairy root culture system of O. pumila with good CPT production ability has been reported for the first time by scientists in Japan, but with too long time span for induction of hairy roots (emerging 80 days after infection)27. In this study, we successfully optimized the procedures of hairy root induction from O. pumila and shortened the time taken for the emergence of hairy roots to only 10–15 days with high efficiency, which greatly simplifies the operation, and obviously improves research efficiency. Meanwhile, CPT content was obviously enhanced in transgenic O. pumila hairy roots using the above optimized system by means of metabolic engineering. Further MTT assay results showed that there are no obvious differences on anti-tumor activity of CPT extracted from transgenic O. pumila hairy root lines and WT plant roots. The above results suggested transgenic O. pumila hairy root lines could be an alternatively promising approach to produce more CPT in place of natural plant resources including O. pumila, which will reduce environmental concerns.
G10H, a cytochrome P450 monooxygenase, was considered to be a key enzyme involved in the biosynthesis of TIAs in different plant species19. Previous studies showed that G10H is a possible bottleneck for TIAs production and a good candidate target for genetic manipulation21. However, there is no report on the effect of overexpression of G10H in CPT-producing plants. In our study, the CPT content of G10H-transformed lines ranged from 1.25 to 1.28 mg/g, with an average CPT content (1.27 mg/g) of all the five G lines being obviously higher than NC and wild-type root (Fig 5). The above results indicate that expression of CrG10H obviously enhanced production of CPT in O. pumila, which was in good agreement with previous results that overexpression of CrG10H increased accumulation of TIAs in C. roseus plants and hairy roots22,23. Our study, for the first time, provides direct evidence that overexpression of heterologous G10H is sufficient to promote CPT synthesis in O. pumila, suggesting that G10H is an effective regulation target for metabolic engineering of CPT synthesis at least in O. pumila. Furthermore, overexpression of single CrG10H showed much more powerful driving effect than single CrSTR in increasing the production of CPT, which suggested that G10H may play a more important role in the regulation of CPT synthesis.
STR condenses tryptamine and the iridoid secologanin to yield strictosidine which is the universal precursor of TIAs including CPT7. Overexpression of STR in transgenic C. roseus showed ten-fold higher STR activity than wild-type cultures, which indicated that STR plays a critical role in biosynthesis of TIAs18. However, the real effect of the overexpression of the STR gene in CPT-producing plants remains unknown. In the present study, the camptothecin content in S lines varied from 0.83 mg/g to 1.20 mg/g, which are all higher than that of wild type root (0.68 mg/g DW). However, the CPT content was increased in two S lines (S8, S14), but decreased in the other two S lines (S16, S26) when compared to the non-transgenic control line (NC, 1.05 mg/g). The above puzzling result makes it hard to generalize the overall effect of a single STR gene on CPT biosynthesis, reflecting some kind of uncertainty in regulating metabolite flux through the biosynthetic pathway by manipulation of only a single enzyme31. The introduction of STR in O. pumila hairy roots resulted in mixed results, and the average effect of STR overexpression is not as good as G10H (Fig 5). One possible explanation for the result is that the effect of STR overexpression may be blocked by another key enzymes such as G10H.
The “push-pull” strategy has been successfully applied to increase pharmaceuticals and flavors yields in many plants such as C. roseus, Solanum lycopersicum, Artemisia annua, Salvia miltiorrhiza and Anisodus acutangulus, respectively12,13,31,33,34. But sometimes overexpression of a single gene encoding a rate-limiting enzyme to increase contents of secondary metabolites leads to unexpected consequences, and simultaneous overexpression of several enzymes within the same pathway is more effective to increases metabolites production31. In our study, SG lines possessed higher average CPT content (1.64 mg/g DW) than S lines (1.04 mg/g DW) and G lines (1.27 mg/g DW) (Fig 5B), and all the tested SG lines have a higher level of camptothecin than other lines, which varied from 1.54 mg/g to 1.77 mg/g. The CPT content of SG lines is relatively higher than the reported CPT content in the 5-week cultured O. pumila hairy roots (0.1% per dry weigh) and the intact plant (approx. 0.03–0.04% dry weight in the leaves, 0.1% dry weight in the young roots)27. Expression of single CrSTR has less effect than CrG10H in increasing the camptothecin content, but co-expression of CrG10H and CrSTR displayed a steady and more powerful pulling effect compared with S lines and G lines. The results suggested that multiple key enzymes can effectively promote the accumulation of camptothecin. Alternatively, introduction of a global transcription factor that positively regulates the pathway is more effective than modifying multiple biosynthetic pathway genes at one time12,31. Recently, we found a considerable increase in tanshinones in transgenic S. miltiorrhiza hairy roots after elicitor treatments35,36. Thus, transgenic hairy root technology coupled with elicitor treatments may be another promising strategy to increase CPT yield in the near future.
Methods
Plant materials
Wild-type O. pumila plants were collected from Fujian Province, China. Shoot tips of wild type plants were rinsed overnight with running tap water, soaked in 70% (v/v) ethanol for 30 sec and then in 0.1% (w/v) mercuric chloride for 10–15 min, and finally thoroughly rinsed four times with sterilized distilled water. The treated shoot tips were cultured on solid Murashige and Skoog (MS) medium to obtain sterile O. pumila plants using a previously reported method8. Sterile O. pumila plants were cultured and maintained at 25°C under a 14 h light/10 h dark photoperiod with light provided by cool white fluorescent lamps at an intensity of 350 μmol m−2 s−1.
Construction of plant expression vectors
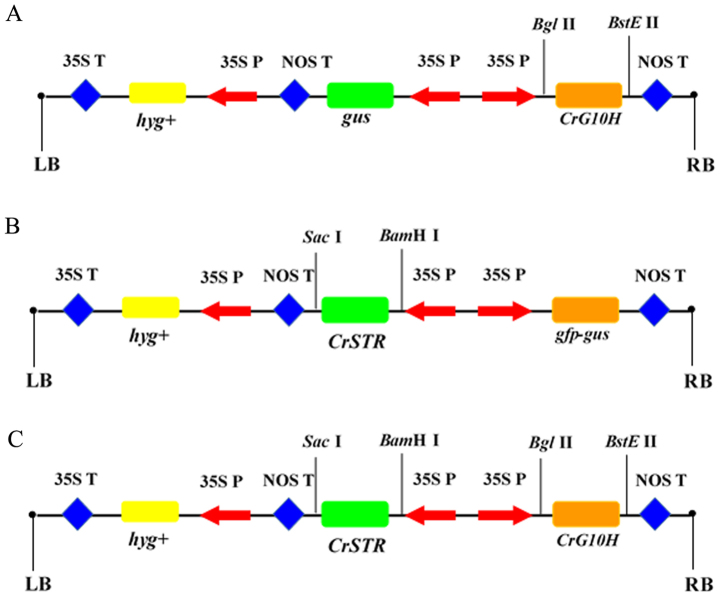
The complete G10H and STR cDNAs were cloned from the sterile seedlings of C. roseus according to the sequences reported in NCBI (X61932.1 for STR and AJ251269.1 for G10H). The full-length ORF of CrG10H cDNA was inserted into pCAMBIA1304+12,13,30 in place of the mGFP5 and gusA genes to generate a pCAMBIA1304+-CrG10H expression vector containing the CrG10H gene under the digestion of BglII and BstEII (Takara) (Fig. 7A). Similarly, the full-length CrSTR cDNA was inserted into pCAMBIA1304+ in place of the gus gene to generate a pCAMBIA1304+-CrSTR expression vector containing the CrSTR gene under the digestion of SacI and BamHI (Takara) (Fig. 7B). On the basis of pCAMBIA1304+-CrSTR, the full-length CrG10H cDNA was used to replace the mGFP5 and gusA gene in pCAMBIA1304+-CrSTR under the digestion of BglII and BstEII (Takara Biotech Co., Ltd) to generate the expression vector pCAMBIA1304+-CrSTR-CrG10H containing both CrSTR and CrG10H genes (Fig. 7C). The genes CrSTR or/and CrG10H were under the control of the strong cauliflower mosaic virus (CaMV) 35S promoter. The blank vector pCAMBIA1304+ without exogenous genes was used as the control. The disarmed Agribacterium tumefaciens strain C58C1 harboring both the Agribacterium rhizogenes Ri plasmid pRiA411,12,30 and each of the four plasmids constructed above was used for plant genetic transformation.
Figure 7. The scheme of recombinant vector.
(A): pCAMBIA1304+-CrSTR, (B): pCAMBIA1304+-CrG10H, (C): pCAMBIA1304+-CrSTR+CrG10H 35SP: promoter of CaMV35S, 35ST:terminator of CaMV35S, NOST: terminator of noster, LB: left border of T-DNA, RB: right border of T-DNA, hyg+: hygromycin.
Plant transformation and hairy root culture
Different explants including leaf blades, petioles or stems were isolated from 4-week-old in vitro grown young aseptic O. pumila plants cultured on B5 medium supplemented with 100 mg/L LH, 0.1 mg/L NAA and 0.1 mg/L KT. The isolated explants were cut into small pieces (about 1 cm) followed by pre-incubation on B5 in the dark for 2 days, and then submerged in three A. rhizogenes strains (A4, 15834, and C58C1) for 10 min, blot-dried on sterile filter paper and then placed on B5 medium in the dark for 2 days. The explants were subsequently rinsed with sterile water, and transferred onto B5 medium supplemented with 300 mg/L carbenicillin. The apical tips of hairy root induced from transformed explants were excised and sub-cultured on B5 solid medium with 200 mg/L carbenicillin at 2-week intervals, the concentration of carbenicillin was gradually lowered and finally omitted after 2 month. When cultures had been cleared of agrobacterium, hairy roots were transferred onto B5 solid medium and the apical tips of rapidly growing hairy roots were inoculated into 50 mL liquid B5 media in 250 mL-shake flasks to establish hairy root lines, on a shaker with a gyratory rate of 120 rpm at 25°C for further study.
DNA isolation and PCR analysis
Genomic DNA was isolated from harvested hairy root samples using the cetyltrimethyl ammonium bromide (CTAB) method37. The DNA was then used as the template in PCR analysis for detecting the presence of CrSTR and CrG10H genes in transgenic hairy roots. The sequences of 35SF23 (forward primer) located at the 35S promoter of the pBI121 vector and CrG10HR214 (reverse primer) in the interior of the CrG10H gene for the CrG10H detection were as follows: 5′-GAGGACCTAACAGAACTCGCC-3′ and 5′-ACGATTGTAGTGATCTGGCCTAATT-3′, respectively, and those of primers 35SF23 at the 35S promoter of the pCAMBIA1304 plasmid and CrSTR (reverse primer) in the interior of the CrSTR gene used for the amplification of the CrSTR gene were as follows: 5′-GAGGACCTAACAGAACTCGCC-3′ and 5′- TCCTCCCACACAATGGTCTTTT -3′, respectively. PCR was carried out in a total volume of 25 μL reaction mixture, containing 1 μL of each primer, 0.5 μL of 10 mmol/L dNTPs, 2.5 μL of 10× PCR buffer (Mg2+ plus), 1 μL genomic DNA as template and 1.25 units of Taq DNA polymerase (TaKaRa), following the protocol: the DNA template was denatured at 94°C for 10 min followed by 35 cycles of amplification (94°C for 45 s, 54°C for 60 s, 72°C for 60 s) and inoculated at 72°C for 10 min extension.
cDNA synthesis and qPCR amplification
Total RNA was extracted from the hairy root samples using the RNA prep pure plant kit (Tiangen Biotech.) according to the manufacturer's protocol. The quality and concentration of RNA was checked and stored as described before38. Aliquots of total RNA (1 μg) were used as templates to generate cDNA with Oligo dT primer using avian myeloblastosis virus (AMV) reverse transcriptase (TaKaRa) in Bio-Rad T100 Thermal Cycler (Bio-Rad, USA), and then for further quantitative real-time fluorescence analysis. Gene-specific primers CrG10HQF486 (5′-GAGCGGAGAAGCGGTTGAC-3′), CrG10HQR641 (5′-TTCCCCGCCTCAACCATTA-3′), CrSTRQF171 (5′-AGGGTTCTACACTTCCGTCCAA-3′) and CrSTRQR322 (5′-TCCTCCCACACAATGGTCTTTT-3′) were used to detect the expression level of CrG10H and CrSTR, respectively. And alpha-tubulin gene (TUB-QF: CCAGATAACTTTGTTTTCGG, TUB-QR: GTGAACTCCATTTCATCCAT) was used as reference gene39. The qPCR amplification was performed in an Applied Biosystem Step One (Applied Biosystem). The relative Ct (threshold cycle value) method was used to estimate the initial amount of template present in the reactions by Applied Biosystems SDS 2.0.
Determination of camptothecin by HPLC
The 6-week-old hairy roots were dried to a constant weight in an oven at 50°C, and grinded into powder and weighed. 95% ethanol (analytic grade) was then added to the powder at a ratio of 1:50 (W/V) and the mixture was concentrated to 2 mL, 95% ethanol was added to redissolve it at a ratio of 1:10 (V/V) overnight after a 60 min's ultrasonication. The extracts were clarified twice by centrifugation at 12,000 rpm for 10 min at 4°C, and the supernatant was collected and dried at 50°C using a vortex evaporator. Then the powder was dissolved in 2 mL methanol and passed through a 0.22 mm nylon filter (Pall Corporation). HPLC analysis was performed to determine the contents of CPT on a sepax sapphire C18 reversed-phase symmetry column (4.6 × 250 mm, 5 μM). The mobile phase consisted of 35% acetonitrile (HPLC grade) and 75% double distilled water. The flow rate was 1.0 mL/min, and the injection volume was 20 μL. The chromatogram was monitored at 220 nm on a Hitachi Diode Array Detector L2455. CPT standard substance was dissolved in methanol at 100 μg/ml, the retention time of the CPT was 9.58 min. The accuracy and reproducibility of HPLC analysis were confirmed by analyzing different quantities of the sample including the control group. Samples were quantified using standard curve fitting with linear regression.
Anti-tumor activity of transgenic hairy roots by MTT analysis
The 6-week-old hairy roots from line S14, G1, SG26 and WT were dried and dissolved in methanol using the same extraction method for HPLC determination, and used to measure in vitro anti-tumor activity by MTT assay as described previously40, and methanol as negative control. The crude CPT extracts were diluted to the same concentration with a serial of grades (100 ug/ml, 50 ug/ml, 25 ug/ml, 12.5 ug/ml, 6.25 ug/ml, 3.13 ug/ml) of CPT using methanol and the final concentration of samples were adjusted using RPMI 1640 medium in a ratio of 1:100. K562 cells were cultured and the cell density was adjusted to 2 × 105/mL in a total of 100 ul RPMI 1640 medium supplemented with 10% fetal bovine serum. Equal volume of mixture containing K562 cells and tested samples or methanol was added to the plate respectively and incubated at 37°C for 44 h in a humidified atmosphere consisting of 5% CO2, and 6–8 replicates were set for each sample. Then, 10 ul MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma-Aldrich) was added to the medium at 5 mg/ml, and the medium was incubated for an additional 4 h. And then 100 ul sodium dodecyl sulfonate (SDS) at a concentration of 10% was added to wells of culturing plates and formazan absorbance was measured with a spectrophotometer (Biomek 1000) at test and reference wavelengths of 570 nm.
Statistical analysis
All the experiments including PCR identification, qRT-PCR, HPLC analysis and anti-tumor activity analysis were repeated three times. Results of CPT content are presented as mean values ± S.D. The error bars are due to biological triplicates. The statistical significance of CPT content difference was analyzed by one sample t test and the errors of different hairy root clones were used in the one-way analysis of variance (ANOVA) using SPSS 11.5 software (SPSS, Inc.).
Author Contributions
G.K. and L.C. designed research, L.C., X.N., Q.J., X.T., Y.Y. and C.W. performed research, L.C., G.K. and D.S.Z. analyzed data, G.K., L.C., X.N. and D.Z. wrote the paper.
Acknowledgments
This work was supported by National Natural Science Fund (31201261, 31270007, 30900110), New Century Talent Project (NECT-13-0902), Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (131041), Shanghai Talent Development Fund, Shanghai Education Committee Fund (13ZZ104, 09ZZ138, J50401) and Shanghai Young Teacher Training Project of Shanghai Education Committee Fund.
References
- Wall M. E. et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 88, 3888–3890 (1966). [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S. & Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Bio. Chem. 260, 14873–14878 (1985). [PubMed] [Google Scholar]
- Venditto V. J. & Simanek E. E. Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol. Pharm. 7, 307–349 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel E., Showalter S. D. & Blair D. G. Inhibition of human immunodeficiency virus (HIV-1) replication in vitro bynoncytotoxic doses of camptothecin, a topoisomerase I inhibitor. AIDS Res. Hum. Retroviruses. 7, 65–72 (1991). [DOI] [PubMed] [Google Scholar]
- Sankar-Thomas Y. D. & Lieberei R. Camptothecin accumulation in various organ cultures of Camptotheca acuminata Decne grown in different culture systems. Plant Cell. Tiss. Organ Cult. 106, 445–454 (2011). [Google Scholar]
- Lorence L. & Nessler C. L. Camptothecin, over four decades of surprising findings. Phytochemistry 65, 2735–2749 (2004). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. Molecular characterization and expression analysis of a new cDNA encoding strictosidine synthase from Ophiorrhiza japonica. Mol. Biol. Rep. 36, 1845–1852 (2009). [DOI] [PubMed] [Google Scholar]
- Kai G. Y. et al. Effect of three plant hormone elicitors on the camptothecin accumulation and gene transcript profiling in Camptotheca acuminata seedlings. Inter. J. Sci. 3, 86–95 (2014). [Google Scholar]
- Aiyama R., Nagai H., Nokata K., Shinohara C. & Sawada S. A. Camptothecin derivative from Notapodytes foetida. Phytochemistry 27, 3663–3664 (1988). [Google Scholar]
- Li L. et al. Optimization of induction and culture conditions and tropane alkaloid production in hairy roots of Anisodus acutangulus. Biotechnol. Bioproc. E. 13, 606–612 (2008). [Google Scholar]
- Sudo H., Yamakawa T., Yamazaki M., Aimi N. & Saito K. Bioreactor production of camptothecin by hairy root cultures of Ophiorrhiza pumila. Biotechnol. Lett. 24, 359–363 (2002). [Google Scholar]
- Kai G. Y. et al. Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 13, 319–327 (2011). [DOI] [PubMed] [Google Scholar]
- Kai G. Y. et al. Enhancing the production of tropane alkaloids in transgenic Anisodus acutangulus hairy root cultures by over-expressing tropinone reductase I and hyoscyamine-6b-hydroxylase. Mol. BioSys. 8, 2883–2890 (2012). [DOI] [PubMed] [Google Scholar]
- Yamazaki Y. et al. Metabolite profiling of alkaloids and strictosidine synthase activity in camptothecin producing plants. Phytochemistry 62, 461–470 (2003). [DOI] [PubMed] [Google Scholar]
- Yamazaki Y. et al. Biosynthesis of camptothecin. In silico and in vivo tracer study from [1-13C] glucose. Plant Physiol. 134, 161–170 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan T. M. Expression of enzymatically active cloned strictosidine synthase from the higher plant Rauvolfia serpentine in Escherichia coli. FEBS Lett. 257, 127–130 (1989). [DOI] [PubMed] [Google Scholar]
- McKnight T. D., Roessner C. A., Devagupta R., Scott A. I. & Nessler C. L. Nucleotide sequence of a cDNA encoding the vacuolar protein strictosidine synthase from Catharanthus roseus. Nucleic. Acids. Res. 18, 4939 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canel C. et al. Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205, 414–419 (1998). [DOI] [PubMed] [Google Scholar]
- Collu G., Garcia A. A., van Der Heijden R. & Verpoorte R. Activity of the cytochrome P450 enzyme geraniol-10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci. 162, 165–172 (2002). [Google Scholar]
- Sun Y. et al. Pyrosequencing of the Camptotheca acuminate transcriptome reveals putative genes involved in camptothecin biosynthesis and transport. BMC Genomics. 12, 533 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L. & Memelink J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289, 295–297 (2000). [DOI] [PubMed] [Google Scholar]
- Wang C. T., Liu H., Gao X. S. & Zhang H. X. Overexpression of G10H and ORCA3in the hairy roots of Catharanthus roseus improves catharanthine production. Plant Cell Rep. 29, 887–894 (2010). [DOI] [PubMed] [Google Scholar]
- Pan Q. et al. Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PLoS One. 7, e43038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. et al. Pyrosequencing of the Camptotheca acuminate transcriptome reveals putative genes involved in camptothecin biosynthesis and transport. BMC Genomics. 12, 533 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X. N. et al. Enhancement of camptothecin production in Camptotheca acuminata hairy roots by overexpressing ORCA3 gene. JAPS 1, 85–88 (2011). [Google Scholar]
- Tafur S., Nelson J. D., Delong D. C. & Svoboda G. H. Antiviral components of Ophiorrhiza mungos isolation of camptothecin and 10-methoxycamptothecin. Lloydia 39, 261–262 (1976). [PubMed] [Google Scholar]
- Saito K. et al. Feasible production of camptothecin by hairy root culture of Ophiorrhiza pumila. Plant Cell Rep. 20, 267–271 (2001). [Google Scholar]
- Dhakulkar S., Ganapathi T. R., Bhargava S., & Bapat V. A. Induction of hairy roots in Gmelina arborea Roxb. and production of verbascoside in hairy roots. Plant Sci. 169, 812–818 (2005). [Google Scholar]
- Zhang L. et al. Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. Proc. Natl. Acad. Sci. U. S. A. 101, 6786–6791 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai G. Y. et al. Molecular cloning and characterization of two tropinone reductases in Anisodus acutangulus and enhancement of tropane alkaloid production in AaTRI-transformed hairy roots. Biotechnol. Appl. Biochem. 54, 177–186 (2009). [DOI] [PubMed] [Google Scholar]
- Peebles C. A. et al. The expression of 1-deoxy-D-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab. Eng. 13, 234–240 (2011). [DOI] [PubMed] [Google Scholar]
- Sirikantaramas S., Asano T., Sudo H., Yamazaki M. & Saito K. Camptothecin: Therapeutic potential and biotechnology. Curr. Pharm. Biotechnol. 8, 196–202 (2007). [DOI] [PubMed] [Google Scholar]
- Muir S. R. et al. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 19, 470–474 (2001). [DOI] [PubMed] [Google Scholar]
- Aquil S., Husaini A. M., Abdin M. Z. & Rather G. M. Overexpression of the HMG-CoA reductase gene leads to enhanced artemisinin biosynthesis intransgenic Artemisia annuaplants. Planta Med. 75, 1453–1458 (2009). [DOI] [PubMed] [Google Scholar]
- Hao X. L. et al. Effects of methyl jasmonate and salicylic acid on the tanshinone production and biosynthetic genes expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 10.1002/bab.1236 (2014). [DOI] [PubMed] [Google Scholar]
- Shi M. et al. Increased accumulation of the cardio-cerebrovascular disease treatment drug tanshinone in Salvia miltiorrhiza hairy roots by the enzymes 3-hydroxy-3-methylglutaryl CoA reductase and 1-deoxy-D- xylulose 5-phosphate reductoisomerase. Funct. Integr. Genomic. 14, 603–15 (2014). [DOI] [PubMed] [Google Scholar]
- Doyle J. J. & Doyle J. L. Isolation of plant DNA from fresh tissue. Focus 12, 13–15 (1990). [Google Scholar]
- Kai G. Y. et al. Molecular cloning and expression analyzes of a new gene encoding 3-hydroxy-3-methylglutaryl-CoA synthase from Taxus media. Biol. Plant. 50, 359–366 (2006). [Google Scholar]
- Asano T. et al. Suppression of camptothecin biosynthetic genes results in metabolic modification of secondary products in hairy roots of Ophiorrhiza pumila. Phytochemistry. 91, 128–139 (2013). [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55 (1983). [DOI] [PubMed] [Google Scholar]