Abstract
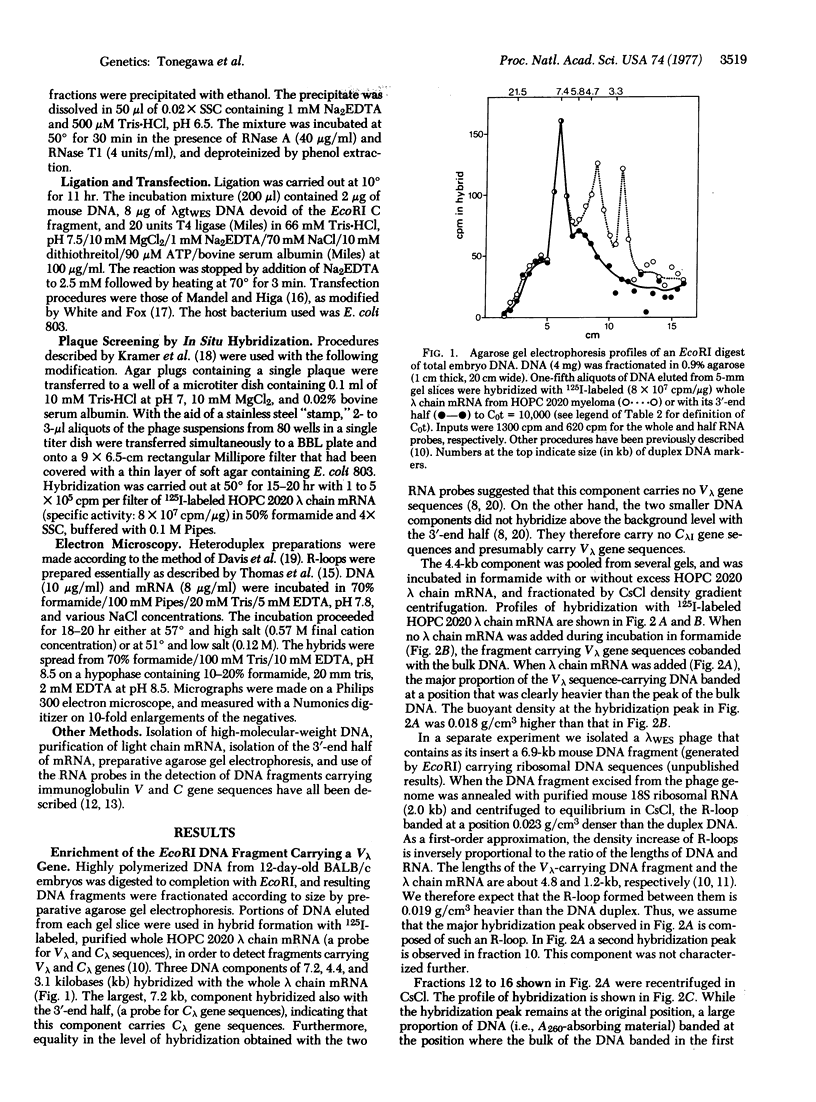
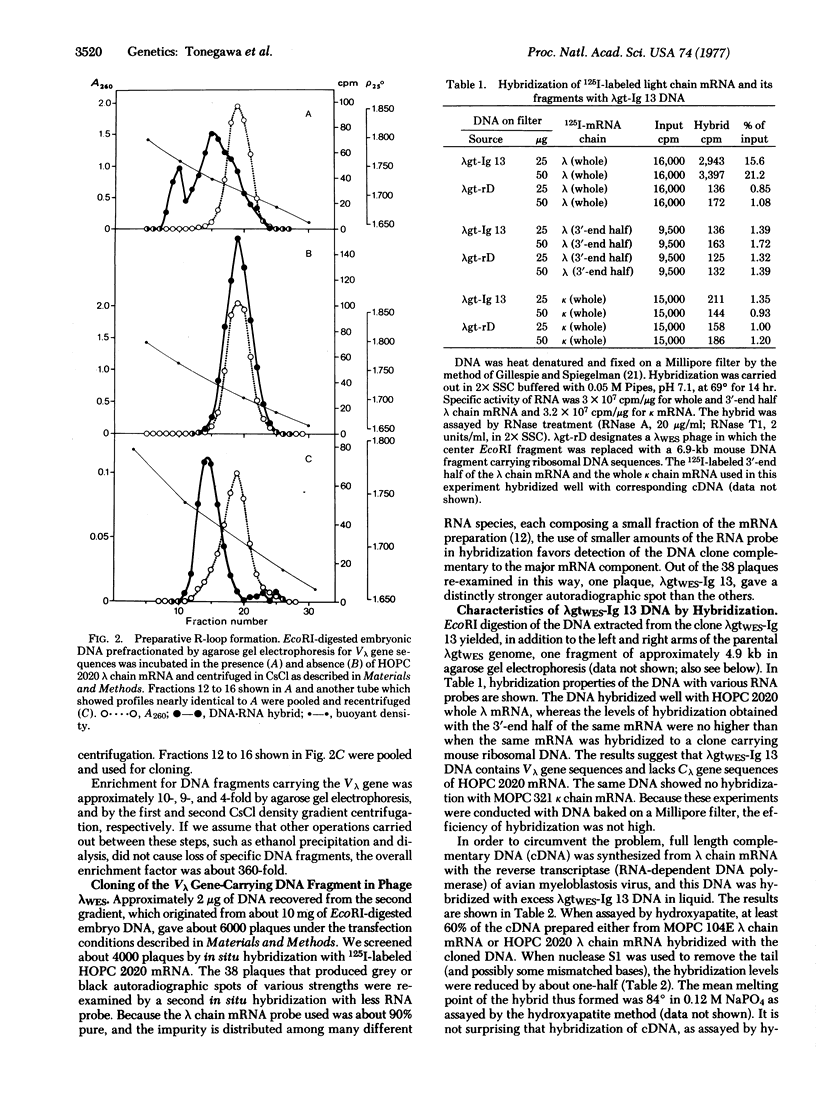
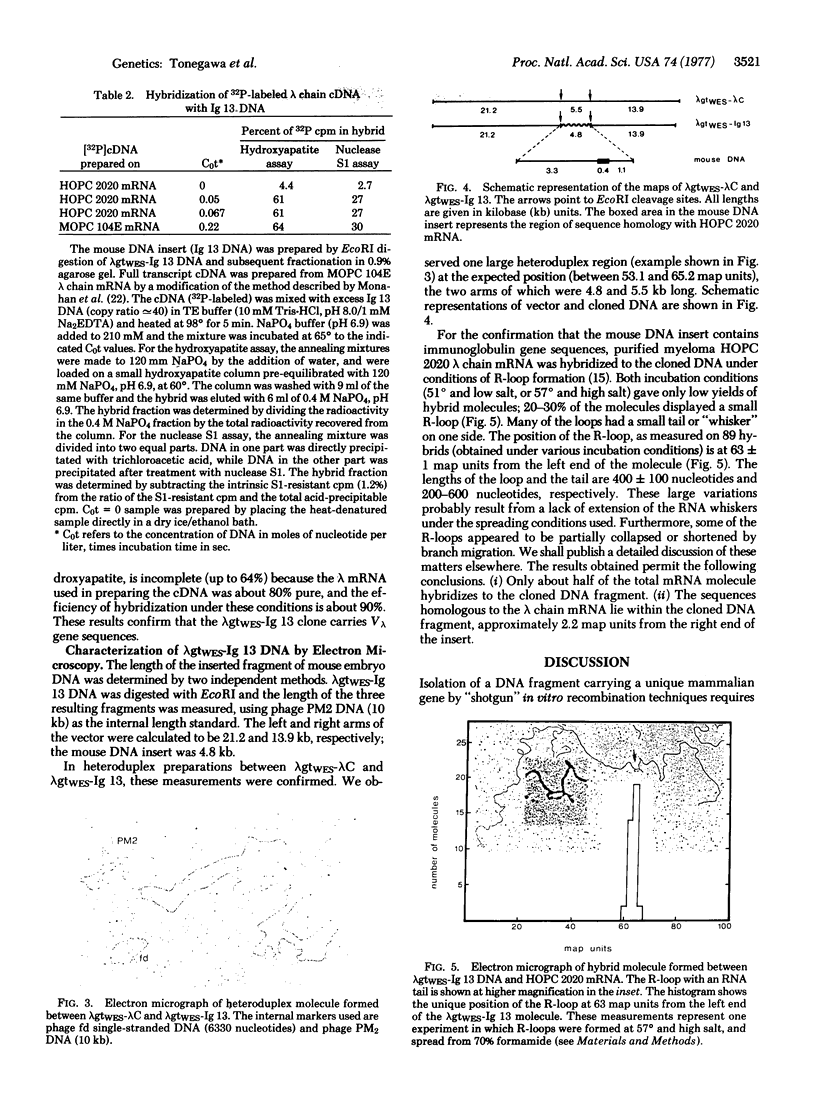
A 4.8-kilobase DNA fragment carrying an immunoglobulin gene coding for a mouse lambda chain variable region (Vlambda gene) was enriched about 350-fold from a total endonuclease EcoRI digest of embryonic DNA by a combination of preparative agarose gel electrophoresis of double-stranded DNA and CsCl density gradient centrifugation of R-loops formed with a purified lambda chain mRNA. DNA fragments thus enriched for the immunoglobulin gene were inserted in vitro in the middle of the genome of the vector phage lambdagt Wam 403, Eam 100, Sam 100 by use of the EcoRI cohesive ends. Transfection of CaCl2-treated Escherichia coli 803 [rk-, mk- (lacking restriction and modification systems for K-12)] with such hybrid DNA and subsequent screening of about 4000 plaques by in situ hybridization with purified 125I-labeled lambda chain mRNA led to isolation of a clone that carries a Vlambda gene (lambdagtWES-Ig 13). Electron microscopy of R-loops confirmed the presence of sequences homologous to part of the lambda chain mRNA in its 5'-end.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Schimke R. T. Partial purification of the ovalbumin gene. Cell. 1976 Mar;7(3):331–338. doi: 10.1016/0092-8674(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Smith H. O., Schaffner W., Gross K. W., Birnstiel M. L. Integration of eukaryotic genes for 5S RNA and histone proteins into a phage lambda receptor. Nucleic Acids Res. 1976 Oct;3(10):2617–2632. doi: 10.1093/nar/3.10.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Enquist L., Tiemeier D., Leder P., Weisberg R., Sternberg N. Safer derivatives of bacteriophage lambdagt-lambdaC for use in cloning of recombinant DNA molecules. Nature. 1976 Feb 19;259(5544):596–598. doi: 10.1038/259596a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Honjo T., Packman S. Quantitation of constant and variable region genes for mouse immunoglobulin lambda chains. Biochemistry. 1976 Jun 29;15(13):2780–2785. doi: 10.1021/bi00658a012. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Symons R. H., Berg P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2904–2909. doi: 10.1073/pnas.69.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. A., Cameron J. R., Davis R. W. Isolation of bacteriophage lambda containing yeast ribosomal RNA genes: screening by in situ RNA hybridization to plaques. Cell. 1976 Jun;8(2):227–232. doi: 10.1016/0092-8674(76)90006-4. [DOI] [PubMed] [Google Scholar]
- Lobban P. E., Kaiser A. D. Enzymatic end-to end joining of DNA molecules. J Mol Biol. 1973 Aug 15;78(3):453–471. doi: 10.1016/0022-2836(73)90468-3. [DOI] [PubMed] [Google Scholar]
- Matthyssens G., Hozumi N., Tonegawa S. Somatic generation of antibody diversity. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):439–448. [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Martin M. A. A general method of gene isolation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1697–1700. doi: 10.1073/pnas.70.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Hozumi N., Matthyssens G., Schuller R. Somatic changes in the content and context of immunoglobulin genes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):877–889. doi: 10.1101/sqb.1977.041.01.097. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Reiteration frequency of immunoglobulin light chain genes: further evidence for somatic generation of antibody diversity. Proc Natl Acad Sci U S A. 1976 Jan;73(1):203–207. doi: 10.1073/pnas.73.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Steinberg C., Dube S., Bernardini A. Evidence for somatic generation of antibody diversity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4027–4031. doi: 10.1073/pnas.71.10.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- White R. L., Fox M. S. Genetic consequences of transfection with heterduplex bacteriophage lambda DNA. Mol Gen Genet. 1975 Nov 24;141(2):163–171. doi: 10.1007/BF00267681. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Smith R. G., Means A. R., O'Malley B. W. The ovalbumin gene. Partial purification of the coding strand. J Biol Chem. 1976 Jul 10;251(13):3868–3874. [PubMed] [Google Scholar]