Abstract
The rates and severity of Clostridium difficile infections, including pseudomembranous colitis, have increased markedly. However, there are few effective treatments for refractory or recurrent C. difficile infections and the outcomes are poor. Fecal microbiota transplantation is becoming increasingly accepted as an effective and safe intervention in patients with recurrent disease, likely due to the restoration of a disrupted microbiome. Cure rates of >90% are being consistently reported from multiple centers. We cured a case of severe refractory C. difficile infection with fecal microbiota transplantation in a patient colonized by vancomycin-resistant enterococcus.
Keywords: Clostridium difficile; Fecal microbiota transplantation; Vancomycin-resistant Enterococcus; Enterocolitis, pseudomembranous; Fecal bacteriotherapy
INTRODUCTION
Clostridium difficile infection is a gastrointestinal disease believed to be causally related to disruption of the intestinal flora under the selective pressure of antibiotic therapy during hospitalization. Since 2000, the incidence of C. difficile infections has increased. The increasing incidence and recurrence of refractory C. difficile infections is increasing the financial stress on healthcare systems, and the associated severe complications are increasing mortality.1,2 The few effective therapeutic approaches to refractory or recurrent C. difficile infection include intravenous immunoglobulin (IVIG), fidaxomicin, rifaximin, and colectomy.2,3,4 Recently, fecal microbiota transplantation (FMT) has been suggested as an effective, alternative treatment.5
Here, we report on a case of severe refractory C. difficile infection cured with FMT in a patient colonized by vancomycin-resistant enterococci (VRE).
CASE REPORT
A 33-year-old man was admitted to our hospital with a 7-day history of fever and watery diarrhea. He had been in a long-term care facility with spastic tetraplegia since a traumatic subdural and epidural hematoma with subarachnoid hemorrhage 6 years previously.
He had a temperature of 38.1℃, respiratory rate of 20 breaths/min, pulse of 110 beats/min, and blood pressure of 100/60 mmHg. On physical examination, he appeared chronically ill and was drowsy. He had a distended abdomen with a bulging contour and diffuse abdominal tenderness. Normal breath sounds and decreased bowel sounds were evident.
Laboratory tests (reference values in parentheses) on admission revealed a white blood cell (WBC) count of 51,900/mm3 with 35,600/mm3 neutrophils, plus 16.0 g/dL hemoglobin, 140×103/mm3 platelets, 54/16 U/L (5-37/5-40) aspartate aminotransferase/alanine aminotransferase, 0.38 mg/dL (0.22-1.3) total bilirubin, 2.9 g/dL (6.4-8.3) total protein, 1.7 g/dL (3.5-5.2) albumin, 18.1 mg/dL (8-23) BUN, 1.0 mg/dL (0.5-1.2) creatinine, 138/2.9/107 mEq/L (135-145/3.5-5/98-110) sodium/potassium/chloride, and 13.4 mg/dL (0-0.3) CRP. The chest x-ray was unremarkable, while an abdominal x-ray showed ileus (Fig. 1A).
Fig. 1.

Simple abdomen x-ray, sigmoidoscopy, and CT findings. (A) The initial simple abdomen x-ray showed ileus. (B) Sigmoidoscopy showed diffuse edematous mucosal change with several yellowish plaques. (C) The initial abdominal CT for extension of colitis revealed marked edematous wall thickening and mural enhancement of the entire colonic loop and rectum.
Three weeks before admission, he caught pneumonia and was treated with ampicillin/sulbactam, ceftriaxone with metronidazole, and meropenem. A clinical diagnosis of antibiotic-associated diarrhea was made. We performed sigmoidoscopy to make a rapid diagnosis and a C. difficile stool culture and toxin assay to evaluate the cause of the nosocomial diarrhea. Sigmoidoscopy showed diffuse edematous mucosal change with several yellowish plaques (Fig. 1B). The stool culture was positive for C. difficile, as were assays for toxins A and B. In addition, VRE was cultured in the stool. Abdominal CT to determine the extent of the colitis revealed marked edematous wall thickening and mural enhancement of the entire colonic loop and rectum (Fig. 1C).
Under a diagnosis of severe C. difficile infection, the antibiotic therapy was switched to vancomycin (125 mg orally four times per day) and metronidazole (500 mg intravenously at every 8 hours), with rectal instillation of vancomycin (500 mg in 100 mL normal saline as a retention enema four times per day) due to the ileus. However, 8 days later, the patient complained of persistent diarrhea (>20 episodes per day), abdominal pain, and fever. The same day, a follow-up sigmoidoscopy revealed multiple elevated yellowish pseudomembranes with hyperemic, edematous mucosa throughout the entire sigmoid colon and rectum (Fig. 2).
Fig. 2.

Sigmoidoscopy findings. Follow-up sigmoidoscopy 7 days later revealed more elevated yellowish pseudomembranes with hyperemic, edematous mucosa in the entire sigmoid colon and rectum.
Under a diagnosis of C. difficile infection refractory to vancomycin and intravenous metronidazole, FMT was planned. The donor was the patient's 37-year-old brother, who had no underlying disease. His feces was screened for parasites, C. difficile, enteropathogenic bacteria, and VRE. His blood was screened for antibodies to human immunodeficiency virus, hepatitis A, B, C and syphilis. FMT was performed on day 13 of hospitalization. At that time, the administration of vancomycin and metronidazole was stopped. For the FMT, 300 g samples of donor feces excreted within the previous 6 hours were transferred to a blender and 400 mL sterile 0.9% NaCl was added. These were mixed until fully homogenized. Then the feces solution was poured through a clean metal sieve and instilled in the patient's gut in several sessions via a rectal syringe. The patient was in the left lateral position during the procedure, and tried to keep his hips elevated for about 6 hours after procedure.
The diarrhea and fever persisted for more than 48 hours after the procedure. The WBC count and CRP did not decrease and the ileus on abdominal x-ray did not change. Three days later (day 16), 150 g of a similarly prepared stool transplant specimen was instilled through a nasoduodenal tube. Our patient had severe pancolitis, which made colonoscopy risky and duodenoscopy for inserting a nasoduodenal tube was not available in our hospital on the weekend when the feces samples were first prepared. Consequently, the first FMT was performed via an enema and a second FMT conducted 3 days later was performed using a nasoduodenal tube inserted at duodenoscopy. All antimicrobials had been stopped since performing the FMT.
Five days after the second FMT, he was afebrile and the diarrhea was reduced in quantity and frequency (Fig. 3). On day 28, a follow-up abdominal x-ray showed improvement of the ileus and the follow-up sigmoidoscopy revealed focal erythematous edematous mucosa with no pseudomembrane (Fig. 4) and a C. difficile toxin assay of his stool was negative. No recurrence was evident for about 3 months, although VRE was cultured from the stool throughout the follow-up period.
Fig. 3.
Hospital course of the patient. Monitoring the outcomes of fecal microbiota transplantation, after two fecal microbiota transplantations (arrow), the patient was afebrile and the number of episodes and amount of diarrhea had decreased. PO, by mouth; IV, intravenous.
Fig. 4.
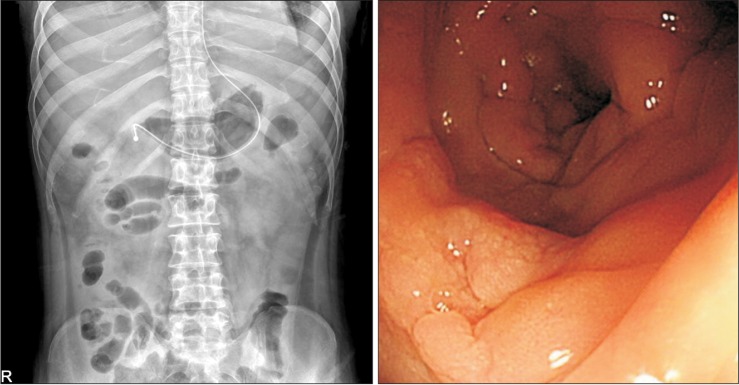
Simple abdomen x-ray, sigmoidoscopy findings. (A) Follow-up abdomen x-ray showed improvement of ileus. (B) Sigmoidoscopy 10 days after the second fecal microbiota transplantation revealed focal erythematous edematous mucosa with no pseudomembrane.
DISCUSSION
The patient was diagnosed with a severe refractory C. difficile infection and VRE colonization and was ultimately cured by FMT.
C. difficile infection alters the balance of the normal gut microflora, allowing the production of C. difficile toxins.6,7 These toxins induce mucosa barrier injury, and can lead to pseudomembranous colitis varying in degree from mild to fulminant. Zar et al. stratified C. difficile infection severity based on an assessment score: one point each for age >60 years, temperature >38.3℃, albumin <2.5 mg/dL, and peripheral WBC count >15,000/mm3 and two points for endoscopic evidence of pseudomembranous colitis or treatment in the intensive care unit.8 Patients with ≥2 points were considered to have severe C. difficile infection. In severe cases such as ours, the standard treatment regimen is vancomycin alone or in combination with metronidazole.
Although the definitions of clinical cure and recurrence vary considerably, when the diarrhea persists after 7-10 days of treatment or is recurrent, other therapeutic options include IVIG, fidaxomicin, rifaximin, and colectomy. IVIG is not readily available and so is relatively expensive, and its effect is controversial.9,10 A recent phase III trial showed that fidaxomicin was not inferior to vancomycin for C. difficile infection.11 Although fidaxomicin is a promising approach, this drug is not available in Korea. The antimicrobials used to treat C. difficile infection can predispose patients to relapse by maintaining the perturbed intestinal flora and might contribute to the emergence of drug resistance.12 Therefore, if medical treatment fails, fecal bacteriotherapy, also called microbiota transplantation, is an alternative treatment.
The term microbiota refers to the community of microorganisms that inhabit a particular region of the body.13 FMT is believed to restore the composition and function of the intestinal microbiota. A recent randomized controlled trial of FMT for recurrent C. difficile infection concluded that the infusion of donor feces was significantly more effective for the treatment of recurrent C. difficile infection than was the use of vancomycin.5
For FMT, the donor should be screened for relevant communicable diseases. The recommended tests are stool tests for C. difficile toxin; culture for enteric pathogens, ova, and parasites; serology tests for human immunodeficiency virus antibody (Ab), HAV IgM, HBs Ag, anti-HBc IgM, anti-HBc IgG, anti-HBs Ab, and anti-HCV Ab; and non-treponemal tests for syphilis.14 The donor feces samples are prepared by diluting them with normal saline, homogenizing them with a blender to achieve a liquid slurry, and then filtering them to remove particulate matter to facilitate administration.5 A meta-analysis of 317 patients showed that the incidence of resolution increased with the volume of FMT given, with 200 and 500 mL producing success rates of 80% and 97%, respectively.15 The route of FMT administration can be a nasoduodenal tube, transcolonoscopic, or enema-based. A recent meta-analysis reported that instillation via a gastroscope or nasojejunal tube seemed less effective than by colonoscopy, although no comparative data were analyzed.15 Further study of the optimal route of instillation of the fecal microbiota is necessary. It is recommended that any antibiotics be stopped 1-3 days before FMT to prevent an antibiotic effect on the transplanted fecal microbiota.
The reported success rate of FMT exceeds 90%.5,15 Our patient was cured clinically with FMT. The sigmoidoscopic examination and toxin assays showed negative conversion, and there were no specific adverse events, except mild abdominal pain after instillation of the donor feces. Reported side effects include diarrhea, cramping, belching, and small bowel bacterial overgrowth, but these are rare.5,13,15,16 Despite these promising results, the acquisition of feces for FMT might be less acceptable for some physicians. In our experience, however, the patients and their families tolerate the procedure, which is cost-effective.
VRE-colonized patients with C. difficile infection have a particularly high risk of skin contamination and environmental shedding of VRE.17 In addition, stool VRE colonization might be a significant risk factor for the recurrence of C. difficile infection.18 The intestinal microbiota, including obligate anaerobic bacteria, might enable the clearance of intestinal VRE and provide a novel approach to prevent the spread of highly antibiotic-resistant bacteria.19 Although our patient did not achieve VRE conversion within the observation period, FMT should be considered in patients with C. difficile infection who are also colonized by VRE.
There have been recent case reports of FMT in a single center in Korea.20 More active therapeutic attempts are needed, especially in patients colonized by VRE, to standardize an applicable, acceptable protocol with maximum efficacy.
Footnotes
Financial support: None.
Conflict of interest: None.
References
- 1.Kim YS, Han DS, Kim YH, et al. Incidence and clinical features of Clostridium difficile infection in Korea: a nationwide study. Epidemiol Infect. 2013;141:189–194. doi: 10.1017/S0950268812000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Bauer MP, van Dissel JT, Kuijper EJ. Clostridium difficile: controversies and approaches to management. Curr Opin Infect Dis. 2009;22:517–524. doi: 10.1097/QCO.0b013e32833229ce. [DOI] [PubMed] [Google Scholar]
- 4.Seo GS. Clostridium difficile infection: what's new? Intest Res. 2013;11:1–13. [Google Scholar]
- 5.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 6.Rashid MU, Weintraub A, Nord CE. Effect of new antimicrobial agents on the ecological balance of human microflora. Anaerobe. 2012;18:249–253. doi: 10.1016/j.anaerobe.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 8.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 9.Salcedo J, Keates S, Pothoulakis C, et al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53:882–884. doi: 10.1093/jac/dkh176. [DOI] [PubMed] [Google Scholar]
- 11.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 12.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 13.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 14.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 16.Sofi AA, Silverman AL, Khuder S, Garborg K, Westerink JM, Nawras A. Relationship of symptom duration and fecal bacteriotherapy in Clostridium difficile infection-pooled data analysis and a systematic review. Scand J Gastroenterol. 2013;48:266–273. doi: 10.3109/00365521.2012.743585. [DOI] [PubMed] [Google Scholar]
- 17.Sethi AK, Al-Nassir WN, Nerandzic MM, Donskey CJ. Skin and environmental contamination with vancomycin-resistant Enterococci in patients receiving oral metronidazole or oral vancomycin treatment for Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2009;30:13–17. doi: 10.1086/592710. [DOI] [PubMed] [Google Scholar]
- 18.Choi HK, Kim KH, Lee SH, Lee SJ. Risk factors for recurrence of Clostridium difficile infection: effect of vancomycin-resistant enterococci colonization. J Korean Med Sci. 2011;26:859–864. doi: 10.3346/jkms.2011.26.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ubeda C, Bucci V, Caballero S, et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun. 2013;81:965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gweon TG, Choi MG, Lee SK, et al. Two cases of refractory Pseudomembranous colitis that healed following fecal microbiota transplantation. Korean J Med. 2013;84:395–399. [Google Scholar]



