Abstract
Advances in physiology and biochemistry have provided fundamental insights into the role of pulmonary surfactant in the pathogenesis and treatment of preterm infants with respiratory distress syndrome. Identification of the surfactant proteins, lipid transporters, and transcriptional networks regulating their expression has provided the tools and insights needed to discern the molecular and cellular processes regulating the production and function of pulmonary surfactant prior to and after birth. Mutations in genes regulating surfactant homeostasis have been associated with severe lung disease in neonates and older infants. Biophysical and transgenic mouse models have provided insight into the mechanisms underlying surfactant protein and alveolar homeostasis. These studies have provided the framework for understanding the structure and function of pulmonary surfactant, which has informed understanding of the pathogenesis of diverse pulmonary disorders previously considered idiopathic. This review considers the pulmonary surfactant system and the genetic causes of acute and chronic lung disease caused by disruption of alveolar homeostasis.
Keywords: alveolar proteinosis, interstitial lung disease, respiratory distress syndrome, alveolar capillary dysplasia, pulmonary fibrosis, pulmonary alveolar microlithiasis
LUNG MORPHOGENESIS AND FORMATION OF THE GAS-EXCHANGE REGION OF THE LUNG
The development of the vertebrate lung represents the remarkable adaptation to air breathing that is required for gas exchange and cellular respiration after birth. Derived from anterior foregut endodermal precursors in the embryo, the lung buds invade the splanchnic mesenchyme and undergo branching morphogenesis to form the conducting airways that lead to an expansive region of peripheral saccules, the fetal precursors of alveolar structures that form after birth (Figure 1) (1). Branching morphogenesis, mediated by complex signaling and transcriptional programs, is accompanied by the proliferation of diverse epithelial and mesenchymal progenitor cells that form the epithelial, stromal, and vascular components of the lung. During the stages of sacculation (before birth) and alveolarization (after birth), an ever-increasing number of peripheral airspaces are formed that are closely associated with an extensive network of microvasculature structures lined by endothelial cells; these structures are closely apposed to the alveolar epithelium to mediate the efficient exchange of carbon dioxide and oxygen (Figure 1).
Figure 1.
Stages of lung morphogenesis. Confocal microscopy was used to image lung architecture in the mouse from (a) day E14 (pseudoglandular) to (b) E16.5 (canalicular), (c) E18.5 (saccular), and (d) PN14 (alveolar period). TTF-1 (green) staining identifies epithelial cells; α-SMA (purple) identifies smooth muscle cells. Endomucin (red) marks pulmonary endothelial cells. Arrows indicate the regions of enlargement illustrated in panel insets. Scale bar = 200 µm for all panels. Immunofluorescent images courtesy of Dr. John Shannon, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center and the University of Cincinnati, Cincinnati, Ohio. Abbreviations: α-SMA, α smooth muscle actin; E, embryonic; PN, postnatal; TTF-1, thyroid transcription factor-1.
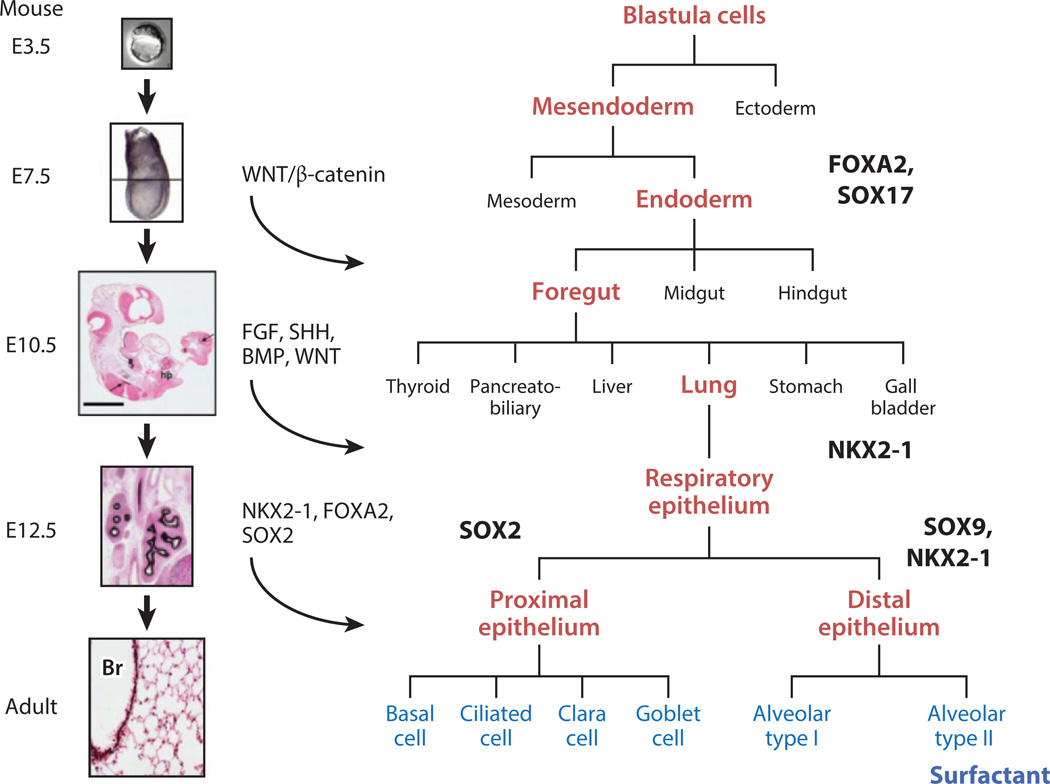
The transcriptional and signaling processes mediating branching morphogenesis of the lung are centered around paracrine interactions among multiple cell types, which specify cell fate, migration, and proliferation. Specification of pulmonary epithelial cell types and mesenchymal progenitors is established in the ventral foregut before formation of the lung bud (2). Foregut endodermal cells require signaling by fibroblast growth factor (FGF), sonic hedgehog (SHH), bone morphogenetic proteins (BMP), and Wingless-type MMTV integration site (WNT) to initiate and maintain normal branching morphogenesis (Figure 2) (for reviews, see 3, 4). Before implantation, progenitor cells in the anterior–ventral region of the embryo are restricted to endodermal cell fate by expression of Forkhead Box (FOXA) 2 and Sry-related HMG-Box (SOX) 17 (Figure 2). The primordial lung bud is first demarcated by the expression of Nkx2 homeobox 1 [NKX2-1, also known as thyroid transcription factor-1 (TTF-1)], a transcription factor required for formation of the lung (Figure 2) (5). Distinct SOX2-expressing progenitor cells are restricted to conducting airways, as SOX2 is required for differentiation of conducting airway epithelial cells (6, 7). Peripheral respiratory epithelial cells that will form the alveoli express SOX9 and NKX2-1 (Figure 2) (8). As gestation advances, airway progenitor cells further differentiate into ciliated, serous/secretory, goblet, basal, and neuroendocrine cells in the conducting airways. Type I and type II alveolar epithelial cells differentiate in the peripheral lung saccules and alveoli. From a clinical perspective, congenital malformations affecting the respiratory tract are caused by disruption of these critical modulators of lung formation. For example, pulmonary malformations are associated with altered FGF signaling, as seen in the following syndromes: Pfeiffer [Online Mendelian Inheritance in Man (OMIM) database #101600], Apert (OMIM #101200), and Crouzon (OMIM #123500) (9, 10); with altered SHH signaling, as seen in VATER/VACTERL (OMIM #192350) and Pallister–Hall syndromes (OMIM #146510) (11); with mutations in transcriptional mechanisms, such as SOX2 (OMIM *184429); as seen in anophthalmia–esophageal–genital syndrome (OMIM #206900) (12); and with mutations in NKX2-1 (OMIM *600635), which cause brain–thyroid–lung syndrome (OMIM #610978) (13, 14). Mutations in FOXF1 (OMIM *601089), a gene expressed in the developing pulmonary mesenchyme, causes severe congenital lung disease, termed alveolar capillary dysplasia with misalignment of pulmonary veins (OMIM #265380) (15). Application of whole exome and genome sequencing of infants with congenital malformations of the respiratory tract will extend our knowledge of the genetic basis of pulmonary disorders that cause severe lung disease after birth.
Figure 2.
Blueprint for specification and differentiation of the respiratory epithelium in the mouse, from day E3.5 to adult. The lungs are derived from progenitor cells located in the ventral region of the embryonic foregut. Endoderm is specified from neuroectoderm and mesoderm prior to the implantation of the embryo. This process is mediated in part by the expression of SOX17 and FOXA2, transcription factors critical for the formation of endoderm. Under the influence of WNT, SHH, FGF, and BMP signaling, the lung buds are distinguished from the gut tube and marked by the expression of NKX2-1 (thyroid transcription factor-1). The esophagus separates from the trachea, the latter marked by expression of SOX2, which is required for the formation and differentiation of airways and airway epithelial cells. Peripheral lung progenitors, expressing both NKX2-1 and SOX9, produce alveolar type I and type II epithelial cells. Surfactant proteins and lipids are produced after the differentiation of type II epithelial cells, which in preparation for birth are also under the control of NKX2-1, FOXA2, and associated transcription factors. Mutations in the genes encoding the transcription factors SOX2 and NKX2-1 cause pulmonary malformations; NKX2-1 regulates genes that are critical for alveolar homeostasis after birth. Abbreviations: Br, bronchiole; E, embryonic.
TRANSITION TO AIR BREATHING/PULMONARY MATURATION
Many of the signaling and transcriptional mechanisms regulating early lung branching remain highly active in late gestation as the fetal lung undergoes sacculation, and airway epithelial cells differentiate to produce pulmonary surfactant (16). During the canalicular, saccular, and alveolar periods of lung morphogenesis, pulmonary mesenchymal cells differentiate to form fibroblasts, lipofibroblasts, pericytes, matrix-associated fibroblasts, smooth muscle, and endothelial cells in the distal lung. From the end of the pseudoglandular period to the beginning of the saccular period of development, the number of peripheral lung tubules increases dramatically and then tubules undergo sacculation as the gas exchange region of the lung forms (Figure 1). Mesenchymal components become relatively less abundant, and as the gas exchange region is created before birth, an extensive pulmonary microvascular capillary bed is formed in close apposition to the epithelial cells (Figure 1). Differentiation of the epithelial cells lining the peripheral lung saccules produces large, squamous, type I epithelial cells that comprise the majority of the alveolar surface and mediate gas exchange. Smaller, cuboidal, alveolar type II epithelial cells account for approximately two-thirds of the alveolar epithelium. Type II epithelial cells produce pulmonary surfactant, which is necessary for reducing surface tension at the air–liquid interface after initiation of ventilation at birth. Many of the genes and transcriptional networks critical for early lung formation have important roles in lung sacculation, including signaling by FOXA2, NKX2-1, SOX9, WNT, β-catenin, SHH, and FGF (Figure 2) (16).
THE PULMONARY SURFACTANT SYSTEM
The seminal study by Avery & Mead in 1959 (17) demonstrated that hyaline membrane disease, now termed infantile respiratory distress syndrome (RDS), was caused by a lack of surfactant lipids in the lungs of the preterm infants who died from this common disorder. Advances in physiology, biochemistry, and molecular biology have identified the chemical composition of pulmonary surfactant as well as the genes and cellular processes involved in surfactant lipid and protein homeostasis. These studies have provided molecular and genetic tools that are useful in identifying the genetic defects underlying the pathogenesis of RDS and the genetic causes of respiratory disease in newborns, infants, and children. Pulmonary surfactant is primarily composed of phospholipids that are enriched with phosphatidylcholine (PC) and phosphatidylglycerol (PG); these phospholipids are produced in increasing amounts in late gestation by type II epithelial cells. Surfactant lipids are routed from the endoplasmic reticulum (ER) to the multivesicular body (MVB) and then to the lamellar body (LB), the surfactant storage organelle in type II epithelial cells (Figure 3). Four surfactant proteins (SPs)—SP-A, SP-B, SP-C, and SP-D—have been identified as critical components of alveolar surfactant, each contributing to lung homeostasis via their distinct protein structures and activities (18). Surfactant lipids and proteins are synthesized primarily by type II epithelial cells, and these are not fully differentiated in preterm infants, resulting in insufficient pulmonary surfactant, which contributes to the pathogenesis of RDS. Surfactant proteins are produced by type II epithelial cells in increasing amounts prior to birth. NKX2-1, a nuclear transcription factor critical for lung morphogenesis (5), binds to and regulates the transcription of surfactant-associated genes and is required for normal surfactant synthesis at birth (19, 20). Knowledge of the surfactant proteins and lipids, including the addition of SP-B and SP-C as critical components of the surfactant-replacement preparations used to treat RDS, enabled widespread use of these preparations to prevent and treat RDS. The intratracheal administration of formulations containing surfactant lipids, SP-B, and SP-C has dramatically improved morbidity and mortality from RDS in preterm infants (21, 22).
Figure 3.
Biosynthesis of surfactant likely involves distinct pathways for surfactant proteins and lipids. SP-B and SP-C are trafficked from the endoplasmic reticulum to lamellar bodies via the Golgi complex and MVB; in contrast, surfactant phospholipids are likely directly transported from the endoplasmic reticulum to specific lipid importers (ABCA3) in the lamellar body–limiting membrane. Surfactant proteins and lipids are assembled into bilayer membranes that are secreted into the alveolar airspace, where they form a surface film at the air–liquid interface. Cyclical expansion and compression of the bioactive film results in the incorporation (large green arrow) and loss (red arrows) of lipids and proteins from the multilayered surface film. Surfactant components removed from the film are degraded in alveolar macrophages or are taken up by type II epithelial cells for recycling or degradation in the lysosome (red arrows). The MVB plays a key part in the integration of pathways for surfactant synthesis, recycling, and degradation. Abbreviations: ABCA3, ATP-binding cassette transporter A3; GM-CSF, granulocyte macrophage colony–stimulating factor; MVB, multivesicular body; PC, phosphatidylcholine; PG, phosphatidylglycerol; SP/SFTP, surfactant protein.
SURFACTANT METABOLISM
Surfactant lipids are synthesized by type II epithelial cells in the ER. Metabolic substrates for lipogenesis are derived from precursors taken up from the circulation, from de novo synthesis of lipids, and from reuptake of lipids by type II epithelial cells. Within type II epithelial cells, PC is transferred to LBs in a process requiring adenosine triphosphate (ATP)-binding cassette transporter A3 (ABCA3), a membrane-spanning transporter of the ATP-binding cassette family of proteins that is located on the limiting membrane of the LB. Surfactant precursor proteins pro-SP-B and pro-SP-C are processed proteolytically by napsin, cathepsin, and pepsinogen during trafficking to MVBs and LBs. The active hydrophobic proteins SP-B and SP-C are assembled with surfactant phospholipids into bilayer membranes that are stored in LBs (for a review, see 18). The contents of LBs are secreted into the airway via a process stimulated by catecholamines, purinoreceptor agonists, and cell stretch. Control of secretory processes requires GPR116, an orphan G protein–coupled receptor located on respiratory epithelial cells, which modulates the secretory process (23, 24).Genetic deletion of Gpr116 in mice causes excess secretion of surfactant with accumulation in the airspaces after birth. After secretion, LBs unwind and interact with SP-A and SP-D to produce tubular myelin and multilayered surface films that spread over the alveolus and reduce surface tension. SP-A and SP-B are required for formation of tubular myelin (25, 26) and have important roles in innate host defense in the alveolus. SP-D regulates extracellular forms of surfactant and has an important role in controlling the size of the surfactant lipid pool (27, 28). SP-D and SP-A are members of the collectin family of host defense proteins that bind pulmonary microbial pathogens and products, including viruses, fungi, and bacteria, and serve as critical innate host defense proteins with anti-inflammatory properties (29–31). Pulmonary surfactant is recycled, catabolized or reutilized actively by alveolar type II epithelial cells in a process influenced by surfactant proteins. Alveolar macrophages play a critical part in surfactant uptake and degradation in a process that depends upon signaling by granulocyte macrophage colony–stimulating factor (GM-CSF) or CSF2 (for a review, see 32). Autoantibodies against GM-CSF or mutations in GM-CSF receptors cause the disorder known as pulmonary alveolar proteinosis (PAP). Figure 3 provides an integrated schematic of important aspects of the processes critical for surfactant homeostasis in the alveolus.
UNIQUE BIOPHYSICAL ACTIVITY OF PULMONARY SURFACTANT
The close apposition of alveolar type I epithelial cells to underlying capillary endothelial cells forms a highly diffusible air–blood barrier across which gas exchange occurs. The stabilization of alveolar structure during breathing-induced expansion and contraction is achieved by the formation and maintenance of a phospholipid-rich film (pulmonary surfactant) that spreads over the thin liquid layer (the aqueous hypophase) that covers the alveolar epithelial cell surface (Figure 3) (33). The unique biophysical properties of surfactant prevent alveolar collapse (atelectasis) at low lung volumes by reducing surface tension, which is generated by the aqueous hypophase, to very low levels (<2 mN/m). During alveolar expansion, surface tension increases (to a maximum of 20–25 mN/m), stabilizing the alveolus at higher lung volumes. The unique biophysical properties of the surface film are directly related to the incorporation of dipalmitoyl phosphotidylcholine (DPPC), a saturated phospholipid that allows acyl chains to be very tightly packed as the film is compressed during exhalation. Incorporation of cholesterol and phospholipids with the unsaturated acyl chains helps to maintain the fluidity of the surface film at body temperature. SP-B and SP-C facilitate remodeling of newly secreted surfactant membranes by promoting the incorporation and spreading of lipids as the surface film expands during inhalation. Neonatal lethality in knockout mice indicates that SP-B is indispensable for this process (25). SP-C deficiency is not lethal and the function of SP-C is less well understood, but it is likely required for optimal function of the surface film. Lipid–protein complexes are removed from the surface film during compression and are degraded by alveolar macrophages or are recycled in type II epithelial cells; the recycling process depends at least partly on SP-D, which enhances uptake of surfactant lipids by type II epithelial cells (34). Overall, maintenance of the surface film is a highly dynamic process that requires integration of pathways involved in synthesis and assembly, secretion, recycling, and degradation of surfactant. Dysregulation can lead to alterations in the size, composition, or both of the alveolar surfactant pool, resulting in PAP (surfactant accumulation) or RDS (surfactant insufficiency). Thus, sensing of the size or composition, or both, of the alveolar pool is essential for pathway integration and maintenance of alveolar homeostasis. Recent evidence has suggested that GPR116 may be a component of the sensing machinery (23, 24), but knowledge about the signaling pathway that couples this receptor to surfactant metabolism is lacking.
GENETIC DISORDERS OF SURFACTANT HOMEOSTASIS AND ALVEOLAR DEVELOPMENT
The identification of the genes and cellular processes involved in maturation and function of the pulmonary surfactant system, as well as in alveolar development, has provided genetic tools that are useful for diagnosing rare lung diseases presenting in newborns, infants, and children.
Abnormalities in Surfactant Packaging, Function, and Degradation
Pathological and genetic analyses of full-term infants with refractory respiratory failure after birth have identified the role of mutations in the genes encoding SP-B (SFTPB), SP-C (SFTPC), and ABCA3 (ABCA3) in the pathogenesis of respiratory failure and diffuse, chronic interstitial lung disease (ILD) in newborn infants and children (35–37). Classification of the histopathology associated with these disorders (Table 1) includes neonatal or congenital PAP, infantile desquamative interstitial pneumonia (DIP), chronic pneumonitis of infancy, nonspecific interstitial pneumonia (NSIP), and usual interstitial pneumonia (UIP) (in older children and adolescents). The histopathology of these disorders often overlaps and depends on the nature of the mutations, the age of the child, and the clinical therapy provided. Mutations in SFTPB and ABCA3 are inherited as autosomal recessive genes, usually presenting in full-term newborn infants as cyanosis, grunting, retractions, and atelectasis. Pulmonary disease associated with these mutations is unremitting despite the use of intensive care and surfactant replacement; this contrasts with RDS that is associated with prematurity in newborns and responds to surfactant replacement. Mutations in the SFTPC gene are inherited as autosomal dominant disorders that are associated with chronic ILD in older infants, children, and adults; infants and children with chronic ILD often present with symptoms of diffuse lung disease including tachypnea, retractions, hypoxemia, and digital clubbing. Mutations in the genes encoding receptors for GM-CSF (CSF2RA, CSF2RB) are inherited as autosomal recessive genes and are associated with progressive dyspnea and congenital PAP. These disorders are classified as surfactant metabolism dysfunctions, pulmonary (SMDP) types 1, 2, 3, and 4, and are summarized in Table 2.
Table 1.
Histopathology of genetic lung disease
| Histopathology | Age groups | Characteristics | Associated genetic mutations or acquired disorder |
|---|---|---|---|
| Growth abnormalities | Newborns, infants, and children | Alveolar simplification with poorly septated airspaces; malformed lobules | FOXF1, NKX2-1 |
| Pulmonary alveolar proteinosis, neonatal onset | Newborns and infants | Intraalveolar accumulation of granular, eosinophilic, or PAS-positive lipoproteinaceous material, with or without accumulation of large, foamy alveolar macrophages hyperplastic alveolar type 2 cells, and septal thickening | SFTPB, SFTPC, ABCA3, NKX2-1 |
| Pulmonary alveolar proteinosis, pediatric and adult onset | Children and adults | Intraalveolar accumulation of granular, eosinophilic, or PAS-positive lipoproteinaceous material, with or without accumulation of large, foamy alveolar macrophages | CSF2RA, CSF2RB, anti-CSF2 |
| Chronic pneumonitis of infancy | Newborns and infants | Septal thickening with mild lymphocytic inflammation and muscularization of the alveolar septa; intraalveolar accumulation of foamy macrophages; focal proteinosis and cholesterol clefts; hyperplastic type 2 cells | SFTPC |
| Desquamative interstitial pneumonia, infantile | Newborns and infants | Intraalveolar accumulation of alveolar macrophages, with or without hyperplasia of alveolar type 2 cells | ABCA3, SFTPB, SFTPC, NKX2-1 |
| Nonspecific interstitial pneumonia | Children and adults | Interstitial pneumonitis with diffuse lymphocytic infiltrates and varying degrees of fibrosis | SFTPC, ABCA3, NKX2-1 |
| Usual interstitial pneumonia | Children and adults | Alternating areas of normal lung with fibroblastic foci; progressing to dense fibrosis, with remodeling and scarring of the lung in adults | SFTPC, ABCA3, SFTPA2, TERT, TERC |
Abbreviations: PAS, periodic acid–Schiff.
Table 2.
Comparison of genetic disorders affecting surfactant metabolism
| Gene | SFTPB | SFTPC | ABCA3 | CSF2RA and CSF2RB |
|---|---|---|---|---|
| Chromosome | 2p11 | 8p21 | 16p13 | Xp22 and 22q12 |
| Protein | SP-B | SP-C | ABCA3 | CSF2RA and CSF2RB |
| Phenotype | Surfactant dysfunction 1 | Surfactant dysfunction 2 | Surfactant dysfunction 3 | Surfactant dysfunctions 4 and 5 |
| Inheritance | Autosomal recessive | Autosomal dominant or sporadic, with variable penetrance | Autosomal recessive | Autosomal recessive |
| Mechanism | Loss of function | Dominant negative or toxic gain of function | Loss of function | Loss of function |
| Pathogenesis | Lack of SP-B | Lack of mature SP-C; misfolded protein with ER stress | Defective phospholipid transport into LBs | Defective GM-CSF signaling |
| Age of onset | Neonatal | Infancy to adult | Neonatal > childhood | Childhood or neonatal > childhood > adult |
| Clinical syndrome | RDS | ILD > RDS | RDS > ILD | Respiratory distress and/or insufficiency; dyspnea; tachypnea |
| Outcome | Neonatal lethal | Highly variable | Lethal if neonatal; variable severity in childhood | Variable severity |
| Histopathology | PAP > DIP | Depends on age and mutation Infants: CPI > PAP or DIP Children/adults: NSIP, UIP |
Depends on age and mutation Infants: PAP, DIP Children/adolescents: NSIP, UIP |
PAP |
| LB phenotypes | Abnormal: large MVBs with no normal LBs | Variable: normal LBs and/or ↑ in large fusing LBs | Variable: small, dense LBs > normal LBs | NDa |
Abbreviations: ABCA3, ATP-binding cassette transporter A3; CPI, chronic pneumonitis of infancy; CSF2R, granulocyte-macrophage colony–stimulating factor receptor; DIP, desquamative interstitial pneumonia; ER, endoplasmic reticulum; ILD, interstitial lung disease; LB, lamellar body; MVB, multivesicular body; NSIP, nonspecific interstitial pneumonia; PAP, pulmonary alveolar proteinosis; RDS, respiratory distress syndrome; SP/SFTP, surfactant protein; UIP, usual interstitial pneumonia.
ND, not determined for type II cells; large, foamy alveolar macrophages filled with surfactant material or large vacuoles containing neutral lipids, or both, were observed with electron microscopy.
Mutations in SFTPB associated with fatal lung disease in newborn infants
The surfactant protein SP-B is encoded by SFTPB on human chromosome 2p11.2 (OMIM *178640), which produces a 381-amino-acid preproprotein that is expressed and processed by type II epithelial cells. Mutations in SFTPB are associated with a complete loss of SP-B protein as well as with secondary effects on the processing of pro-SP-C to its mature peptide that result in the accumulation of partially processed, inactive pro-SP-C in the alveoli (35, 38, 39). SP-B is processed proteolytically to the active, 79-amino-acid, amphipathic peptide that is closely associated with lipids in LBs and alveolar surfactant (for a review, see 40). Studies in Sftpb−/− mice have demonstrated that the effects of SP-B are specific to the lung, with newborn mice dying from atelectasis and respiratory failure at the time of birth in the absence of SP-B (25). SP-B has critical roles in the intracellular packaging and processing of surfactant lipids and proteins. SP-B is required for the formation of LBs, proteolytic processing of pro-SP-C, formation of tubular myelin, and generation of the surface-active films required to reduce surface tension (18; for a review, see 40). Infants who are deficient in SP-B may have a family history of perinatal respiratory failure in siblings because the mutations are inherited as autosomal recessive alleles. A number of distinct SFTPB mutations have been identified in infants with neonatal respiratory failure, enabling both prenatal and postnatal genetic diagnoses (39, 41). Rarely, less severe mutations in SP-B production are consistent with the diagnosis of chronic lung disease in infants (42). SFTPB-related lung disease (SMDP1 #265120) is generally fatal in the first months of life, although some infants have been supported by lung transplantation (43, 44). Surfactant replacement and other ventilatory support are not effective therapies for this disorder. Histopathological diagnoses described in SP-B deficiency are associated primarily with neonatal PAP or infantile DIP. Histological findings include (a) variable amounts of amorphous, eosinophilic and/or periodic acid–Schiff (PAS)-positive, lipoproteinaceous material in the alveolar spaces; (b) foamy alveolar macrophages; (c) alveolar epithelial hyperplasia with prominent alveolar type II cells; and (d) thickened interstitial septa (Figure 4).
Figure 4.
Histopathology of genetic disorders of surfactant homeostasis. (a) Histopathological specimen from the lung of a neonate with a lethal SFTPB mutation, demonstrating the typical pattern of pulmonary alveolar proteinosis composed of intraalveolar, foamy, eosinophilic, lipoproteinaceous material and thickened alveolar septa. (b) Histopathological specimen from the lung of an infant with a SFTPC mutation and chronic pneumonitis of infancy, showing muscularization of the thickened alveolar septa; patchy, intraalveolar, granular, proteinosis material; and alveolar type II cell hyperplasia. (c) Histopathological specimen from the lung of a neonate with a lethal ATP-binding cassette transporter A3 (ABCA3) mutation, demonstrating granular, eosinophilic, alveolar proteinosis material mixed with macrophages; thickened alveolar septa; and alveolar type II cell hyperplasia. (d) Histopathological specimen from the lung of a child with a mutation in the αchain of the granulocyte macrophage colony–stimulating factor (GM-CSF) receptor (CSF2RA), demonstrating both foamy and globular intraalveolar pulmonary alveolar proteinosis but with alveolar septa that appear thin and more normal. (e) Histopathological specimen from an infant with an NKX2-1 mutation, demonstrating features consistent with the genetic surfactant disorders including thickened, muscularized alveolar septa; intraalveolar accumulation of foamy macrophages (as seen in desquamative interstitial pneumonia); and hyperplastic alveolar type II cells. (f) Histopathological specimen from an infant with an NKX2-1 (TTF-1) mutation, demonstrating a mixed phenotype consisting of a diffuse alveolar growth abnormality that is superimposed on chronic interstitial inflammation and fibrosis and that resembles nonspecific interstitial pneumonia. All specimens are stained with hematoxylin and eosin; scale bars = 200 µm for all panels.
Mutations in SFTPC associated with interstitial lung disease
SFTPC is located on chromosome 8p21.3 (OMIM *178620) and encodes the preprotein pro-SP-C that is expressed selectively in alveolar type II epithelial cells in the lung (for a review, see 18). Like SP-B, SP-C is produced by proteolytic processing of its 197-amino-acid precursor during transit from the ER to MVBs and LBs, where it is stored with phospholipids. The active SP-C peptide of 35 amino acids consists of a highly hydrophobic α helix that spans lipid membranes and contributes to the spread of surfactant in the alveoli. The preprotein contains a C-terminal BRICHOS domain that is involved in the proper folding and trafficking to the secretory pathway (45, 46). While SP-C is not required for survival of newborn Sftpc−/− mice, a lack of SP-C causes progressive lung inflammation, emphysema, and remodeling in some mouse strains as well as marked susceptibility to infection, inflammation, and fibrosis, indicating its role in innate immune responses in this animal model (47–49). Acute and chronic lung disease are associated with mutations in SFTPC, which cause missense mutations in the BRICHOS domain and result in the misfolding, misrouting, and/or misprocessing of pro-SP-C that then accumulates within type II alveolar cells, causing cell toxicity (45, 50–52).Cellular responses to the misfolded pro-SP-C products include ER stress, the activation of reactive oxygen species, autophagy, and injury related to the accumulation of misfolded protein in abnormal cellular compartments. SFTPC-related disease (SMDP2 #610913) is typically inherited as an autosomal dominant disease with variable penetrance (41, 53–55), although sporadic mutations also have been identified (41, 54). Infants with SFTPC mutations usually exhibit features of chronic pneumonitis of infancy, including diffuse alveolar injury with regenerating alveolar epithelia lined by hyperplastic type II epithelial cells, variable amounts of alveolar proteinosis material containing cholesterol clefts, foamy macrophages, and interstitial thickening with lymphocytic inflammation and muscularization of the alveolar walls (Figure 4) (35, 36, 56). Patients with SPC-related disease who present later in childhood exhibit chronic interstitial lung disease, usually classified as NSIP or UIP (36, 37); older adults often present with ILD or pulmonary fibrosis (55). Variability and presentation are influenced by the mutations as well as by environmental factors including viral infections (57, 58). The age at presentation and severity vary greatly within the same kindred (55). Definitive diagnosis is made by identifying mutations in the SFTPC gene. The observation that cell injury is caused by the misfolding of pro-SP-C provided critical insight into the role that injury to alveolar epithelial cells has in the pathogenesis of ILD (for a review, see 51). Although mutations in SFTPC are not a common cause of ILD in adults (59), 25% of patients in a Dutch cohort with familial pulmonary fibrosis carried SFTPC mutations (60). Both pharmaceutical and genetic approaches to inhibit the production or misfolding of the mutant protein are being studied. Hydroxychloroquine, as well as lung transplantation, have been used for therapy (37, 58, 61).
Mutations in ABCA3 associated with acute and chronic lung disease
Mutations in ABCA3 (chromosome 16p13.3; OMIM #601615) are the most common cause of hereditary respiratory failure in newborns. ABCA3 is a large membrane-spanning protein sharing structural similarities with the cystic fibrosis transmembrane conductance regulator (CFTR). Mutations have been identified throughout the ABCA3 gene that cause abnormal processing, misrouting, or impaired lipid transport (62–65) as well as secondary effects on processing SP-B and SP-C (66, 67). Lung histology is consistent with congenital PAP or infantile DIP (Figure 4) (35, 68, 69). Ultrastructural studies of type II cells from these patients demonstrate the absence of normal LBs and the presence of electron-dense inclusions within small vesicular structures, both of which are characteristic of the disorder (35, 68, 70). While ABCA3 is expressed in various tissues, abnormalities are found only in the lung. Pulmonary disease associated with ABCA3 deficiency is generally fatal (68, 69), although a number of infants have been treated by lung transplantation (43). Prenatal and postnatal diagnoses are best made by identifying mutant ABCA3 alleles. Diseases related to ABCA3 and SFTPB are similar in onset and clinical course and are generally associated with severe respiratory failure, although several ABCA3 mutations have been associated with chronic ILD in older infants, children, and adolescents (67, 71–73). Histopathological findings vary depending upon the age of the patient and the mutation and often overlap with those found in deficiencies of SP-B and SP-C. Biopsy specimens obtained from young infants are associated with PAP and DIP; those obtained from older infants are associated with DIP and NSIP; and those from older children are associated with NSIP, which often exhibits additional focal proteinosis, cholesterol clefts, and thickened alveolar septa. Clinical symptoms in older infants and children include cough, tachypnea, dyspnea, exercise intolerance, digital clubbing, failure to thrive, and hypoxemia. Treatment modalities include systemic corticosteroids, hydroxychloroquine, and lung transplantation.
Role of GM-CSF signaling defects in pulmonary alveolar proteinosis
Although surfactant catabolism by alveolar macrophages represents a relatively small contribution to surfactant recycling and metabolism, studies in transgenic mice in which GM-CSF (Csf) and GM-CSF receptors (Csf2ra and Csf2rb) were mutated or deleted, found that the mice developed a syndrome virtually identical to that associated with idiopathic PAP (iPAP) in adult patients (74, 75). Analysis of surfactant catabolism in mouse models has demonstrated that GM-CSF signaling activated PU.1 and was required not only for the maturation of alveolar macrophages but also for their function, including their ability to catabolize surfactant lipids and proteins (76). Defects in surfactant catabolism related to a lack of GM-CSF signaling result in the accumulation of surfactant in the alveolus. Most patients with iPAP present with progressive dyspnea and radiographic findings termed crazy paving, which are caused by heterogeneous alveolar infiltrates resulting from the accumulation of surfactant. The seminal findings by Kitamura et al. (77) demonstrated that most patients with adult iPAP produced neutralizing antibodies against GM-CSF (CSF2; chromosome 5q31.1; OMIM #13906), defining the disorder as an acquired, autoimmune disease (OMIM #610910). Pathological findings consist of heterogeneous accumulation of PAS-positive diastase-resistant surfactant material in the peripheral lung parenchyma associated with foamy macrophages that are rich in neutral lipids. In general, alveolar structures are preserved in iPAP (Figure 4). Subsequently, both pediatric and adult onset of PAP have been associated with mutations in the GM-CSF receptors CSF2RA (chromosome Xp22.33; OMIM #306250) (78–80) or CSF2RB (chromosome 22q12.3; OMIM #138981) (81–83). Patients with these mutations develop congenital PAP with clinical and histopathological findings identical to those of acquired (autoimmune) iPAP. Congenital PAP is inherited as an autosomal recessive disorder (OMIM #300770 and #614370). CSF2RA is located on the X chromosome’s pseudoautosomal region 1. Sequential bronchoalveolar lavage has been utilized to treat acquired autoimmune iPAP for many years (84). The recognition that defective GM-CSF signaling underlies the disorder has provided new diagnostic approaches for measuring GM-CSF autoantibodies and other serum markers of the disease (e.g., SP-D and KL6). Functional assays have been developed to assess the function of GM-CSF-dependent macrophages. The use of inhaled GM-CSF and rituximab, the latter used to inhibit production of autoantibodies, has been actively studied for treatment of iPAP (85–88). Similarly, novel treatments based on restoring GM-CSF receptor activity using gene or cell transfer are being actively studied in animal models for the potential development of new therapeutic strategies to treat congenital PAP (89, 90).
Role of NKX2-1 in the Pathogenesis of Brain-Lung-Thyroid Syndrome
Analysis of the functions of NKX2-1 (chromosome 14q13.3;OMIM#600635) in transgenic mouse models has demonstrated its critical role in initiating lung morphogenesis and perinatal lung maturation (5, 20). NKX2-1 is a transcriptional regulator of many of the genes involved in surfactant function and lung maturation (19). NKX2-1 is a highly conserved member of a larger family of homeodomain-containing transcription factors involved in organogenesis and cell differentiation in various organs. NKX2-1, encoding TTF-1, is selectively expressed throughout development in forebrain, thyroid, and lung (91). Mutations in NKX2-1 cause a variably penetrant disorder that affects the central nervous system of newborns—usually manifesting as a choreiform movement disorder, thyroid agenesis, or hypoplasia with associated hypothyroidism—and produces both acute and chronic respiratory disease (OMIM #610878) (13, 14). NKX2-1 is located on chromosome 14q13.3 near NKX2–8 and PAX9, genes that are also expressed in the respiratory epithelium. Haploinsufficient mutations in NKX2-1 are associated with pulmonary disease in infants and with variable inhibitory effects on the expression of target genes (e.g., the surfactant proteins) (13, 92, 93). The clinical presentation of NKX2-1-related pulmonary disease is also variable, ranging from neonatal RDS with and without pulmonary hypertension to ILD in older children and pulmonary fibrosis in adults (13, 14, 92–96). Recurrent respiratory infections are frequently encountered (14). Clinically, the diagnosis is supported by congenital hypothyroidism together with lung disease in a term infant. The pulmonary histopathology is heterogeneous, both among and within individual cases (13, 14, 92). In some cases, histological features are consistent with those seen in the genetic surfactant disorders and include thickening of the alveolar septa, hyperplasia of alveolar type II epithelial cells, and accumulation of foamy alveolar macrophages (Figure 4). In other cases, there are clear growth abnormalities with alveolar simplification and cyst formation. Homogeneous regions of alveolar septal thickening with fibrosis and chronic interstitial inflammation, resembling NSIP, have also been found in several cases (Figure 4). Genetic analysis of the NKX2-1 locus is useful for diagnosis by identifying point mutations and deletions in the gene. Recently, an infant who presented with features consistent with brain-lung-thyroid disorder and an intact NKX2-1 coding region was found to have a deletion in a noncoding segment of DNA located in a potential regulatory region of the NKX2-1 locus, demonstrating that alterations in noncoding regulatory regions of the NKX2-1 gene may also be responsible for this syndrome (13, 14, 97).
Role of FOXF1 in the Pathogenesis of Alveolar Capillary Dysplasia
FOXF1 (chromosome 16q24.1, OMIM #601089) is a member of the forkhead ortholog family of transcription factors that is expressed in the embryonic splanchnic mesenchyme. Deletion of Foxf1 in the mouse has been shown to cause severe pulmonary vascular and gastrointestinal malformations that are lethal at birth (98). Subsequently, mutations in FOXF1 have been identified as the most common genetic cause of alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV) (15). These infants generally present soon after birth as full-term infants with severe pulmonary hypertension and respiratory failure. ACD/MPV is suspected when severe cyanosis is refractory to supportive and pharmacological therapies in the absence of structural heart disease, with or without a family history of respiratory failure in the immediate postnatal period. Patients with ACD/MPV often have abnormalities in other organs, including gastrointestinal, genitourinary, and cardiovascular malformations (15, 99–103). Infants with ACD/MPV are often placed on extracorporeal membrane oxygenation but fail to respond to intensive care. More than 30 FOXF1 mutations have been identified in patients with ACD/MPV (104). Most are sporadic mutations, although several have been inherited as autosomal recessive disorders with maternal inheritance consistent with paternal imprinting, thus enabling prenatal and postnatal genetic diagnoses to be made (104). Pulmonary pathology in ACD/MPV is characterized by abnormalities in the growth and development of the respiratory parenchyma, which result in immature lobular development with reduced alveolar and capillary formation as well as immature thickened alveolar septa or mesenchyme. Overall, these features imply severe growth arrest of the distal lung and suggest that pulmonary immaturity, with reduced levels of surfactant and poor oxygen diffusion, causes respiratory distress and failure in these cases. In addition, the pulmonary veins are thin-walled, dilated, congested, and mislocated or malpositioned within the pulmonary segments. Instead of coursing independently through the parenchyma, the pulmonary veins are found adjacent to the pulmonary arteries as part of the bronchoarterial compartment (Figure 5). The arteries and arterioles are muscularized and can be immunostained with antibodies to α smooth muscle actin (Figure 5). As indicated above, both the alveolar compartment and the capillary bed are malformed and reduced. The capillaries are dilated and are generally located in abnormally thickened interstitial regions of the distal lung instead of being in close proximity to the alveolar epithelium (Figure 5).
Figure 5.
Histopathology of alveolar capillary dysplasia with misalignment of the pulmonary veins. Histopathological specimen from the lung of a neonate with a lethal FOXF1 mutation. (a) The pulmonary veins are large, dilated, congested vessels that are mislocated and lie adjacent to the pulmonary artery in the bronchoarterial compartment. (b) The pulmonary medial wall of the arteries is hypertrophic with increased immunostaining for α smooth muscle actin. (c) The pulmonary lobes and segments are hypoplastic, with abnormal formation of the parenchyma. There are decreased numbers of acinar and alveolar structures, with thickened interacinar and alveolar septa. The capillary bed is abnormal and has a decreased number of capillaries; these are disorganized and located primarily in the center of the thickened interstitial compartment (arrows). (d) Immunostaining for von Willebrand factor, a marker of endothelial cells, highlights the abnormal capillary network (arrows). Specimens in panels a and c were stained with hematoxylin and eosin; panels b and d were stained with immunoperoxidase and nuclear fast red counterstain. Scale bars for panels a and b = 200 µm; scale bars for panels c and d = 100 µm. Abbreviations: Art, artery; Br, bronchiole.
GENETIC DISORDERS ASSOCIATED WITH ALVEOLAR CELL INJURY: ADULT ONSET OF INTERSTITIAL LUNG DISEASE
Interstitial pulmonary fibrosis (IPF) is a progressive disease that involves chronic injury to the alveolar parenchyma, resulting in the replacement of normal lung tissue with excess connective tissue, and limiting oxygen diffusion across the alveolar–capillary membranes. Recently, genetic disorders that compromise alveolar cell function or cause cell injury have been associated with familial IPF (Table 3).
Table 3.
Comparison of genetic disorders affecting growth, patterning, and fibrotic remodeling of the lungs
| Gene | NKX2-1 | FOXF1 | SFTPA2 | SLC34A2 | TERC and TERT |
|---|---|---|---|---|---|
| Chromosome | 14q13 | 16q24 | 10q22 | 4p15 | 3q26 and 5p15 |
| Protein | NKX2-1 (TTF-1) | FOXF1 | SP-A | SLC34A2 | TERC and TERT |
| Phenotype | Brain-thyroid-lung syndrome | ACD/MPV | IPF | IPF | IPF |
| Inheritance | Sporadic or autosomal dominant with variable penetrance | Sporadic or autosomal dominant → maternal inheritance with paternal imprinting | Autosomal dominant | Autosomal recessive | Autosomal dominant with incomplete penetrance |
| Mechanism | Unknown | Unknown | Loss of function | Loss of function | Loss of function |
| Pathogenesis | Unknown | Unknown | Misfolded protein with ER stress | Reduced phosphate clearance | Telomere shortening |
| Age of onset | Neonatal > childhood | Neonatal | Adults | Adults | Adults |
| Clinical Syndrome | RDS > ILD | RDS | Familial IPF; lung cancer | Familial IPF | Familial IPF |
| Outcome | Lethal if neonatal; variable severity in childhood | Lethal if neonatal | Survival into adulthood | Survival into adulthood | Survival into adulthood |
| Histopathology | Growth disorder with alveolar simplification and lobular remodeling; PAP, DIP, NSIP | ACD/MPV; growth disorder with impaired lobe formation | UIP, pulmonary adenocarcinoma | Pulmonary microlithiasis | UIP |
Abbreviations: ACD/MPV, alveolar capillary dysplasia with misalignment of pulmonary veins; DIP, desquamative interstitial pneumonia; ER, endoplasmic reticulum; FOX, forkhead box; ILD, interstitial lung disease; IPF, interstitial pulmonary fibrosis; NKX2-1, Nkx2 homeobox 1; NSIP, nonspecific interstitial pneumonia; PAP, pulmonary alveolar proteinosis; RDS, respiratory distress syndrome; SP/SFTP, surfactant proteins; TERC, telomerase RNA compartment; TERT, telomerase reverse transcriptase; TTF-1, thyroid transcription factor-1; UIP, usual interstitial pneumonia.
Mutations in SFTPA Associated with Familial Pulmonary Fibrosis
SP-A is encoded by two genes, SFTPA1 and SFTPA2, located near SFTPD (105) on chromosome 10q22.3 (OMIM #178630 and *178642, respectively). Heterozygous mutations in SFTPA2 have recently been recognized as a rare cause of adult-onset IPF (OMIM #178500) and lung cancer in older individuals (106). Mutations in SFTPA2 likely disrupt normal routing and processing of the protein, supporting the concept that, like mutations in SFTPC, SP-A-related disease results from misfolding of the protein in alveolar type II epithelial cells. The mutant proteins are retained in the ER, which leads to activation of the unfolded protein response and ER-associated degradation pathways, chronic ER stress, and cell injury (107). Recent studies have supported the role of mutant forms of both SFTPA2 and SFTPA1 in the activation of latent transforming growth factor β (TGF-β), which results in a profibrotic response (108, 109).
Mutations in the Phosphate Transporter SLC34A2 Associated with Pulmonary Microlithiasis
Pulmonary microlithiasis (OMIM #265100) is a rare disorder in which patients present with dyspnea, interstitial lung disease, and the characteristic micronodular calcifications seen on radiographs and computed tomography (CT) scans (for a review, see 110). It is an autosomal recessive disorder that has been mapped to locus 4p15.31-p15.2, which contains the SLC34A2 gene (OMIM *604217); allelic variants include both deletions and point mutations in this gene (111, 112). Although most patients present in middle age, this diagnosis has been made in infants. SLC34A is a sodium-dependent phosphate transporter expressed at high levels in alveolar type II epithelial cells. The disease is common in patients of Chinese, Turkish, and Japanese descent (113). The clinical course is variable but often results in slow deterioration of the lung, and ends in severe pulmonary fibrosis, respiratory insufficiency, and cardiac failure. Many patients are asymptomatic. The disease is usually detected on chest X-rays during routine examination or by family history. Histopathological findings include PAS-positive intraalveolar microliths that consist of concentric lamellae arranged around a central nucleus that has an amorphous or granular appearance. These structures are composed primarily of calcium phosphate, which accumulates in the alveolus. The microliths gradually grow from smaller to larger structures that eventually fill the entire alveolar space, resulting in injury to the alveolar walls, and are replaced by fibrotic tissue. Recent anecdotal reports support the use of disodium etidronate to treat this rare cause of interstitial lung disease, although lung transplantation has been beneficial (110, 114).
Mutations in Telomerases Associated with Familial Pulmonary Fibrosis
Severe, progressive, lung disease with pathological diagnoses of adult-onset ILD and pulmonary fibrosis are associated with mutations in two essential components of the telomerase enzyme complex: telomerase reverse transcriptase or TERT (chromosome 5p15.33; OMIM +187270; #614743), and telomerase RNA component or TERC (chromosome 3q26.2; OMIM *602322; #614743), which regulates the maintenance of telomeres during cell proliferation (115–117). Diagnosis of familial pulmonary fibrosis was initially recognized by associating mutations in the TERT or TERC genes with severe lung disease in patients with dyskeratosis congenita, further highlighting the importance of epithelial cell injury and repair in the pathogenesis of chronic alveolar diseases (118–124). Inheritance is primarily autosomal dominant, with incomplete penetrance. Patients with these mutations have shortened telomeres and variable penetrance of pulmonary fibrosis, liver fibrosis, and bone marrow failure. Pulmonary fibrosis associated with mutations in TERT is diagnosed primarily in older adults and correlates with a history of smoking. It is a progressive, lethal disease with a mean life expectancy of 3 years after diagnosis (125). Radiographic and histological findings are consistent with UIP. Heterozygous mutations in TERT have been found in 8–15% of patients with IPF of adult onset; less than 1–3% had heterozygous mutations in TERC (117). Altogether, autosomal dominantmutations in TERT, TERC, SFTPC, and SFTPA2 account for only 15–20% of familial IPF cases, indicating novel genes are involved that have not yet been identified (126, 127).
SUMMARY
Knowledge regarding the cellular and molecular mechanisms controlling the pulmonary surfactant system has enabled recognition and diagnosis of hereditary disorders underlying lung diseases previously described as idiopathic. Insights regarding the pathogenesis of PAP have led to new diagnostic and therapeutic advancements that are leading to treatment of both acquired and hereditary PAP. Although diagnostic criteria for hereditary disorders that disrupt surfactant function and cause lung remodeling are now well established, a deeper understanding of the biological processes leading to pulmonary cell injury and tissue remodeling will be required to enable new therapies for these life-threatening diseases in the future.
ACKNOWLEDGMENTS
The authors acknowledge the secretarial support of Ann Maher; imaging support by Joe Kitzmiller; and ongoing collaborations with Dr. Lawrence Nogee, Department of Pediatrics, Johns Hopkins University, Baltimore, Maryland; Dr. Aaron Hamvas, Department of Pediatrics, Washington University, St. Louis, Missouri; and Dr. Bruce Trapnell, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center and the University of Cincinnati, Cincinnati, Ohio.
The authors also acknowledge grants from the National Institute of Health to J.A.W. (HL108907, HL110964, HL095580, and HL122642), and to T.E.W. (HL1093923, HL086492).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Jeffrey A. Whitsett, Email: jeff.whitsett@cchmc.org.
Susan E. Wert, Email: susan.wert@cchmc.org.
Timothy E. Weaver, Email: tim.weaver@cchmc.org.
LITERATURE CITED
- 1.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. PNAS. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, et al. Lung organogenesis. Curr. Top. Dev. Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 6.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tompkins DH, Besnard V, Lange AW, Keiser AR, Wert SE, et al. Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2011;45:101–110. doi: 10.1165/rcmb.2010-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, et al. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. PNAS. 2013;110:E4456–E4464. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajihosseini MK, Wilson S, De Moerlooze L, Dickson C. A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer-syndrome-like phenotypes. PNAS. 2001;98:3855–3860. doi: 10.1073/pnas.071586898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zackai EH, McDonald-McGinn DM, Stolle C, Huff DS. Craniosynostosis with tracheal sleeve: a patient with Pfeiffer syndrome, tracheal sleeve and additional malformations in whom an FGFR2 mutation was found. Clin. Dysmorphol. 2003;12:209. doi: 10.1097/01.mcd.0000080414.95344.ae. [DOI] [PubMed] [Google Scholar]
- 11.Kang S, Graham JM, Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister–Hall syndrome. Nat. Genet. 1997;15:266–268. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- 12.Williamson KA, Hever AM, Rainger J, Rogers RC, Magee A, et al. Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum. Mol. Genet. 2006;15:1413–1422. doi: 10.1093/hmg/ddl064. [DOI] [PubMed] [Google Scholar]
- 13.Guillot L, Carre A, Szinnai G, Castanet M, Tron E, et al. NKX2-1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “Brain-Lung-Thyroid Syndrome”. Hum. Mutat. 2010;31:E1146–E1162. doi: 10.1002/humu.21183. [DOI] [PubMed] [Google Scholar]
- 14.Hamvas A, Deterding RR, Wert SE, White FV, Dishop MK, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2-1. Chest. 2013;144:794–804. doi: 10.1378/chest.12-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am. J. Hum. Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Wang Y, Besnard V, Ikegami M, Wert SE, et al. Transcriptional programs controlling perinatal lung maturation. PLOS ONE. 2012;7:e37046. doi: 10.1371/journal.pone.0037046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA. J. Dis. Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 18.Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu. Rev. Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol. Cell. Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFelice M, Silberschmidt D, DiLauro R, Xu Y, Wert SE, et al. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J. Biol. Chem. 2003;278:35574–35583. doi: 10.1074/jbc.M304885200. [DOI] [PubMed] [Google Scholar]
- 21.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 22.Polin RA, Carlo WA Comm. Fetus Newborn. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–163. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 23.Bridges JP, Ludwig MG, Mueller M, Kinzel B, Sato A, et al. Orphan G protein-coupled receptor GPR116 regulates pulmonary surfactant pool size. Am. J. Respir. Cell Mol. Biol. 2013;49:348–357. doi: 10.1165/rcmb.2012-0439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang MY, Hilton MB, Seaman S, Haines DC, Nagashima K, et al. Essential regulation of lung surfactant homeostasis by the orphan G protein-coupled receptor GPR116. Cell Rep. 2013;3:1457–1464. doi: 10.1016/j.celrep.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. PNAS. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, et al. Altered surfactant function and structure in SP-A gene targeted mice. PNAS. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikegami M, Whitsett JA, Jobe A, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene-targeted mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L468–L476. doi: 10.1152/ajplung.2000.279.3.L468. [DOI] [PubMed] [Google Scholar]
- 28.Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L552–L561. doi: 10.1152/ajplung.00142.2004. [DOI] [PubMed] [Google Scholar]
- 29.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Investig. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr. Opin. Pharmacol. 2006;6:277–283. doi: 10.1016/j.coph.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Ariki S, Nishitani C, Kuroki Y. Diverse functions of pulmonary collectins in host defense of the lung. J. Biomed. Biotechnol. 2012;2012:532071. doi: 10.1155/2012/532071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N. Engl. J.Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Gil J, Weaver TE. Pulmonary surfactant pathophysiology: current models and open questions. Physiology. 2010;25:132–141. doi: 10.1152/physiol.00006.2010. [DOI] [PubMed] [Google Scholar]
- 34.Ikegami M, Grant S, Korfhagen T, Scheule RK, Whitsett JA. Surfactant protein-D regulates the postnatal maturation of pulmonary surfactant lipid pool sizes. J. Appl. Physiol. 2009;106:1545–1552. doi: 10.1152/japplphysiol.91567.2008. [DOI] [PubMed] [Google Scholar]
- 35.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr. Dev. Pathol. 2009;12:253–274. doi: 10.2350/09-01-0586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dishop MK. Paediatric interstitial lung disease: classification and definitions. Paediatr. Respir. Rev. 2011;12:230–237. doi: 10.1016/j.prrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am. J. Respir. Crit. Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vorbroker DK, Profitt SA, Nogee LM, Whitsett JA. Aberrant processing of surfactant protein C in hereditary SP-B deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 1995;268:L647–L656. doi: 10.1152/ajplung.1995.268.4.L647. [DOI] [PubMed] [Google Scholar]
- 39.Nogee LM, Wert SE, Proffit SA, Hull WM, Whitsett JA. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am. J. Respir. Crit. Care Med. 2000;161:973–981. doi: 10.1164/ajrccm.161.3.9903153. [DOI] [PubMed] [Google Scholar]
- 40.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 41.Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu. Rev. Physiol. 2004;66:601–623. doi: 10.1146/annurev.physiol.66.032102.134711. [DOI] [PubMed] [Google Scholar]
- 42.Dunbar AE, 3rd, Wert SE, Ikegami M, Whitsett JA, Hamvas A, et al. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr. Res. 2000;48:275–282. doi: 10.1203/00006450-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Hamvas A. Evaluation and management of inherited disorders of surfactant metabolism. Chin. Med. J. 2010;123:2943–2947. [PubMed] [Google Scholar]
- 44.Palomar LM, Nogee LM, Sweet SC, Huddleston CB, Cole FS, Hamvas A. Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure. J. Pediatr. 2006;149:548–553. doi: 10.1016/j.jpeds.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am. J. Respir. Cell Mol. Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson H, Nordling K, Weaver TE, Johansson J. The Brichos domain-containing C-terminal part of pro-surfactant protein C binds to an unfolded poly-val transmembrane segment. J. Biol. Chem. 2006;281:21032–21039. doi: 10.1074/jbc.M603001200. [DOI] [PubMed] [Google Scholar]
- 47.Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J. Biol. Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- 48.Glasser SW, Witt TL, Senft AP, Baatz JE, Folger D, et al. Surfactant protein C-deficient mice are susceptible to respiratory syncytial virus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L64–L72. doi: 10.1152/ajplung.90640.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glasser SW, Senft AP, Maxfield MD, Ruetschilling TL, Baatz JE, et al. Genetic replacement of surfactant protein-C reduces respiratory syncytial virus induced lung injury. Respir. Res. 2013;14:19. doi: 10.1186/1465-9921-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L721–L729. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kropski JA, Lawson WE, Young LR, Blackwell TS. Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis. Model. Mech. 2013;6:9–17. doi: 10.1242/dmm.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurm T, Kaltenborn E, Kern S, Griese M, Zarbock R. SFTPC mutations cause SP-C degradation and aggregate formation without increasing ER stress. Eur. J. Clin. Investig. 2013;43:791–800. doi: 10.1111/eci.12107. [DOI] [PubMed] [Google Scholar]
- 53.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N. Engl. J. Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 54.Nogee LM, Dunbar AE, 3rd, Wert S, Askin F, Hamvas A, Whitsett JA. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest. 2002;121(Suppl.):20S–21S. doi: 10.1378/chest.121.3_suppl.20s. [DOI] [PubMed] [Google Scholar]
- 55.Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am. J. Respir. Crit. Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 56.Katzenstein AL, Gordon LP, Oliphant M, Swender PT. Chronic pneumonitis of infancy. A unique form of interstitial lung disease occurring in early childhood. Am. J. Surg. Pathol. 1995;19:439–447. [PubMed] [Google Scholar]
- 57.Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J. Cell Biol. 2006;172:395–407. doi: 10.1083/jcb.200508016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avital A, Hevroni A, Godfrey S, Cohen S, Maayan C, et al. Natural history of five children with surfactant protein C mutations and interstitial lung disease. Pediatr. Pulmonol. 2014;49:1097–1105. doi: 10.1002/ppul.22971. [DOI] [PubMed] [Google Scholar]
- 59.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am. J. Respir. Crit. Care Med. 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 61.Hepping N, Griese M, Lohse P, Garbe W, Lange L. Successful treatment of neonatal respiratory failure caused by a novel surfactant protein C p.Cys121Gly mutation with hydroxychloroquine. J. Perinatol. 2013;33:492–494. doi: 10.1038/jp.2012.131. [DOI] [PubMed] [Google Scholar]
- 62.Cheong N, Madesh M, Gonzales LW, Zhao M, Yu K, et al. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J. Biol. Chem. 2006;281:9791–9800. doi: 10.1074/jbc.M507515200. [DOI] [PubMed] [Google Scholar]
- 63.Matsumura Y, Ban N, Ueda K, Inagaki N. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J. Biol. Chem. 2006;281:34503–34514. doi: 10.1074/jbc.M600071200. [DOI] [PubMed] [Google Scholar]
- 64.Matsumura Y, Ban N, Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L698–L707. doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- 65.Weichert N, Kaltenborn E, Hector A, Woischnik M, Schams A, et al. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir. Res. 2011;12:4. doi: 10.1186/1465-9921-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brasch F, Schimanski S, Muhlfeld C, Barlage S, Langmann T, et al. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am. J. Respir. Crit. Care Med. 2006;174:571–580. doi: 10.1164/rccm.200509-1535OC. [DOI] [PubMed] [Google Scholar]
- 67.Bullard JE, Wert SE, Nogee LM. ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Semin. Perinatol. 2006;30:327–334. doi: 10.1053/j.semperi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N. Engl. J. Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 69.Somaschini M, Nogee LM, Sassi I, Danhaive O, Presi S, et al. Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J. Pediatr. 2007;150:649–653.e1. doi: 10.1016/j.jpeds.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Edwards V, Cutz E, Viero S, Moore AM, Nogee L. Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct. Pathol. 2005;29:503–509. doi: 10.1080/01913120500323480. [DOI] [PubMed] [Google Scholar]
- 71.Doan ML, Guillerman RP, Dishop MK, Nogee LM, Langston C, et al. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 72.Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- 73.Flamein F, Riffault L, Muselet-Charlier C, Pernelle J, Feldmann D, et al. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum. Mol. Genet. 2012;21:765–775. doi: 10.1093/hmg/ddr508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooke KR, Nishinakamura R, Martin TR, Kobzik L, Brewer J, et al. Persistence of pulmonary pathology and abnormal lung function in IL-3/GM-CSF/IL-5 βc receptor-deficient mice despite correction of alveolar proteinosis after BMT. Bone Marrow Transplant. 1997;20:657–662. doi: 10.1038/sj.bmt.1700958. [DOI] [PubMed] [Google Scholar]
- 75.Reed JA, Ikegami M, Robb L, Begley CG, Ross G, Whitsett JA. Distinct changes in pulmonary surfactant homeostasis in common β-chain and GM-CSF-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L1164–L1171. doi: 10.1152/ajplung.2000.278.6.L1164. [DOI] [PubMed] [Google Scholar]
- 76.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 77.Kitamura T, Tanaka N, Watanabe J, Uchida Kanegasaki S, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, et al. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRα gene in the X chromosome pseudoautosomal region 1. J. Exp. Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, et al. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki T, Sakagami T, Young LR, Carey BC, Wood RE, et al. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. Am. J. Respir. Crit. Care Med. 2010;182:1292–1304. doi: 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dirksen U, Nishinakamura R, Groneck P, Hattenhorst U, Nogee L, et al. Human pulmonary alveolar proteinosis associated with a defect inGM-CSF/IL-3/IL-5 receptorcommon β chain expression. J. Clin. Investig. 1997;100:2211–2217. doi: 10.1172/JCI119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanaka T, Motoi N, Tsuchihashi Y, Tazawa R, Kaneko C, et al. Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. J. Med. Genet. 2011;48:205–209. doi: 10.1136/jmg.2010.082586. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki T, Maranda B, Sakagami T, Catellier P, Couture CY, et al. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur. Respir. J. 2011;37:201–204. doi: 10.1183/09031936.00090610. [DOI] [PubMed] [Google Scholar]
- 84.Luisetti M, Kadija Z, Mariani F, Rodi G, Campo I, Trapnell BC. Therapy options in pulmonary alveolar proteinosis. Ther. Adv. Respir. Dis. 2010;4:239–248. doi: 10.1177/1753465810378023. [DOI] [PubMed] [Google Scholar]
- 85.Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med. 2010;181:1345–1354. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luisetti M, Kroneberg P, Suzuki T, Kadija Z, Muellinger B, et al. Physical properties, lung deposition modeling, and bioactivity of recombinant GM-CSF aerosolised with a highly efficient nebulizer. Pulm. Pharmacol. Ther. 2011;24:123–127. doi: 10.1016/j.pupt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Malur A, Kavuru MS, Marshall I, Barna BP, Huizar I, et al. Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasis. Respir. Res. 2012;13:46. doi: 10.1186/1465-9921-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leth S, Bendstrup E, Vestergaard H, Hilberg O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology. 2013;18:82–91. doi: 10.1111/j.1440-1843.2012.02274.x. [DOI] [PubMed] [Google Scholar]
- 89.Lachmann N, Happle C, Ackermann M, Luttge D, Wetzke M, et al. Gene correction of human induced pluripotent stem cells repairs the cellular phenotype in pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med. 2014;189:167–182. doi: 10.1164/rccm.201306-1012OC. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki T, Mayhew C, Sallese A, Chalk C, Carey BC, et al. Use of induced pluripotent stem cells to recapitulate pulmonary alveolar proteinosis pathogenesis. Am. J. Respir. Crit. Care Med. 2014;189:183–193. doi: 10.1164/rccm.201306-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Price M, Lazzaro D, Pohl T, Mattei MG, Ruther U, et al. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992;8:241–255. doi: 10.1016/0896-6273(92)90291-k. [DOI] [PubMed] [Google Scholar]
- 92.Galambos C, Levy H, Cannon CL, Vargas SO, Reid LM, et al. Pulmonary pathology in thyroid transcription factor-1 deficiency syndrome. Am. J. Respir. Crit. Care Med. 2010;182:549–554. doi: 10.1164/rccm.201002-0167CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kleinlein B, Griese M, Liebisch G, Krude H, Lohse P, et al. Fatal neonatal respiratory failure in an infant with congenital hypothyroidism due to haploinsufficiency of the NKX2-1 gene: alteration of pulmonary surfactant homeostasis. Arch. Dis. Child. Fetal Neonatal Ed. 2011;96:F453–F456. doi: 10.1136/adc.2009.180448. [DOI] [PubMed] [Google Scholar]
- 94.Iwatani N, Mabe H, Devriendt K, Kodama M, Miike T. Deletion of NKX2.1 gene encoding thyroid transcription factor-1 in two siblings with hypothyroidism and respiratory failure. J. Pediatr. 2000;137:272–276. doi: 10.1067/mpd.2000.107111. [DOI] [PubMed] [Google Scholar]
- 95.Maquet E, Costagliola S, Parma J, Christophe-Hobertus C, Oligny LL, et al. Lethal respiratory failure and mild primary hypothyroidism in a term girl with a de novo heterozygous mutation in the TITF1/NKX2.1 gene. J. Clin. Endocrinol. Metab. 2009;94:197–203. doi: 10.1210/jc.2008-1402. [DOI] [PubMed] [Google Scholar]
- 96.Salerno T, Peca D, Menchini L, Schiavino A, Petreschi F, et al. Respiratory insufficiency in a newborn with congenital hypothyroidism due to a new mutation of TTF-1/NKX2.1 gene. Pediatr. Pulmonol. 2014;49:E42–E44. doi: 10.1002/ppul.22788. [DOI] [PubMed] [Google Scholar]
- 97.Barnett CP, Mencel JJ, Gecz J, Waters W, Kirwin SM, et al. Choreoathetosis, congenital hypothyroidism and neonatal respiratory distress syndrome with intact NKX2-1. Am. J. Med. Genet. A. 2012;158A:3168–3173. doi: 10.1002/ajmg.a.35456. [DOI] [PubMed] [Google Scholar]
- 98.Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, et al. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev. Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 99.Rabah R, Poulik JM. Congenital alveolar capillary dysplasia with misalignment of pulmonary veins associated with hypoplastic left heart syndrome. Pediatr. Dev. Pathol. 2001;4:167–174. doi: 10.1007/s100240010125. [DOI] [PubMed] [Google Scholar]
- 100.Alameh J, Bachiri A, Devisme L, Truffert P, Rakza T, et al. Alveolar capillary dysplasia: a cause of persistent pulmonary hypertension of the newborn. Eur. J. Pediatr. 2002;161:262–266. doi: 10.1007/s00431-002-0927-7. [DOI] [PubMed] [Google Scholar]
- 101.Antao B, Samuel M, Kiely E, Spitz L, Malone M. Congenital alveolar capillary dysplasia and associated gastrointestinal anomalies. Fetal Pediatr. Pathol. 2006;25:137–145. doi: 10.1080/15513810600908230. [DOI] [PubMed] [Google Scholar]
- 102.Miranda J, Rocha G, Soares P, Morgado H, Baptista MJ, et al. A novel mutation in FOXF1 gene associated with alveolar capillary dysplasia with misalignment of pulmonary veins, intestinal malrotation and annular pancreas. Neonatology. 2013;103:241–245. doi: 10.1159/000346062. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen L, Riley MM, Sen P, Galambos C. Alveolar capillary dysplasia with misalignment of pulmonary veins with a wide spectrum of extrapulmonary manifestations. Pathol. Int. 2013;63:519–521. doi: 10.1111/pin.12102. [DOI] [PubMed] [Google Scholar]
- 104.Sen P, Yang Y, Navarro C, Silva I, Szafranski P, et al. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum. Mutat. 2013;34:801–811. doi: 10.1002/humu.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kolble K, Lu J, Mole SE, Kaluz S, Reid KB. Assignment of the human pulmonary surfactant protein D gene (SFTP4) to 10q22-q23 close to the surfactant protein A gene cluster. Genomics. 1993;17:294–298. doi: 10.1006/geno.1993.1324. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am. J. Hum. Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maitra M, Wang Y, Gerard RD, Mendelson CR, Garcia CK. Surfactant protein A2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J. Biol. Chem. 2010;285:22103–22113. doi: 10.1074/jbc.M110.121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maitra M, Cano CA, Garcia CK. Mutant surfactant A2 proteins associated with familial pulmonary fibrosis and lung cancer induce TGF-β1 secretion. PNAS. 2012;109:21064–21069. doi: 10.1073/pnas.1217069110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Maitra M, Dey M, Yuan WC, Nathanielsz PW, Garcia CK. Lung fibrosis-associated surfactant protein A1 and C variants induce latent transforming growth factor β1 secretion in lung epithelial cells. J. Biol. Chem. 2013;288:27159–27171. doi: 10.1074/jbc.M113.475335. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Ferreira Francisco FA, Pereira e Silva JL, Hochhegger B, Zanetti G, Marchiori E. Pulmonary alveolar microlithiasis. State-of-the-art review. Respir. Med. 2013;107:1–9. doi: 10.1016/j.rmed.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 111.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am. J. Hum. Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huqun Izumi S, Miyazawa H, Ishii K, Uchiyama B, et al. Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am. J. Respir. Crit. Care Med. 2007;175:263–268. doi: 10.1164/rccm.200609-1274OC. [DOI] [PubMed] [Google Scholar]
- 113.Yin X, Wang H, Wu D, Zhao G, Shao J, Dai Y. SLC34A2 gene mutation of pulmonary alveolar microlithiasis: report of four cases and review of literatures. Respir. Med. 2013;107:217–222. doi: 10.1016/j.rmed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Ozcelik U, Yalcin E, Ariyurek M, Ersoz DD, Cinel G, et al. Long-term results of disodium etidronate treatment in pulmonary alveolar microlithiasis. Pediatr. Pulmonol. 2010;45:514–517. doi: 10.1002/ppul.21209. [DOI] [PubMed] [Google Scholar]
- 115.Garcia CK, Wright WE, Shay JW. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res. 2007;35:7406–7416. doi: 10.1093/nar/gkm644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat. Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gansner JM, Rosas IO. Telomeres in lung disease. Transl. Res. 2013;162:343–352. doi: 10.1016/j.trsl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 118.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 119.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. PNAS. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. PNAS. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alder JK, Cogan JD, Brown AF, Anderson CJ, Lawson WE, et al. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLOS Genet. 2011;7:e1001352. doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J. Med. Genet. 2008;45:654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 123.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fernandez BA, Fox G, Bhatia R, Sala E, Noble B, et al. A Newfoundland cohort of familial and sporadic idiopathic pulmonary fibrosis patients: clinical and genetic features. Respir. Res. 2012;13:64. doi: 10.1186/1465-9921-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLOS ONE. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garcia CK. Idiopathic pulmonary fibrosis: update on genetic discoveries. Proc. Am. Thorac. Soc. 2011;8:158–162. doi: 10.1513/pats.201008-056MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Devine MS, Garcia CK. Genetic interstitial lung disease. Clin. Chest Med. 2012;33:95–110. doi: 10.1016/j.ccm.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]