Abstract
Individuals with autism spectrum disorders (ASD) have difficulty understanding other minds (Theory of Mind; ToM), with atypical processing evident at both behavioural and neural levels. Individuals with conduct problems and high levels of callous-unemotional (CU) traits (CP/HCU) exhibit reduced responsiveness to others' emotions and difficulties interacting with others, but nonetheless perform normally in experimental tests of ToM. The present study aimed to examine the neural underpinnings of ToM in children (aged 10–16) with ASD (N = 16), CP/HCU (N = 16) and typically developing (TD) controls (N = 16) using a non-verbal cartoon vignette task. Whilst individuals with ASD were predicted to show reduced fMRI responses across regions involved in ToM processing, CP/HCU individuals were predicted to show no differences compared with TD controls. The analyses indicated that neural responses did not differ between TD and CP/HCU groups during ToM. TD and CP/HCU children exhibited significantly greater medial prefrontal cortex responses during ToM than did the ASD group. Within the ASD group, responses in medial prefrontal cortex and right temporoparietal junction (TPJ) correlated with symptom severity as measured by the Autism Diagnostic Observation Schedule (ADOS). Findings suggest that although both ASD and CP/HCU are characterized by social difficulties, only children with ASD display atypical neural processing associated with ToM.
Introduction
Theory of Mind (ToM) describes the ability to attribute mental states in order to explain or predict behaviour (Premack & Woodruff, 1978). Research indicates that individuals with autism spectrum disorders (ASD) have impairments in ToM (Baron-Cohen, Leslie & Frith, 1985; Senju, Southgate, White & Frith, 2009). For example, children with autism have difficulties attributing mental states such as beliefs or intentions to explain characters' actions or communication in simple stories (Baron-Cohen, O'Riordan, Jones, Stone & Plaisted, 1999; Happé, 1994; White, Hill, Happé & Frith, 2009). Social difficulties are mirrored by atypical neural processing, with most fMRI studies to date reporting reduced neural responses in adults and children with ASD relative to controls across a network of regions implicated in ToM (posterior superior temporal sulcus (pSTS)/temporoparietal junction (TPJ), medial prefrontal cortex (mPFC) and temporal poles) (e.g. Castelli, Frith, Happé & Frith, 2002; Lombardo, Chakrabarti, Bullmore & Baron-Cohen, 2011; Mason, Williams, Kana, Minshew & Just, 2008; Wang, Lee, Sigman & Dapretto, 2006).
In contrast, individuals with conduct problems and high levels of callous-unemotional (CU) traits (CP/HCU) appear to show intact ToM but reduced affective reactivity to others' emotions (Blair, 2005; Jones, Happé, Gilbert, Burnett & Viding, 2011; Schwenck, Mergenthaler, Keller, Zech, Salehi, Taurines, Romanos, Schecklmann, Schneider, Warnke & Freitag, 2011). Several studies have reported reduced psychophysiological reactivity to distress cues in children with CP/HCU (e.g. de Wied, van Boxtel, Matthys & Meeus, 2012; Kimonis, Frick, Munoz & Aucoin, 2007). Neuroimaging studies have found reduced amygdala response to fearful faces in children with CP/HCU (Jones, Laurens, Herba, Barker & Viding, 2009; Marsh, Finger, Fowler, Adalio, Jurkowitz, Schechter, Pine, Decety & Blair, 2013; Marsh, Finger, Mitchell, Reid, Sims, Kosson, Towbin, Leibenluft, Pine & Blair, 2008; Viding, Sebastian, Dadds, Lockwood, Cecil, De Brito & McCrory, 2012) and reduced responses to others' pain or distress across amygdala, anterior insula and dorsal/rostral anterior cingulate cortex (Lockwood, Sebastian, McCrory, Hyde, Gu, De Brito & Viding, 2013; Marsh et al., 2013; Sebastian, McCrory, Cecil, Lockwood, De Brito, Fontaine & Viding, 2012a). However, cognitive-experimental studies in adults with psychopathy and children with CP/HCU indicate intact ToM across a range of measures (Dolan & Fullam, 2004; Richell, Mitchell, Newman, Leonard, Baron-Cohen & Blair, 2003; Jones et al., 2011), although this has not yet been explored with fMRI.
Whilst behavioural evidence suggests that ToM is impaired in ASD but intact in CP/HCU, no study has directly compared the neural basis of ToM across these groups. Indeed, only one imaging study has directly compared ASD and anti-social traits, determining differential contributions to structural brain development in children, with only ASD traits associated with cortical thinning in superior temporal regions recruited during performance (Wallace, Shaw, Lee, Clasen, Raznahan, Lenroot, Martin & Giedd, 2012). Imaging methods can pick up subtle differences not always detectable at the cognitive or behavioural levels (e.g. Carter, Williams, Minshew & Lehman, 2012; Kana, Keller, Cherkassky, Minshew & Just, 2009), and as such provide a clearer picture of whether atypical processing associated with ToM is limited to ASD.
Here we used a cartoon-based vignette task (Sebastian, Fontaine, Bird, Blakemore, De Brito, McCrory & Viding, 2012b; Sebastian et al., 2012a) to explore the neural bases of ToM in ASD, CP/HCU and TD children. We compared responses during cartoons requiring ToM (understanding intentions) versus physical causality (PC) (understanding cause and effect without ToM demands). The CP/HCU and TD groups reported here contributed data to a previous paper (Sebastian et al., 2012a), which focused on a third, affective condition, tapping understanding of emotions within an intentional, narrative context. In the previous study, the focus was on the CP/HCU group and contrasting affective versus ToM conditions to examine affective processing in children with conduct problems. The current study focuses on the comparison of ASD and CP/HCU groups in ToM processing relative to the PC control condition. Since affective processing in an intentional context involves the integration of ToM and empathy-related processes (Shamay-Tsoory, 2011), and hence requires intact ToM, the affective condition was not analysed in the current study, since interpretation of any observed differences would be problematic given likely ToM deficits in the ASD group. Previous use of the present vignette task (Sebastian et al., 2012a, 2012b), comparing ToM versus PC conditions in typically developing populations has yielded reliable and replicable responses in the ‘mentalizing network’ comprising the pSTS extending to the TPJ, precuneus, temporal poles, and mPFC, in line with other studies using non-verbal cartoon stimuli (Brunet, Sarfati, Hardy-Bayle & Decety, 2000; Carter et al., 2012; Gallagher, Happé, Brunswick, Fletcher, Frith & Frith, 2000; Kana, Libero, Hu, Deshpande & Colburn, 2014; Völlm, Taylor, Richardson, Corcoran, Stirling, McKie, Deakin & Elliott, 2006).
Studies of ToM in adult ASD samples have reported reductions in mPFC response (Castelli et al., 2002; Happé, Ehlers, Fletcher, Frith, Johansson, Gillberg, Dolan, Frackowiak & Frith, 1996; Kana et al., 2009; Kennedy & Courchesne, 2008; Mason et al., 2008; Murdaugh, Shinkareva, Deshpande, Wang, Pennick & Kana, 2012; Watanabe, Yahata, Abe, Kuwabara, Inoue, Takano et al., 2012) and reductions or reduced selectivity of pSTS/TPJ (Castelli et al., 2002; Lombardo et al., 2011; Mason et al., 2008), pSTS (Pelphrey, Morris & McCarthy, 2005) and temporal poles (Castelli et al., 2002) relative to control participants. Several studies have reported negative correlations between autistic symptoms or ToM impairment and functional responses in pSTS (Kana et al., 2009; Pelphrey et al., 2005), mPFC (Kennedy & Courchesne, 2008) and right TPJ (Lombardo et al., 2011).
Three fMRI studies examining ToM in ASD have been conducted with child samples. One study, using static cartoon stimuli in children aged 7 to 16 with and without ASD, reported significantly reduced activation in mPFC, STS, left temporal pole, and precuneus during ToM (Carter et al., 2012). The two other studies have reported similar results, including negative correlations between autistic social symptoms and superior temporal responses (Wang et al., 2006), and between social responsiveness and medial PFC activation (Wang, Lee, Sigman & Dapretto, 2007).
This study aimed to test whether neural processing associated with ToM is abnormal in ASD in contrast to CP/HCU. Despite social deficits in both groups, they are rarely directly compared in the existing literature, and have never been compared with fMRI. To our knowledge, ours is also the first fMRI study to examine the neural correlates of ToM in children with CP/HCU, and the first to extend use of a non-verbal cartoon vignette task to a sample of children with ASD. Use of non-verbal vignettes to assay ToM is an advantage because individuals with ASD are reported to show impairments in deriving meaning from verbal stimuli (e.g. Randi, Newman & Grigorenko, 2010). In line with previous studies, we predicted reduced activation across the ‘mentalizing network’ in individuals with ASD compared to both CP/HCU and TD control groups. In particular, atypical responses were predicted in mPFC, pSTS/TPJ, and temporal poles in ASD. By contrast, based on cognitive-experimental evidence, we predicted intact responses in the CP/HCU group.
Method
Participants
Participants were a community sample of adolescent males (aged 10–16) who were typically developing (TD, N = 16), had conduct problems plus callous unemotional traits (CP/HCU, N = 16), or an autism spectrum disorder (ASD, N = 16). Details of TD and CP/HCU participant recruitment are reported elsewhere (Sebastian et al., 2012a).
The ASD group were identified through their previous participation in research studies at King's College London or UCL. They were originally recruited from the community via schools and parent groups, and had a clinical diagnosis of autism or Asperger syndrome. They were assessed using the Autism Diagnostic Observational Schedule (Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles & Rutter, 2000) and, for nine participants, a full developmental interview (either the Autism Diagnostic Interview - Revised (ADI-R) or the 3di; Table S1) (Lord, Rutter & Couteur, 1994; Skuse, Warrington, Bishop, Chowdhury, Lau, Mandy & Place, 2004). For the remainder, developmental history was assessed using the Social Communication Questionnaire (based on the ADI; Eaves, Wingert, Ho & Mickelson, 2006). No cut-off for conduct problems or CU traits was imposed in the ASD group, but all ASD participants scored below the median for CU traits reported by Sebastian et al. (2012a), and ASD and TD groups did not differ on CP symptoms (from the Child and Adolescent Symptom Inventory-4R – Conduct Disorder subscale, CASI-CD; Gadow & Sprafkin, 2009). In line with previous studies (e.g. Bird, Silani, Brindley, White, Frith & Singer, 2010), participants who did not meet full criteria on all ASD assessment measures but had a clinical diagnosis were retained in the analyses. This allowed us to recruit sufficient numbers of high-functioning participants who could tolerate scanning. No participants were currently taking psychoactive medication, except for occasional melatonin (N = 1), which was not used for 24 hours prior to scanning.
Participants with CP/HCU scored in the clinically relevant range on the CASI-CD subscale (> 2 at 10–14 years; > 5 at 15–16 years), and comprised the top 50% of the sample from Sebastian et al. (2012a) in terms of scores on the Inventory of Callous-Unemotional traits (ICU; Essau, Sasagawa & Frick, 2006). TD controls all scored below the clinical threshold for conduct problems and below 45 on the ICU. Parent/guardian data screening for psychiatric and neurological conditions, general psychopathology (including conduct problems and CU traits), and demographic data (including parent-defined ethnicity, handedness and socioeconomic status, based on National Statistics Socio-economic Classification NS-SEC coding; http://www.ons.gov.uk) was available for all participants and is displayed in Table1. To ensure that case groups were representative of ASD and CP/HCU, co-occurring symptoms of generalized anxiety disorder, major depression, substance abuse, or ADHD did not result in exclusion, but were measured using the CASI and their effects explored in the analyses. All participants completed the Wechsler Abbreviated Scale of Intelligence (2-subtest version;Wechsler, 1999), the Alcohol Use Disorders Identification Test (AUDIT; Saunders, Aasland, Babor, de la Fuente & Grant, 1993), and the Drug Use Disorders Identification Test (DUDIT; Berman, Bergman, Palmstierna & Schlyter, 2005).
Table 1.
Demographic information
| Demographic variables | Typically developing (N = 16) | CP/HCU (N = 16) | ASD (N = 16) | p-value | Difference |
|---|---|---|---|---|---|
| Age (years) | 13.51 (1.65) | 14.15 (1.88) | 14.18 (1.63) | .47 | |
| Socioeconomic status | 2.70 (0.85) | 3.19 (1.07) | 2.69 (0.95) | .26 | |
| Full-scale IQ | 106.69 (12.67) | 98.13 (11.98) | 107.31 (13.23) | .08 | |
| Verbal T score | 56.94 (10.52) | 49.13 (8.74) | 55.25 (8.70) | .06 | |
| Matrix reasoning T score | 50.13 (8.61) | 48.38 (9.27) | 52.88 (9.94) | .39 | |
| Race/ ethnicity (N) | |||||
| White | 14 | 13 | 14 | ||
| Black | 1 | 1 | 1 | ||
| Mixed race | 1 | 2 | 1 | .83 | |
| Handedness (N) | |||||
| Right | 11 | 14 | 15 | ||
| Left | 4 | 2 | 1 | ||
| Ambidextrous | 1 | 0 | 0 | .14 | |
| Inventory of Callous Unemotional traits (parent rated) | 16.87 (5.72) | 46.46 (7.02) | 27.24 (8.99) | <.001 | TD<ASD<CP/HCU |
| Child and Adolescent Symptom Inventory (parent rated) | |||||
| Conduct disorder symptoms | 0.61 (0.85) | 10.29 (5.45) | 1.25 (1.39) | <.001 | TD=ASD<CP/HCU |
| ADHD symptoms | 9.88 (6.20 | 31.25 (9.09) | 23.50 (9.91) | <.001 | TD<ASD<CP/HCU |
| Generalised anxiety symptoms | 3.75 (3.19) | 8.13 (5.17) | 9.38 (3.72) | .001 | TD<ASD=CP/HCU |
| Major depressive symptoms | 2.75 (1.98) | 5.47 (3.34) | 6.75 (4.48) | .006 | TD<ASD=CP/HCU |
| Autism spectrum symptoms | 1.40 (2.35) | 4.27 (3.99) | 13.88 (7.07) | <.001 | TD<CP/HCU<ASD |
| Alcohol use and disorders | 1.19 (1.76) | 4.75 (7.26) | 0.33 (0.62) | .02 | ASD<TD=CP/HCU |
| Drug use and disorders | 0.00 (0.00) | 1.06 (2.62) | 0.13 (0.50) | .11 | |
Nineteen children with ASD were scanned. Data from three ASD participants were excluded due to excessive motion, leaving a final ASD sample of 16 to be compared with the 16 TD and 16 CP/HCU participants described above. Written informed parental consent and written assent from participants was obtained. Groups were matched on age, IQ, SES, gender and handedness (see Table1).
Experimental task
The task involved 30 cartoons, 10 each for ToM, physical causality (PC) and Affective ToM conditions. As noted in the introduction, the present study focuses on ToM and PC conditions only. Each cartoon was silent and static, and depicted two people in everyday scenarios (e.g. pouring a drink; going for a walk).
The task was structured as in Sebastian et al. (2012a, 2012b). In total, 30 cartoons (10 of each condition) were used, presented in sets of six, with a 15-second fixation period between sets. The six cartoons in each set included two cartoons from each condition, which were always yoked together. The order in which the ToM, Affective ToM and PC cartoon pairs in each set were presented was randomized for each participant. Each cartoon exemplar was presented once only.
Each cartoon involved four sequential frames. The first screen (3 seconds) displayed ‘What happens next?’ This was followed by three sequentially presented story frames, each presented for 2 seconds. The final screen, displayed for 5 seconds, showed a choice of two possible endings for the cartoon. During this time participants made their choice using a keypad. The inter-stimulus interval was 1 second, so each trial lasted 15 seconds in total.
For ToM cartoons, selecting the correct ending required understanding behaviour based on intentions (e.g. using an umbrella to help reach a door handle). PC cartoons required an understanding of cause and effect reasoning (e.g. understanding that a hat cannot blow against the wind). Affective ToM cartoons involved understanding behaviour based on emotion.
fMRI data acquisition
Images were acquired using a Siemens Avanto 1.5-T MRI scanner. These included a 5.5-minute T1 weighted structural scan, and 184 multislice T2-weighted echo planar volumes with blood oxygenation level-dependent contrast, taken during 1 9-minute run of the cartoon task. Acquisition parameters were: 35 2 mm slices with a 1 mm gap; echo-time = 50 milliseconds; repetition time = 2975 milliseconds; slice tilt = −30 (T > C): flip angle = 90o; field of view = 192 mm; matrix size = 64 × 64. Fieldmaps were also obtained and used to adjust functional scans for deformations due to magnetic field in-homogeneities during pre-processing.
fMRI data analysis
SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running in MATLAB R2007b was used to analyse imaging data. Five volumes were removed from the beginning of the sequence and two from the end, to allow for T1 equilibration and the final ‘Thank you’ screen. Voxel displacement maps were created from the fieldmaps for each participant, and used during the realign and unwarp stage of pre-processing. Images were normalized using warps created from segmented structural scans which had been co-registered to the functional data, written with a voxel size of 2 × 2 × 2 mm, and smoothed using an 8-mm Gaussian kernel. Images showing visible motion-related distortions were removed and interpolated using adjacent scans to prevent distortion of the between-subjects mask. Interpolated scans were then regressed out in the first-level design matrix. Movement artefacts were detected in 17 participants (TD: N = 3, ASD: N = 7, CP/HCU: N = 7), and always constituted less than 10% of each subject's data.
The first-level design matrix deconstructed the time series into segments corresponding to each of the three cartoon types (11 seconds each), periods of fixation (15 seconds) or instructions (3 s) and inter-stimulus intervals (1 s). The regressors were modelled as box-car functions and convolved with a canonical hemodynamic response function. Realignment parameters and interpolated scans were also included as regressors. The contrast of interest (ToM > PC) was estimated in each participant. Contrast images were then used in second-level analyses; with group (TD; CP/HCU; ASD) as the between-subjects factor in a one-way ANOVA.
Main effects for ToM > PC were thresholded at p < .05 family-wise error (FWE) corrected at peak level. For interactions with group, a priori regions of interest (bilateral medial/ventromedial prefrontal cortex, temporal poles and pSTS/TPJ) were defined using the aal atlas in the WFU Pickatlas toolbox for SPM (Maldjian, Laurienti, Kraft & Burdette, 2003; Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer & Joliot, 2002). Results within these ROIs were thresholded at p < .05, small-volume FWE-corrected at the peak level within each ROI. The Marsbar toolbox (Brett, Jean-Luc, Valabregue & Poline, 2002) was used to extract mean responses across significant clusters within ROIs for plotting purposes, and for conducting correlational analyses within the ASD group. An exploratory whole brain analysis was also conducted, with results reported at p < .001, k > 5, uncorrected (see Table S3).
Results
Behavioural data
Mean error rates and reaction times are displayed in Table2. For error rates, a Group (TD, CP/HCU, ASD) × Condition (ToM, PC) mixed model ANOVA revealed no significant main effect of condition, F(1, 45) =.78, p = .381, η2partial =.017. There was no significant main effect of group, F(2, 45) = 1.95, p = .154, η2partial =.080, or Group × Condition interaction, F(2, 45) =.09, p = .917, η2partial =.004, which would have complicated interpretation of fMRI data. For reaction times, there was a significant main effect of condition, F(1, 45) = 5.68, p = .021, η2partial =.112. Post-hoc analysis revealed significantly faster responses to PC versus ToM across all groups, t(47) = 2.40, p = .02, Cohen's d = .75. There was no main effect of group, F(2, 45) = 2.52, p = .092, η2partial =.101, nor Group × Condition interaction, F(2, 45) =.64, p = .532, η2partial =.028.
Table 2.
Behavioural data: mean (SD). Abbreviations: RT = reaction time; msec = milliseconds; ns = not significant
| Behavioural Data | Typically developing (N = 16) | CP/HCU (N = 16) | ASD (N = 16) | Main effect of group | Group × condition |
|---|---|---|---|---|---|
| ToM errors (%) | 15.63 (12.09) | 10.00 (11.55) | 10.00 (10.33) | ns | ns |
| Physical Causality (PC) errors (%) | 12.50 (12.91) | 8.75 (8.06) | 8.75 (8.06) | ||
| ToM RT (msec) | 2080 (465) | 2393 (405) | 2313 (499) | ns | ns |
| Physical Causality (PC) RT (msec) | 1965 (404) | 2224 (349) | 2270 (465) |
ToM > PC: main effects
Regions surviving whole brain FWE-correction at p < .05 for ToM > PC are detailed in Table3. Significant clusters were detected in the bilateral TPJ, extending to occipito-temporal cortex, bilateral temporal poles, left parahippocampal gyrus and posterior cingulate cortex. No responses were observed in the medial prefrontal cortex (mPFC) when data from all three groups were included, although at an uncorrected threshold of p < .001, k > 5, a cluster was seen in ventromedial PFC (MNI: x = 4, y = 50, z = −14, k = 15; Figure1a). When main effects were re-run with only TD and CP/HCU groups, a cluster in the anterior rostral mPFC (MNI: x = 6, y = 54, z = 14; k = 5) survived FWE-correction at p < .05 (Figure1b). Reverse contrasts are included in Table S2.
Table 3.
Regions showing a main effect at p < .05 with FWE correction at the peak level for ToM > PC. Abbreviations: TPJ = temporoparietal junction; BA = Brodmann area; k = cluster size; ext. = extending to. Where more than one BA is shown, the peak voxel falls in the first BA but the cluster extends to the others listed
| Peak voxel (MNI) |
|||||||
|---|---|---|---|---|---|---|---|
| Brain region ToM > PC | BA | L/R | x | y | z | k | z-value |
| TPJ, ext. to occipitotemporal cortex | 39, 22, 19 | R | 50 | −58 | 18 | 424 | 6.48 |
| TPJ, ext. to occipitotemporal cortex | 22 | L | −36 | −54 | 15 | 188 | 6.12 |
| 39, 19 | L | −44 | −60 | 18 | 6.05 | ||
| Parahippocampal gyrus | 37 | L | −30 | −42 | −8 | 47 | 5.68 |
| Temporal pole, ext. to fusiform gyrus | 21 | R | 58 | 4 | −20 | 181 | 5.64 |
| 21, 20 | R | 54 | 0 | −26 | 5.64 | ||
| 20 | R | 48 | −8 | −26 | 4.92 | ||
| Temporal pole | 21 | L | −60 | −2 | −20 | 19 | 5.21 |
| Posterior cingulate cortex/ precuneus | 30 | L | −14 | −58 | 16 | 13 | 5.03 |
| Posterior cingulate cortex | 23, 30, 31 | Midline | 0 | −50 | 22 | 6 | 4.79 |
Figure 1.
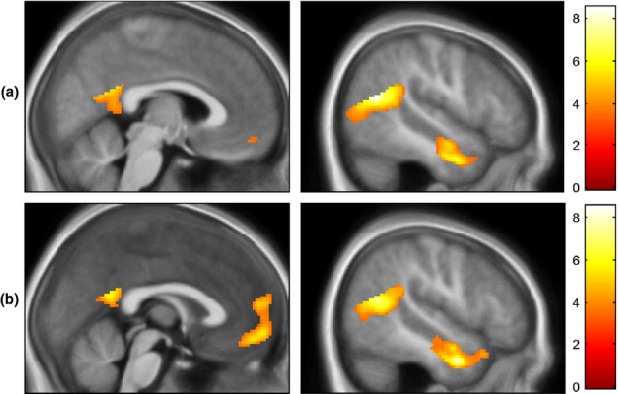
Main effects for ToM > PC for (a) All three groups combined and (b) the TD and CP/HCU groups only. Results are overlaid on an average structural for all participants. Results are shown at a threshold of p < .001, k > 5, uncorrected. Colour-bar represents t-values.
ToM > PC: interactions with group
We first examined whether TD and CP/HCU responses differed within ROIs and across the whole brain for ToM > PC. As predicted, no differences between groups survived small-volume FWE-correction within ROIs, and only one small cluster (middle frontal gyrus, k = 6) was significant in the whole brain at a liberal threshold of p < .001, k > 5 (Table S3). Because no regions, bar this one area, showed group differences at corrected or liberal thresholds for either TD > CP/HCU or CP/HCU > TD, we compared ASD against TD and CP/HCU groups combined (set up in contrasts as 0.5 (TD) 0.5 (CP/HCU) and −1 (ASD)).
Within a priori ROIs, three clusters in medial/ventromedial PFC showed a Group × Condition interaction in the predicted direction (TD = CP/HCU > ASD for ToM > PC) at p < .05, small-volume FWE-corrected (Table4). No other ROIs yielded significant results.
Table 4.
Clusters within masked regions showing a Condition × Group interaction at p < .05, SVC-FWE corrected. BA = Brodmann area, k = cluster size, SVC-FWE = small volume family-wise error corrected
| Peak voxel (MNI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region included in mask | BA | L/R | x | y | z | k | z-value | SVC-FWE peak p-value | Cohen's d |
| TD + CP/HCU > ASD | |||||||||
| Medial prefrontal cortex | 10 | L | −8 | 60 | 8 | 3 | 3.91 | .035 | 1.47 |
| 10 | L | −12 | 54 | 16 | 1 | 3.81 | .048 | 1.43 | |
| Ventromedial prefrontal cortex | 10 | L | −4 | 60 | −4 | 2 | 3.56 | .035 | 1.31 |
Parameter estimates were extracted by averaging across voxels in the clusters surviving small-volume FWE-correction using Marsbar. Figure2 illustrates that neural responses of children with ASD were significantly reduced compared with TD and CP/HCU children. Results for group comparisons were very similar when symptom scores which significantly differed between groups (ADHD, depression and anxiety) or IQ were included as covariates.
Figure 2.
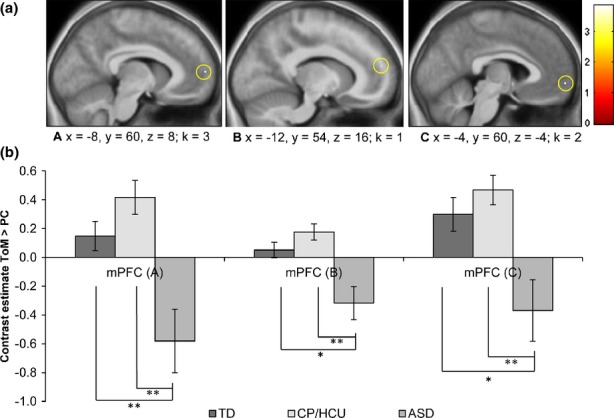
(a) Clusters surviving small-volume corrected FWE thresholds for the condition × group interaction (TD = CP/HCU > ASD). Colour bars represent t-values. (b) Parameter estimates averaged across voxels in the cluster using Marsbar (Maldijan et al., 2003). A: (x = −8, y = 60, z = 8), k = 3; B: (x = −12, y = 54, z = 15), k = 1, C: (x = −4, y = 60, z = −4), k = 2. Error bars indicate standard errors. Analyses indicated significant differences between TD vs. ASD and CP/HCU vs. ASD (* = p < .05; ** = p < .005).
Correlational analyses in the ASD group
To explore whether differences in functional response for the clusters exhibiting Group × Condition interactions were also associated with severity of symptoms within the ASD group, we investigated the relationship between parameter estimates across clusters for ToM > PC and combined social and communication subscales of the ADOS. An inverse correlation was observed between mPFC response (cluster C) and social and communication subscales combined (r = −.69, p = .004; Figure3). This result survives correction for multiple comparisons across the three correlations performed. Results for the other mPFC clusters were in the predicted direction, but not significant.
Figure 3.
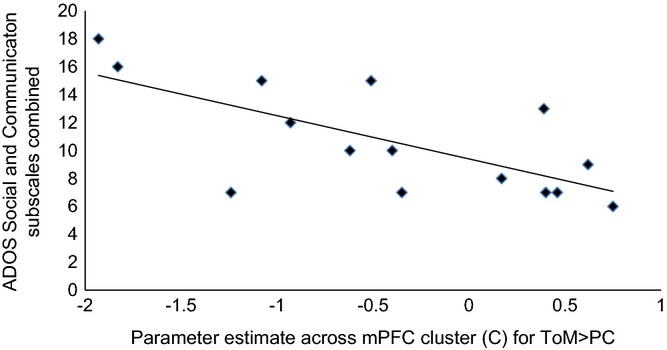
Correlation between functional response in the mPFC (cluster C in Figure2) for ToM > PC and combined ADOS Social and Communication subscales (r = −.69, p = .004).
Bilateral TPJ was also of a priori interest because of its relevance to ToM (e.g. Saxe & Kanwisher, 2003), and previous reports of correlations between functional response during ToM and ASD symptoms (Kana et al., 2009; Lombardo et al., 2011). Marsbar was used to extract mean contrast estimates within the ASD group for right and left TPJ clusters showing a main effect of ToM > PC at whole brain FWE-corrected levels across the whole sample. There was no significant correlation between ADOS social and communication subscales combined; however, the social subscale was inversely correlated with rTPJ functional response (r = −.57, p = .027; Figure4). Cook's distance and leverage values were within acceptable limits.
Figure 4.
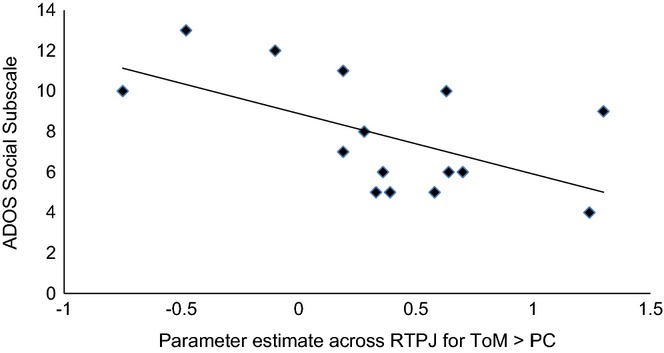
Correlation between functional response in the RTPJ for ToM > PC and ADOS Social subscale (r = −.57, p = .027).
Discussion
This study is the first to compare the neural basis of ToM processing in two groups of children who present with marked social deficits – those with ASD and those with CP/HCU. Compared to CP/HCU children, children with ASD exhibited reduced activation in the medial prefrontal cortex during ToM processing. The same pattern was observed when ASD children were compared to TD controls. There were no differences across ROIs between TD and CP/HCU groups. These findings indicate that whilst individuals with ASD show atypical neural processing, CP/HCU do not have a functional neural impairment during ToM. This is consistent with neurocognitive models that identify a core deficit in ASD as ‘knowing’ about others' mental states (Baron-Cohen et al., 1985). By contrast, this process appears spared in CP/HCU children who are instead characterized by not caring about others' feelings (Sebastian et al., 2012a; Jones et al., 2011; Blair, 2005).
Reduced mPFC responses in ASD compared to TD and CP/HCU children are in line with previous studies in both adult (Castelli et al., 2002; Happé et al., 1996; Kana et al., 2009; Kennedy & Courchesne, 2008; Murdaugh et al., 2012; Watanabe et al., 2012) and developmental (Carter et al., 2012; Wang et al., 2007) ASD samples, even when, as in the present study, behavioural responses do not differ. The mPFC is considered of central importance in ToM. Amodio and Frith (2006) propose that this region could be implicated in our ability to reason about other minds in the abstract, and integrate knowledge about their attributes with ongoing processing of intentions. In the ROI analysis, all clusters that survived correction were in the left hemisphere. This was surprising given that neuropsychological studies have implicated the right hemisphere in ToM (e.g. Brownell, Griffin, Winner, Friedman & Happé, 2000). However, lowering the threshold revealed that the mPFC cluster exhibiting reduced responses in ASD vs. TD and CP/HCU children did extend to the right hemisphere.
Extracted parameter estimates from mPFC illustrate that whilst TD and CP/HCU groups exhibited an increase in functional response during ToM relative to PC, the ASD group displayed a relatively reduced response. Though most studies have reported group differences in ASD reflecting a lack of differential response for ToM compared to baseline, one previous study also reported a relative deactivation for this contrast (in the RTPJ when making mentalistic judgements about the Queen's views; Lombardo et al., 2011). Other studies have not provided parameter estimates for contrasts, making it difficult to explore relative deactivation in ASD. Differences in the paradigms used (e.g. whether or not the PC condition involved agents) could also contribute to differences in results across studies (Castelli et al., 2002). One possible explanation for relative reductions in functional response for ToM may be that the presence of people in the logically engaging PC condition provoked more social processing than the ToM condition in the ASD group. Alternatively, mPFC responses could reflect domain general computation selectively engaged in ASD when processing cause and effect related to physical events.
Within the ASD group, autistic symptoms correlated significantly with functional response for ToM > PC in the most ventral of three clusters exhibiting a Group × Condition interaction in the medial prefrontal cortex. As socio-communicative impairment increased, participants' mPFC response for ToM > PC decreased, in line with similar reports in previous studies (e.g. Wang et al., 2007; Watanabe et al., 2012). This finding is consistent with group-level differences, and suggests that severity of autistic symptoms is related to degree of atypicality of social processing in mPFC – although causal direction cannot be assumed.
A further a priori region of interest was the TPJ, strongly implicated in ToM (Gweon, Dodell-Feder, Bedny & Saxe, 2012; Saxe & Kanwisher, 2003) and showing reduced responses during ToM in ASD compared with controls in previous studies (Kana et al., 2009; Lombardo et al., 2011). Consistent with these reports, RTPJ response for ToM > PC showed a negative relationship with ADOS social symptoms. However, no RTPJ clusters exhibited a Group × Condition interaction at small-volume FWE thresholds, or even at a liberal threshold (p < .001, k > 5; Table S3), in contrast to previous studies in adults (Lombardo et al., 2011).
In conclusion, ASD appears characterized by attenuated mPFC activation during ToM processing. By contrast, CP/HCU children show typical patterns of neural response during ToM processing, comparable to that seen in controls. Given the small sample size, these results should be considered preliminary until replicated in a larger population. Future studies could also explore differences in functional or effective connectivity across the social brain network in these groups. Given the need for methods to differentiate ASD from other clinical populations, including CP/HCU, these findings suggest that sensitive indicators of ToM could assist in differentiating these groups in the clinic.
Acknowledgments
This work was supported by awards [53229] from the British Academy and [RES-062-23- 2202] from the Economic and Social Research Council to Professor Viding and Dr McCrory, and award [AR/CRF/B16] from the University of London Central Research Fund to Professor Happé. E. O'Nions was supported by an ESRC 1+3 PhD studentship [ES/H031367/1]. We are grateful to Dr Liz Pellicano for assistance with recruiting participants.
Supporting Information
Table S1.Diagnostic information for the autism spectrum disorder (ASD) group. Abbreviations: ADOS = autism diagnostic observational schedule, ADI-R = Autism Diagnostic Interview Revised; SCQ = Social and Communication Questionnaire, soc = social, comm = communication, RRBI= rigid and repetitive behaviours and interests. N/A = Not applicable; OCD = obsessive compulsive disorder. Best available instrument refers to whether a diagnostic interview was available (ADI or 3Di), as opposed to the SCQ questionnaire.
Table S2. Whole brain main effects (across group) of the reverse contrast Physical Causality>Theory of Mind (PC>ToM). Results are thresholded at p <.05 FWE-corrected at the peak level across the whole brain. Abbreviations: BA = Brodmann area; k = cluster size.
Table S3. Regions showing a Condition x Group interaction for ToM > PC across the whole brain at p <.005 uncorrected, k > 10. BA = Brodmann area; k = cluster size; TD = typically developing controls; CP/HCU = conduct problem with high callous-unemotional traits; ASD = autism spectrum disorder.
References
- Amodio DM. Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. &. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM. Frith U. Does the autistic child have a ‘theory of mind’? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. &. doi: 0010-0277(85)90022-8 [pii] [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, O'Riordan M, Jones R, Stone V. Plaisted K. A new test of social sensitivity: detection of faux pas in normal children and children with Asperger syndrome. Journal of Autism and Developmental Disorders. 1999;29:407–418. doi: 10.1023/a:1023035012436. &. [DOI] [PubMed] [Google Scholar]
- Berman AH, Bergman H, Palmstierna T. Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. European Addiction Research. 2005;11(1):22–31. doi: 10.1159/000081413. &. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U. Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133(Pt. 5):1515–1525. doi: 10.1093/brain/awq060. &. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition. 2005;14(4):698–718. doi: 10.1016/j.concog.2005.06.004. . doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Brett M, Jean-Luc A, Valabregue R. Poline J. 2002. & Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan.
- Brownell H, Griffin R, Winner E, Friedman O. Happé F. Cerebral lateralization and theory of mind. In: Cohen DJ, editor; Baron-Cohen S, Tager-Flusberg H, editors. Understanding other minds: Perspectives from autism and developmental cognitive neuroscience. 2nd edn. Oxford: Oxford University Press; 2000. pp. 311–338. & (Eds.),, &. In. [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC. Decety J. A PET investigation of the attribution of intentions with a nonverbal task. NeuroImage. 2000;11(2):157–166. doi: 10.1006/nimg.1999.0525. &. doi: 10.1006/nimg 1999.0525. [DOI] [PubMed] [Google Scholar]
- Carter EJ, Williams DL, Minshew NJ. Lehman JF. Is he being bad? Social and language brain networks during social judgment in children with autism. PLoS One. 2012;7(10):e47241. doi: 10.1371/journal.pone.0047241. &. doi: 10.1371/journal.pone.0047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F. Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt. 8):1839–1849. doi: 10.1093/brain/awf189. &. [DOI] [PubMed] [Google Scholar]
- de Wied M, van Boxtel A, Matthys W. Meeus W. Verbal, facial and autonomic responses to empathy-eliciting film clips by disruptive male adolescents with high versus low callous-unemotional traits. Journal of Abnormal Child Psychology. 2012;40(2):211–223. doi: 10.1007/s10802-011-9557-8. &. doi: 10.1007/s10802-011-9557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. Fullam R. Theory of mind and mentalizing ability in antisocial personality disorders with and without psychopathy. Psychological Medicine. 2004;34(6):1093–1102. doi: 10.1017/s0033291704002028. &. [DOI] [PubMed] [Google Scholar]
- Eaves LC, Wingert HD, Ho HH. Mickelson EC. Screening for autism spectrum disorders with the social communication questionnaire. Journal of Developmental and Behavioural Pediatrics. 2006;27(2 Suppl):S95–S103. doi: 10.1097/00004703-200604002-00007. &. doi: 10.1097/00004703-200604002-00007 [pii] [DOI] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S. Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13(4):454–469. doi: 10.1177/1073191106287354. &. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Gadow KD. Sprafkin J. The symptom inventories: An annotated bibliography. Stony Brook, NY: Checkmate Plus; 2009. &. [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U. Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. &. doi: S0028-3932(99)00053-6 [pii] [DOI] [PubMed] [Google Scholar]
- Gweon H, Dodell-Feder D, Bedny M. Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Development. 2012;83(6):1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. &. doi: 10.1111/j.1467-8624.2012.01829. [DOI] [PubMed] [Google Scholar]
- Happé FG. An advanced test of theory of mind: understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Developmental Disorders. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Happé F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R. Frith C. ‘Theory of mind’ in the brain: evidence from a PET scan study of Asperger syndrome. NeuroReport. 1996;8(1):197–201. doi: 10.1097/00001756-199612200-00040. &. [DOI] [PubMed] [Google Scholar]
- Jones AP, Happé FGE, Gilbert F, Burnett S. Viding E. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;51(11):1188–1197. doi: 10.1111/j.1469-7610.2010.02280.x. &. doi: 10.1111/j.1469-7610.2010.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ. Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. &. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ. Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–152. doi: 10.1080/17470910802198510. &. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD. Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Social Cogntive and Affective Neuroscience. 2014;9(1):98–105. doi: 10.1093/scan/nss106. &. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP. Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognitive and Affective Neuroscience. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. &. doi: 10.1093/Scan/Nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Munoz LC. Aucoin KJ. Can a laboratory measure of emotional processing enhance the statistical prediction of aggression and delinquency in detained adolescents with callous-unemotional traits? Journal of Abnormal Child Psychology. 2007;35(5):773–785. doi: 10.1007/s10802-007-9136-1. &. doi: 10.1007/s10802-007-9136-1. [DOI] [PubMed] [Google Scholar]
- Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, De Brito SA. Viding E. Association of callous traits with reduced neural response to others' pain in children with conduct problems. Current Biology. 2013;23(10):901–905. doi: 10.1016/j.cub.2013.04.018. &. doi: 10.1016/j.cub.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET. Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. NeuroImage. 2011;56(3):1832–1838. doi: 10.1016/j.neuroimage.2011.02.067. &. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A. Rutter M. The Autism Diagnostic Observation Schedule – Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. &. [PubMed] [Google Scholar]
- Lord C, Rutter M. Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. &. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA. Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. &. doi: S1053811903001691 [pii] [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, Pine DS, Decety J. Blair RJ. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of Child Psychology and Psychiatry. 2013;54(8):900–910. doi: 10.1111/jcpp.12063. &. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS. Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. &. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N. Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. &. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Shinkareva SV, Deshpande HR, Wang J, Pennick MR. Kana RK. Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS One. 2012;7(11):e50064. doi: 10.1371/journal.pone.0050064. &. doi: 10.1371/journal.pone.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP. McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. &. doi: 10.1093/Brain/Awh404. [DOI] [PubMed] [Google Scholar]
- Premack D. Woodruff G. Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences. 1978;1(4):515–526. &. [Google Scholar]
- Randi J, Newman T. Grigorenko EL. Teaching children with autism to read for meaning: challenges and possibilities. Journal of Autism and Developmental Disorders. 2010;40(7):890–902. doi: 10.1007/s10803-010-0938-6. &. doi: 10.1007/s10803-010-0938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richell RA, Mitchell DG, Newman C, Leonard A, Baron-Cohen S. Blair RJ. Theory of mind and psychopathy: can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia. 2003;41(5):523–526. doi: 10.1016/s0028-3932(02)00175-6. &. doi: S0028393202001756 [pii] [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR. Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. &. [DOI] [PubMed] [Google Scholar]
- Saxe R. Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in ‘theory of mind’. NeuroImage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. &. doi: S1053811903002301 [pii] [DOI] [PubMed] [Google Scholar]
- Schwenck C, Mergenthaler J, Keller K, Zech J, Salehi S, Taurines R, Romanos M, Schecklmann M, Schneider W, Warnke A. Freitag CM. Empathy in children with autism and conduct disorder: group-specific profiles and developmental aspects. Journal of Child Psychology and Psychiatry. 2011;53(6):651–659. doi: 10.1111/j.1469-7610.2011.02499.x. &. doi: 10.1111/j.1469-7610.2011.02499.x. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Fontaine NM, Bird G, Blakemore SJ, De Brito SA, McCrory EJ. Viding E. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Social Cognitive and Affective Neuroscience. 2012b;7(1):53–63. doi: 10.1093/scan/nsr023. &. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM. Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry. 2012a;69(8):814–822. doi: 10.1001/archgenpsychiatry.2011.2070. &. doi: 10.1001/archgenpsychiatry 2011.2070. [DOI] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S. Frith U. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325(5942):883–885. doi: 10.1126/science.1176170. &. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17(1):18–24. doi: 10.1177/1073858410379268. . doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Skuse D, Warrington R, Bishop D, Chowdhury U, Lau J, Mandy W. Place M. The developmental, dimensional and diagnostic interview (3di): a novel computerized assessment for autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(5):548–558. doi: 10.1097/00004583-200405000-00008. &. doi: 10.1097/00004583-200405000-00008. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B. Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. &. doi: 10.1006/nimg 2001.0978. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA. McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. American Journal of Psychiatry. 2012;169(10):1109–1116. doi: 10.1176/appi.ajp.2012.12020191. &. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Völlm BA, Taylor ANW, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JFW. Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29(1):90–98. doi: 10.1016/j.neuroimage.2005.07.022. &. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Shaw P, Lee NR, Clasen LS, Raznahan A, Lenroot RK, Martin A. Giedd JN. Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. Journal of Neuroscience. 2012;32(14):4856–4860. doi: 10.1523/JNEUROSCI.6214-11.2012. &. doi: 10.1523/JNEUROSCI.6214-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M. Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. &. doi: 10.1093/Brain/Awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M. Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Archives of General Psychiatry. 2007;64(6):698–708. doi: 10.1001/archpsyc.64.6.698. &. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yahata N, Abe O, Kuwabara H, Inoue H, Takano Y, et al. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PLoS One. 2012;7(6):e39561. doi: 10.1371/journal.pone.0039561. . doi: 10.1371/journal.pone.0039561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- White S, Hill E, Happé F. Frith U. Revisiting the strange stories: revealing mentalizing impairments in autism. Child Development. 2009;80(4):1097–1117. doi: 10.1111/j.1467-8624.2009.01319.x. &. doi: 10.1111/j.1467-8624.2009.01319.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Diagnostic information for the autism spectrum disorder (ASD) group. Abbreviations: ADOS = autism diagnostic observational schedule, ADI-R = Autism Diagnostic Interview Revised; SCQ = Social and Communication Questionnaire, soc = social, comm = communication, RRBI= rigid and repetitive behaviours and interests. N/A = Not applicable; OCD = obsessive compulsive disorder. Best available instrument refers to whether a diagnostic interview was available (ADI or 3Di), as opposed to the SCQ questionnaire.
Table S2. Whole brain main effects (across group) of the reverse contrast Physical Causality>Theory of Mind (PC>ToM). Results are thresholded at p <.05 FWE-corrected at the peak level across the whole brain. Abbreviations: BA = Brodmann area; k = cluster size.
Table S3. Regions showing a Condition x Group interaction for ToM > PC across the whole brain at p <.005 uncorrected, k > 10. BA = Brodmann area; k = cluster size; TD = typically developing controls; CP/HCU = conduct problem with high callous-unemotional traits; ASD = autism spectrum disorder.


