Abstract
The Stillbirth Collaborative Research Network (SCRN) was organized to study the scope and causes of stillbirth (SB) in the United States. The objective of this report is to describe the approach used for the placental examination performed as part of the study. The SCRN consists of a multidisciplinary team of investigators from five clinical sites, the National Institute of Child Health and Human Development, and the Data Coordination and Analysis Center. The study is a population-based cohort and nested case-control study, with prospective enrollment of women with SB and live births (LB) at the time of delivery. Detailed and standardized postmortem examination was performed on SB and placental examination in both groups. A total of 663 women with SB and 1932 women with LB were enrolled into the case-control study. In the SB group, there were 707 fetuses. Of these cases, 654 (98.6%) had placental examination. Of these LB controls, 1804 (93.4%) had placental examination. This is the largest prospective study to include population-based SB and LB, using standardized postmortem and placental examination, medical record review, maternal interview, collection of samples, and a multidisciplinary team of investigators collaborating in the analyses. Thus it has the potential to provide high-level evidence regarding the contribution of placental abnormalities to stillbirth.
Keywords: Stillbirth, placenta, umbilical cord, cause of death
In the United States, ~26,000 stillbirths occur every year. Although there was a 35% decline in infant mortality between 1985 and 2001, the stillbirth rate declined by only ~17%, from 7.8 to 6.5 deaths per 1000 births, over that time period. As a result, the number of stillbirths has reached a level that is about equivalent to the number of infant deaths. These statistics highlight the importance of improving our understanding of the causes of stillbirth and our approach to prevention. Examination of the placenta is central to the evaluation of stillbirth. The College of American Pathologists in 1991 and 1997 described in detail the utility of placental examination in a variety of conditions, including stillbirth, and described basic methodology for examining placentas.1–4 In 2007, The American College of Obstetricians and Gynecologists Committee on Genetics recommended macroscopic and microscopic examination of the placenta, including membranes and umbilical cord, to corroborate postmortem findings or explain apparent fetal abnormality.5 The Committee further emphasized that the ease of obtaining consent to examine the placenta should make its examination a routine clinical tool in stillbirth evaluation. Despite these recommendations, fetal postmortem and placental examinations are performed in only 15 to 40% of stillbirths.6 It is likely that both the scarcity of perinatal pathologists and the lack of a standardized approach in the evaluation of stillbirths have contributed to the limited use of this readily available resource.
The National Institute of Child Health and Human Development (NICHD) convened a workshop on March 26, 2001, with perinatal pathologists and other experts in the field to set a national agenda for stillbirth research.7,8 The group identified several significant gaps in knowledge to include the lack of a standard protocol for postmortem investigation of stillbirths, including laboratory, toxicological, and genetic tests and the paucity of geographic population-based, detailed investigations of reproductive and fetal risks associated with stillbirth. In 2003, the NICHD established the Stillbirth Collaborative Research Network (SCRN) to study the extent and causes of stillbirth in the United States. The purpose of this report is to describe the SCRN and the approach to pathological examination of the placenta.
STUDY DESIGN
Stillbirth was defined as a fetal death at 20 weeks’ gestation or greater. The SCRN further defined fetal death as a newborn having Apgar scores of 0/0 at 1 and 5 minutes with no other signs of life by direct observation. The specific aims of the SCRN were to: (1) develop a standardized approach to determine the causes of stillbirth to include an improved review of clinical history, and a standardized postmortem examination protocol and pathological examination of the placenta, as well as other postmortem tests to illuminate genetic, maternal, and other environmental influences; (2) obtain a geographic population-based determination of the incidence of stillbirth, defined as fetal death at 20 weeks’ gestation or greater, and compare this with the incidence derived from vital statistics; and (3) implement a geographic population-based case-control study to elucidate maternal biological and environmental factors, in combination with genetic predisposition, that influence the risk of stillbirth.
The SCRN encompassed a Data Coordinating and Analysis Center (DCAC), Research Triangle Park (RTI) International, North Carolina, and five clinical sites: Brown University, Rhode Island; Emory University, Georgia; University of Texas Medical Branch at Galveston, Texas; University of Texas Health Sciences Center at San Antonio, Texas; and University of Utah Health Sciences Center, Utah. The target was for the five clinical sites to enroll 700 stillbirths whose mother was a resident in their geographic catchment areas at the time of delivery to provide 500 stillbirths (fetal deaths 20 weeks or greater gestation) with complete placental and fetal postmortem examinations. Also, a representative sample of ~1400 live birth controls was to be enrolled for placental examination and collection of other study data, with oversampling of ~500 additional live births delivered at <32 weeks’ gestational age and of African descent to allow matching by race/ethnicity and gestational age as needed. These cases and controls were enrolled from predefined catchment areas in five geographically diverse regions involving 59 hospitals, averaging >80,000 deliveries per year overall. Enrollment occurred following admission to the hospital, with sites having between 6 and 14 study hospitals. Hospitals were chosen and active surveillance measures were established to ensure identification and access to at least 90% of deliveries of both stillborn and live-born infants to residents of the designated geographic areas. Participants underwent a standardized protocol including maternal interview, medical record abstraction, biospecimen collection, placental pathology, and, for cases, postmortem examination.
SCRN investigators developed hypotheses in the following content areas: fetal and placental pathology, surveillance and epidemiology, maternal disease mechanisms, immunology and infectious diseases, and genetics. These hypotheses drove the design requirements of the study including case selection and ascertainment, number and type of controls needed, the timing and approach to data collection and management, and the placenta, blood, and fetal tissue biospecimens to be collected from the mother and the stillborns or live-borns. Enrollment to the case-control study began in March 2006 and was completed in September 2008.
A first step in the implementation of the study was to design standard, comprehensive fetal postmortem and placental examination protocols that could be used for assessment. These protocols delineated the techniques of performing and reporting fetal-placental examinations as well as the procedures for biospecimen collection and laboratory testing.
This report outlines the methods used in the development of the SCRN pathology protocols; describes in detail the SCRN protocol for the examination, sampling, and evaluation of the placenta specimens; and discusses how the design of the SCRN study and placental data collection may contribute to the field of perinatal pathology. The protocols and methodology employed in the fetal postmortem examination and perinatal neuropathology are topics of separate reports.
METHODS
The primary SCRN anatomic pathologist at each of the five SCRN clinical centers was responsible for protocol adherence and quality assurance of all SCRN-related pathology procedures. To develop uniform data collection procedures, SCRN anatomic pathologists convened twice in face-to-face meetings during protocol development and prior to subject recruitment to review principles of anatomic examination, develop consensus on examination procedures, examine representative lesions, and develop uniform definitions and reporting categories for macroscopic and microscopic findings. In addition, there were regularly scheduled conference calls to monitor progress and to review and approve written protocols and data collection tools. A third face-to-face meeting took place after initiation of enrollment to examine the procedures in more detail and reinforce consensus. During the data collection phase, conference calls were continued to monitor progress and solve problems as they arose.
The pathology protocols featured primary data collection rather than abstraction from clinical pathology reports. For research purposes, pathologists were not asked to make diagnoses but were instructed to record specific observations and findings based on criteria defined a priori. The data elements included as questions on the forms, and their response categories, were selected to provide a sufficient level of scientific detail without overburdening the resources of the pathologist. Within the data forms, check boxes were provided at the beginning of each major section to indicate “no abnormalities” or “no available data” for that section, thereby allowing the questions in the specific section to be skipped en masse as appropriate. At the end of each major section, a free text “Notes” field was provided for entering additional findings or details to supplement the standard questions. Designing the placental data collection forms to efficiently accommodate multifetal gestations with varying placentation was a particular challenge, and is discussed below.
The placental examination was conducted in several stages—collection and initial assessment; imaging; macroscopic examination; selection of locations and collection of tissue samples; processing of samples into tissue blocks; and microscopic examination of the glass slides prepared from the tissue blocks.
Collection and Initial Assessment of the Placenta
Hospital labor and delivery personnel collected and stored intact and fresh placentas for cases and controls in dedicated refrigerators until the arrival of the study staff to the facility. The study staff abstracted a brief maternal history from the medical records and information on the delivery, and these were given to the pathologists with the placenta. In addition, in study cases, the SCRN pathologists received a copy of the postmortem consent forms and a copy of the pertinent medical records. These documents were placed and transported in sealed envelopes. When the placenta and/or fetus were delivered, the SCRN pathology research staff checked and confirmed the SCRN identification information. The clinical accession number of the specimen was recorded in the study forms.
Once the placenta was checked in, the pathologist made an initial determination of the placentation. We designed data extraction forms that could be used in all types of placentation for singleton and multiple gestations by considering four elements: (1) umbilical cord(s), (2) placental disc(s), (3) chorioamniotic sac(s) (placental membranes), and (4) dividing membrane(s). The number of umbilical cords was used as a marker for the number of fetuses present in a gestation (Fig. 1). The SCRN pathologist recorded whether the specimen was fresh or fixed (including type of fixative) and whether the specimen was previously examined or sampled. Whenever possible, the placentas were examined and specimens obtained fresh. The SCRN pathologist performed as much of the SCRN protocol as was practicable. Also, the degree of fragmentation using the categories given in Table 1 was recorded.
Figure 1.
For the purpose of examining various types of placentas consistently, we designed the data extraction forms that could be used in all types of placentation for singleton and multiple gestations. This system considered four elements: (1) umbilical cord(s), (2) placental disc(s), (3) chorioamniotic sac(s) (placental membranes), and (4) dividing membrane(s). The number of umbilical cords was used as a marker for the number of fetuses present in a gestation. In singleton pregnancies, the cord, disc, and sac were all identified as “A.” In multifetal pregnancies, when the delivery personnel marked the cords, the cord ID for the firstborn was designated as “A,” for the second-born as “B,” and so on. If birth order was unknown, cords were arbitrarily designated using the letters M, N, and so on. Identifiers for placental membranes (sacs) were designated with one or more letters (e.g., A, B, AB; M, N, MN) depending on the placentation. Dividing membranes were indicated with two letters (e.g., AB; MN) and placental discs were each indicated with a single letter when discs were distinct and two (or more) letters when fused (with or without a dividing membrane, depending on the number of sacs). Forms for tissue samples and the microscopic examination used the cord ID. For a fused disc, if a dividing membrane could not be observed, an imaginary perpendicular midway between the umbilical cord insertion points was used to distinguish “side A” and “side B” for purposes of sampling and performing the microscopic examination.
Table 1.
Grading System for Fragmented Placenta Specimens
| General Description | Grade | Definition |
|---|---|---|
| Intact | 0 | Completely intact specimen (one piece) |
| Partially fragmented | I | A specimen where only the membranes are fragmented |
| Partially fragmented | II | A specimen that contains two or more pieces ≥5cm; pieces are large enough that some of the detailed sampling procedures can be implemented |
| Completely fragmented | III | Specimens consisting of completely small fragments (<5cm) |
Imaging of Placental Specimens
The SCRN pathologists used digital cameras with a minimum resolution of 3 megapixels, a 50-mm or equivalent macro lens, and a separate tungsten or fluorescent light source to take images of the placentas. A centimeter ruler and a label with the specimen's network identification number and, when applicable, surgical accession numbers were included in photographs. The image format used in the majority of the facilities was Joint Photographic Experts Group (JPEG). The size of each image files ranged from 2 to 8 MB. Routine images taken from the placenta samples are illustrated in Fig. 2.
Figure 2.
Main images taken from the placenta and documentation of sampling. (A) The first image was that of the fetal surface after trimming the membranes and the umbilical cord. If there were any significant membranous or umbilical cord lesions in relation to the placental disc, these were documented before trimming the placental disc. (B) Maternal surface with irregular lobules (cotyledons). (C) Position of the protractor on the maternal surface. (D) Sliced placenta with the umbilical cord marker in place for orientation.
Macroscopic Examination of Placental Specimen
SCRN pathologists attempted to complete the placental macroscopic examination within 3 working days of receipt of the placenta. The placenta was placed on a cutting surface with the maternal side down, and an attempt was made to reconstruct the fetal membranes to asses their completeness and to determine the shortest distance from the membrane rupture site to the nearest edge of the disc. In addition, the insertion type (marginal, circumvallate, and/or circummarginate) and color and sheen of the membranes were evaluated. In multifetal pregnancies with dividing membranes, the pathologist assessed the thickness of the dividing membranes and the attachment of the dividing membranes to the disc.
Next, the location and type of the umbilical cord insertion were examined and noted. Rather than using these terms such as central, eccentric, marginal, vela-mentous (membranous) or furcate, we characterized the location by recording coordinates of the insertion site and other placental landmarks, and the shortest distance from the insertion site to the edge of the placental disc. Because in velamentous and/or furcate insertions fetal blood vessels can lose their supportive Wharton's substance and enter placental membranes (velamentous) or the placental disc (furcate), the length of the unprotected vessels was measured. In addition, these vessels were serially sectioned to evaluate for any intravascular thrombi.
All the cord segments submitted were individually measured and their lengths summed. The umbilical cord segment attached to the placental disc was designated as the placental (proximal) end. In umbilical cords with uniform coiling, a coil count was obtained from a single segment at least 5 cm long, usually from the segment attached to the placental disc. If coiling was not uniform, the total number of 360-degree coils was counted over all segments and divided by the total length. The cord coil index was calculated as the number of coils divided by the length in centimeters.9–11
Examination of the cord included false knots, true knots, umbilical cord twists, edema, hemorrhage, lacerations, or avulsions. True knots or any other lesions that might have compromised fetal circulation were evaluated for evidence of circulatory compromise, which included careful macroscopic and microscopic examination of the entire placenta. Then the cord was separated from the placental disc, leaving a 1-cm stump at the insertion site. Next the umbilical cord was divided at 5-cm intervals, the cut sections inspected and the diameters of the thickest and thinnest segments and the number of umbilical arteries and veins were recorded.12,13
Placental discs were weighed after the blood was completely drained, extraneous clots were removed, and membranes and umbilical cords were trimmed. The longest and shortest dimensions of the placental disc were measured. Extra lobes and unusual placental shapes were noted and measured separately. Then the pathologist made full thickness cuts 1 cm wide leaving a 2-cm-wide strip containing the umbilical cord insertion site. The cut surfaces of each slice were examined. In placentas with uneven thickness, the thickest and thinnest segments were measured; otherwise, one measurement was recorded. Prior to and after slicing the placenta, distinct lesions and other characteristics of interest were separately photographed and sampled.
Selection of Locations for Parenchymal Sampling
The locations for collection of parenchymal tissue for blocks and frozen samples were selected using a random sampling method. The purpose of the random sampling method was to generate representative samples spread from the cord insertion site to the periphery and collected in a uniform manner among pathologists.
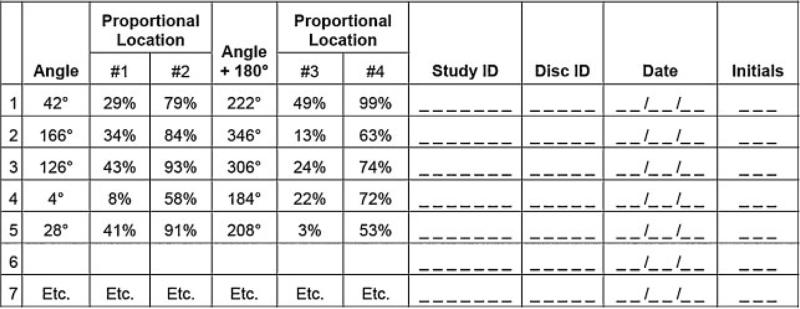
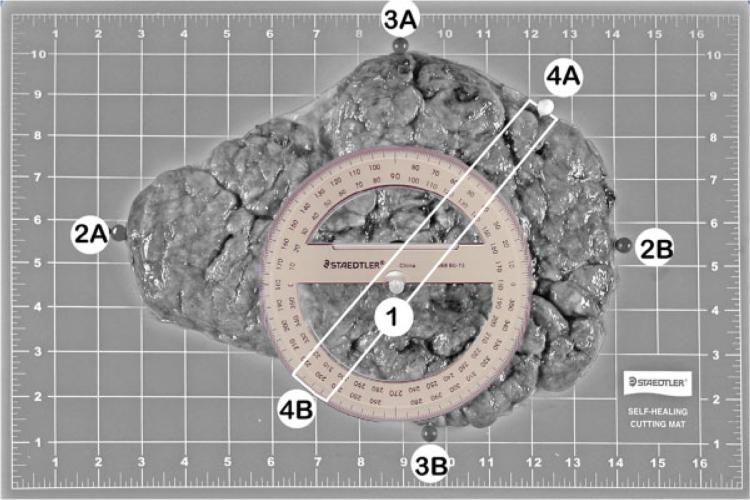
Using a random method, four locations were selected for collection of sample blocks and frozen storage. The placenta was placed on the cutting board with the maternal side up. The cutting board was marked with an imprinted grid to allow the pathologist to record specimen orientation, including landmarks of the perimeter of the disc and locations of the umbilical cord insertion, randomly selected samples, and lesions. The longest dimension of the placenta was aligned on the horizontal axis and the short diameter on the vertical axis so that the upper placental margin lay on an intersection point of the grid (Fig. 3). The grid coordinates of the superior, inferior, and lateral margins of the placental disc and umbilical cord insertion point were then recorded. Additional landmarks were recorded for dividing membranes in multifetal gestations. Coordinates were also recorded for the locations of any focal lesions for which samples were collected. To streamline the process, coordinates were recorded to the tenth of a major grid interval, as sighted by eye; minor markings were ignored in this process. Thus, the coordinates for the pushpin labeled #1 in Fig. 3 might be recorded as, say, (8.8, 4.4), or something similar. It was decided that this would provide rapid assessment and sufficient precision for practical purposes. Next, a protractor was placed on the placenta with its center point placed on the center of the umbilical cord insertion and its 180- to 0-degree axis set parallel to the horizontal axis (Fig. 3). The angle of the first full-thickness incision was determined by a random assignment from the RTI DCAC (Fig. 4).
Figure 3.
Mock-up of a selection list of random angles and proportional locations for sampling from the placental disc.
Figure 4.
This image illustrates the coordinates to be used for the sampling of the placental disc; the same image was repeated after coordinate markers and protractor were added to the placental disc. Frequently, these two images were combined. The maternal surface of the placenta shows the landmarks and placement of the protractor. The placenta is oriented with the long axis horizontal. Locations of the landmarks are marked with pushpins: (1) location of the umbilical cord insertion point (on the fetal side of the placenta); (2A and 2B) leftmost and rightmost borders; (3A and 3B) topmost and bottommost borders. For multiple gestations, additional landmarks were recorded regarding the position of dividing membranes. Coordinates for these landmarks were recorded in the data collection forms. The pins labeled 4A and 4B show the borders marking the randomly assigned sampling axis. The protractor is centered at the landmark corresponding to the location of the umbilical cord insertion point. The white lines indicate the central 2-cm strip, which is used for the histological samples.
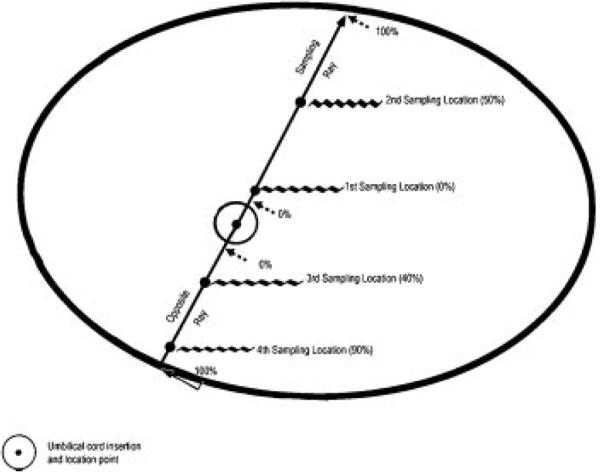
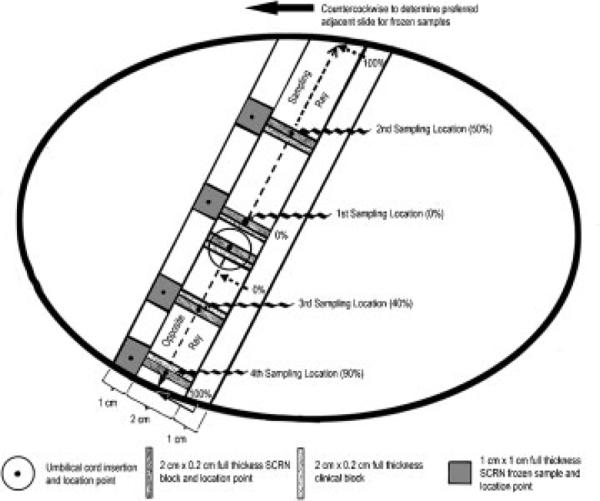
The first two incisions were made 2 cm apart on either side of the umbilical cord, defining a central 2-cm-wide strip, as shown in Figs. 5 and 6. Parallel full-thickness incisions were then made at 1-cm intervals to the edge of the placenta. Four SCRN study samples were collected for histology from the central 2-cm strip at randomly assigned distances from the umbilical cord insertion site, expressed as the percent of the distance from the edge of the insertion site to the margin of the placenta on each side (i.e., along the sampling ray and its opposite side). On each side, the locations of the two samples were based on a (proportional) systematic selection with a random start. The first location was selected using a random percentage between 0 and 49, and the second was determined by adding 50 to the first percentage. These were applied to the sampling ray as shown schematically in Fig. 6. The third and fourth locations were generated similarly and applied to the opposite ray. Again, to streamline the procedure, the specific locations corresponding to the assigned percentages were sighted by eye, rather than by performing precise measurements. A clinical sample was taken adjacent to each of the SCRN study samples, as shown schematically in Fig. 6. Four sections of placental parenchyma intended for storage at 808C were obtained at the corresponding locations along the 1-cm tissue slice lying adjacent to the central strip in a counterclockwise direction to the sampling ray (Fig. 6).
Figure 5.
Locating random percentages along a sampling ray and its opposite ray. Schematic of the maternal surface of the placenta, viewed from above, showing locations of 0% and 50% on the sampling ray and 40% and 90% on the opposite ray. The location for 0% is slightly beyond the edge of the umbilical cord insertion point (to leave room for a clinical block); 100% is at the margin.
Figure 6.
Location of samples relative to the sampling locations and placental slices, overlaid on the diagram in Fig. 5. Schematic of the maternal surface of the placenta, viewed from above. The drawing is to relative scale for a near-term, 22-cm-diameter placenta, assumed to be ~2 cm thick. Specimens for histology (2 × 0.2 cm × full-thickness depth) are cut from the central, 2-cm-wide slice that contains the umbilical cord insertion site. The histological specimens are cut with the 2-cm side perpendicular to the sampling axis. The Stillbirth Collaborative Research Network (SCRN) block is obtained at the sampling location, and the clinical block is collected immediately adjacent to the SCRN block, on the side toward the umbilical cord insertion. An additional SCRN block is cut through the center of the umbilical cord insertion point, and a clinical block is collected immediately adjacent to the SCRN block. Additional slices are made every 1 cm to the edge of the placenta in each direction. SCRN frozen samples are collected preferentially from the adjacent 1-cm slice that is immediately counterclockwise to the first sampling ray. The location point of each frozen sample should line up with the sampling locations whenever possible (there are rules regarding exceptions in certain situations); 1 × 1 cm × full-thickness frozen samples are shown, because this placenta is assumed to be 2-cm thick, yielding ~2 g of placental parenchyma per sample.
It should be noted that this method of selection, although random, does not produce a simple random sample of the placental parenchyma. The adopted procedure samples a progressively greater proportion of the parenchyma at a given proportional distance from the umbilical cord as that distance decreases. Additional rules for selection of sample locations were applied in special situations including very small placentas, placentas with marginal or very eccentric umbilical cord insertions, and placentas for which the umbilical cord was missing and the insertion point unknown.
In multiple gestations, a separate random sampling axis was selected for each umbilical cord. In multiples with dividing membranes, for each umbilical cord, the proportional locations were defined from the umbilical cord insertion to the margin or to the dividing membrane, as appropriate, depending on the direction of the random sampling axis. In multiples without discernable dividing membranes, the proportional locations were defined from the umbilical cord insertion to the margin or to a hypothetical perpendicular located midway between the umbilical cord insertion points.
Collection and Processing of Samples
Samples were collected for placental histology, karyotype analysis, DNA analysis, bacteriologic culture, and analysis of microbial DNA, toxicological analysis, and storage for future use. All the samples that were collected are summarized in Table 2.
Table 2.
Different Placental Samples for Various Analyses
| Type of Sample | Purpose of Sample | Location | No. of Samples Per Sac |
|---|---|---|---|
| Fixed in 10% formalin | Histological analysis (samples obtained in two sets each—one clinical and one research) | Umbilical cord | Two sections—proximal and distal |
| Placental parenchyma | Four randomly selected sections from the center slice (2 cm wide) | ||
| Umbilical cord insertion site | One section perpendicular to the fetal surface | ||
| Placental membranes | 1 membrane roll | ||
| Dividing membrane(s) roll(s) | |||
| T section | Insertion of the dividing membranes | ||
| Collected at room temperature to be stored in 4°C | Toxicological analysis | Umbilical cord | 3.5-g unfixed cord sample |
| Frozen at –80°C | Various analyses requiring frozen tissue | Placental parenchyma | Four randomly selected sections from the adjacent to the center slide; these are divided |
| DNA extraction | Placental parenchyma | One 2-g sample | |
| Microbiologic analysis | Placental parenchyma | 1 × 1-cm piece 4 sections from the adjacent slice (1 cm wide) to the center slice | |
| Microbiologic analysis (not at all research centers) | Swab for aerobic cultures at room temperature for local processing | Between the placental membranes | Swabs from the placental membranes |
| Microbiologic analysis (not at all the research centers) | Swab for future DNA analysis for mycoplasma and ureaplasma—frozen | Between the placental membranes | Swabs from the placental membranes |
| Karyotype analysis for each stillborn | Cell culture growth media | Placental parenchyma | One 0.5- to 2-g sample |
Additional samples were collected for any abnormal finding. Focal and diffuse lesions were separately described and samples were submitted for preparation of paraffin blocks.
Processing of Tissue Blocks
The pathologists completed the microscopic examination from the set of tissue blocks used for the clinical evaluation. This set of tissue blocks was processed in a routine manner. Slides representing the full thickness of the placenta were cut from the tissue block(s) and stained with hematoxylin and eosin. Depending on indications, additional slides were prepared and special stains were used. When these additional methods were used and deemed essential in reaching a final diagnosis, they were documented with digital photomicrographs, if possible.
If an SCRN research block could not be prepared because of any reason, five unstained slides and one paraffin curl at least 25 mm thick were prepared from the clinical block and submitted to the tissue repository in lieu of the SCRN block. When brown pigment was identified during the microscopic examination of the placental membranes, it was recommended that an iron stain be performed using Prussian blue reaction.
In partially fragmented placentas (grade II), the relatively larger pieces were brought together and the specimen was reconstructed to its complete form in a single layer to the extent possible. After separating the membranes and the umbilical cord segments, the fragments of parenchyma were weighed. Then the examination proceeded similarly to that for intact placentas, and as much information as possible was collected.
When the specimen was completely fragmented into small pieces (grade III), the fragments were sifted through; membranes, cord segments and blood clots were separated; and the remaining tissue was examined. The tissue fragments were weighed together. If there were any identifiable pieces, appropriate measurements were obtained (i.e., umbilical cord diameter).
Microscopic Examination of Placenta Specimens
Although there is no standardized recommendation for a specific method to examine the placentas microscopically, a few publications have published some guidelines.1–4,14–17 The data elements collected in the microscopic examination are listed in Fig. 7. To describe the distribution of the lesions, a semiquantitative terminology was accepted after discussions. This is illustrated in Table 3. Although the initial classification included the categories “multifocal” and “patchy,” a consensus soon developed that there was no practical difference between “multifocal” and “patchy.” Thus a decision was made to combine these two categories in the data analysis.
Figure 7.
Data elements collected in the placenta microscopic examination. EVT, extravillous trophoblast; LB, liveborn; SB, stillborn; RBC, red blood cells.
Table 3.
Categorization of Distribution Patterns of Lesions in the Terminal Villi and Other Parts of the Placenta
| Distribution Pattern | Lesions in Terminal Villi | Lesions in Other Locations |
|---|---|---|
| Focal | Aggregates of ≤15 villi in ≤3 foci in a single microscopic section | Present in one area on one single slide |
| Patchy | Aggregates of ≤15 villi in >3 foci involving >1 microscopic section | Forming patches or clusters when multifocal lesions coalesce to form larger aggregates; in this pattern, the distribution is uneven |
| Multifocal | Aggregates of ≤15 villi in >3 foci in a single microscopic section | Present in more than one area and/or in multiple slides |
| Diffuse | Involving >5% of all terminal villi | The lesions involved the full thickness and all the sections to the same degree |
Preliminary Data
A total of 663 women with stillbirth and 1932 women with an all live-birth outcome were enrolled into the case-control study. Of the women with stillbirth, 620 delivered a single stillborn infant, 42 delivered twins (13 sets with two stillborn and 29 sets with one stillborn), and one delivered triplets (one stillborn and two live born), for a total of 707 infants. Of these women, 654 (98.6%) consented to placental examination, and in 632 (95.3%), the examination was considered to have been adequate (i.e., without significant limitations). Signifi-cant limitations included the review of only slides or a report from a non-SCRN pathologist, or the placenta having been discarded in labor and delivery before it could be collected by the study staff. Of the 1932 women with an all live-birth outcome, 1871 delivered singletons, 58 delivered twins, and three delivered triplets, for a total of 1996 infants. Of these women, 1804 (93.4%) consented to placental examination, and in 1347 (69.7%), the examination was considered adequate. Among live-birth controls, the most common significant limitation was that the placenta had been discarded in labor and delivery before the study staff consented the patient (n = 446, 23.1% of those who consented). For those women with adequate placental examinations, grade I, II, and III placental fragmentation was reported in 13.1%, 9.1%, and 2.7% of cases and 12.3%, 6.2%, and 2.2% of controls, respectively.
DISCUSSION
Fetal death is the natural demise of a product of human conception before the complete expulsion or extraction from its mother, regardless of the duration of pregnancy. Reporting of such losses is regulated by state guidelines. Many states use fetal weight of 350 g or more or if weight is unknown, a gestational age of 20 completed weeks or more, calculated from the date of the last menstrual period, as the requirements for reporting. In the past, these losses were mostly ignored and unfortunately unreliable, and incomplete information was entered as the cause of the demise.18,19 Although postmortem examination of the stillborn can yield significant information, it is costly, may not be reimbursed by insurers, and requires specific expertise. On the other hand, the placenta, as the fetomaternal interface, can also provide invaluable information to understand the cause of fetal deaths. In addition, placental examinations are reimbursable and do not require special consent for examination.
Studies describing placental findings in stillbirth are scant.20–22 In one review of 146 postmortem examinations performed on macerated and nonmacerated stillbirths, significant findings were identified in 53% of the placentas.23 These findings were similar to a later study where placental findings supported or confirmed the clinical impression in 61.5% of the 310 cases.24 Due to the vagueness of some of the terminology, there were inconsistencies between the pathological and clinical information in 11% of the cases.24 Other studies have documented abnormalities in ~37 to 40% of placentas from stillbirth, with inflammatory lesions predominating in the 18- to 24-week gestational age range.25
In his review of causes of stillbirth, Bendon26 listed cord lesions such as prolapse, vessel constriction, knots, nuchal cord, and cord entanglement in multifetal pregnancies as most frequent causes of death. In addition, retroplacental hematoma in conjunction with placental abruption, large parenchymal infarcts, massive perivillous fibrin deposition, and parenchymal thrombi also have been linked to fetal death.27
In a retrospective review of 120 autopsy reports of singleton stillborn fetuses and placentas from 23 to 40 weeks of gestation, Kidron et al.28 identified placental pathology in 54 (51%) cases with direct cause or major contributor to death in the etiology of maternal vascular supply abnormalities, 28 (26%) cases in the etiology of fetal vascular supply abnormalities, and 13 (12%) in the etiology of inflammatory lesions. Maternal vascular supply abnormalities were more common in preterm stillbirths, and fetal vascular supply abnormalities were more common among term stillbirths. In 88% of stillbirths, a direct cause or a major contributor to death was found in the placentas. The stillbirth remained unexplained in only 8% of cases.
In contrast to these previous studies, the SCRN case-control study has enrolled contemporaneous live-born controls and stillborns with placental pathological examination. Furthermore, given the oversampling of preterm births <32 weeks, the SCRN study will provide a resource for examining placental pathology in preterm and term birth as well as stillbirth. Prior studies have reported normal measurements from placental samples and established expected growth curves.29,30 Although these values are available, the morphological features expected to be observed in small-for-gestational-age and large-for-gestational-age placentas are still not clearly delineated. Also, preliminary information suggests that in addition to the macroscopic size and shape abnormalities, variations in the villous morphology reflect abnormalities in vascular growth and plays a major role in the ultimate size and function of the placenta.31 The design of the SCRN study will allow us to examine what is typical in the newborn placenta and what findings are associated with stillbirth, according to gestational age.
The application of a uniform placental examination protocol as well as primary data collection with standardized forms allows for objective collection of study data. The associated specimen collection and storage in the central tissue repository will provide a valuable resource for further study. Our approach to examination, data collection, and sampling was to devise a method that was scientifically sound yet simple to implement. We created a workable system of data collection forms to accommodate variations in placentation, and we allowed skipping questions to speed the data collection when major sections of the examination were either normal or not applicable. We recorded specific findings rather than diagnostic categories that can be subject to varying criteria among pathologists and change with time. We used an ordinal categorization to document the distribution of placental lesions.
Although the adopted procedure for sampling the placental parenchyma is not a stereological sampling procedure,32,33 it has several positive attributes. It is implemented with simple tools (a protractor and a random selection list) and accommodates a wide range of placental sizes for gestations from 20 weeks to term. The (proportional) systematic random selection along the sampling axis guarantees a spread of the samples from umbilical cord insertion toward the periphery of each placenta. It eliminates systematic differences in sampling between pathologists. The recording of sample coordinates allows modeling of any potential gradients in the placental response from the umbilical cord insertion to the margin of the disc, and the recording of landmark coordinates and other measurements enables some approximate normalization for placental size. The availability of photographs of the placenta on the cutting grid allows for additional, more detailed measurements if needed. Once the SCRN pathologists, through repetition, became familiar with the sampling procedure, it became routine and was not considered unduly burdensome.
Finally, these anatomic pathology data are linked to extensive prospectively collected social, medical, and biological data as well as biological specimens that will allow correlation with placental macroscopic and microscopic findings. We anticipate that the correlation of pathological data with clinical and epidemiological data will improve the understanding of the mechanisms leading to stillbirth.
Acknowledgments
FUNDING
Supported in part by grant funding from the Stillbirth Collaborative Research Network sites: U10-HD045953 (Brown University, Rhode Island); U10-HD045925 (Emory University, Georgia); U10-HD045952 (University of Texas Medical Branch at Galveston, Texas); U10-HD045955 (University of Texas Health Science Center at San Antonio, Texas); U10-HD045944 (University of Utah Health Sciences Center, Utah); and U01-HD-45954 (RTI International, North Carolina); and by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
REFERENCES
- 1.Driscoll SG, Langston C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on Methods for Placental Examination. Arch Pathol Lab Med. 1991;115:704–708. [PubMed] [Google Scholar]
- 2.Kaplan C, Lowell DM, Salafia C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on the Definition of Structural Changes Associated with Abnormal Function in the Maternal/Fetal/Placental Unit in the Second and Third Trimesters. Arch Pathol Lab Med. 1991;115:709–716. [PubMed] [Google Scholar]
- 3.The examination of the placenta: patient care and risk management. College of American Pathologists Conference XIX. Northfield, Illinois, September 6–7, 1990. Proceedings. Arch Pathol Lab Med. 1991;115:660–721. [PubMed] [Google Scholar]
- 4.Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1997;121:449–476. [PubMed] [Google Scholar]
- 5.Committee on Genetics ACOG Committee Opinion No. 383: Evaluation of stillbirths and neonatal deaths. Obstet Gynecol. 2007;110:963–966. doi: 10.1097/01.AOG.0000263934.51252.e0. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Kirby R, Culhane JF. Stillbirth: a review. J Matern Fetal Neonatal Med. 2004;16:79–94. doi: 10.1080/14767050400003801. [DOI] [PubMed] [Google Scholar]
- 7.Hankins G, Willinger M, Spong CY. Stillbirth: introduction. Semin Perinatol. 2002;26:1–2. [Google Scholar]
- 8.Spong CY, Erickson K, Willinger M, Hankins GD, Schulkin J. Stillbirth in obstetric practice: report of survey findings. J Matern Fetal Neonatal Med. 2003;14:39–44. doi: 10.1080/jmf.14.1.39.44. [DOI] [PubMed] [Google Scholar]
- 9.Machin GA, Ackerman J, Gilbert-Barness E. Abnormal umbilical cord coiling is associated with adverse perinatal outcomes. Pediatr Dev Pathol. 2000;3:462–471. doi: 10.1007/s100240010103. [DOI] [PubMed] [Google Scholar]
- 10.van Diik CC, Franx A, de Laat MWM, Bruinse HW, Visser GHA, Nikkels PGJ. The umbilical coiling index in normal pregnancy. J Matern Fetal Neonatal Med. 2002;11:280–283. doi: 10.1080/jmf.11.4.280.283. [DOI] [PubMed] [Google Scholar]
- 11.Kalish RB, Hunter T, Sharma G, Baergen RN. Clinical significance of the umbilical cord twist. Am J Obstet Gynecol. 2003;189:736–739. doi: 10.1067/s0002-9378(03)00715-4. [DOI] [PubMed] [Google Scholar]
- 12.Heifetz SA. The umbilical cord: obstetrically important lesions. Clin Obstet Gynecol. 1996;39:571–587. doi: 10.1097/00003081-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Doornebal N, de Vries TW, Bos AF, de Vries NKS. Screening infants with an isolated single umbilical artery for renal anomalies: nonsense? Early Hum Dev. 2007;83:567–570. doi: 10.1016/j.earlhumdev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM. First Series, Fascicle 3. American Registry of Pathology and Armed Forces Institute of Pathology; Washington, DC: 2004. Atlas of Non-tumor Pathology-Placental Pathology. [Google Scholar]
- 15.Benirschke K, Kaufmann P. The Pathology of the Human Placenta. 5th ed. Springer; New York: 2006. [Google Scholar]
- 16.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, Society for Pediatric Pathology, Perinatal Section, Amniotic Fluid Infection Nosology Committee Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 17.Redline RW, Boyd T, Campbell V, et al. Society for Pediatric Pathology, Perinatal Section, Maternal Vascular Perfusion Nosology Committee Maternal vascular under-perfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 18.Hoyert DL, Martin JA. Vital statistics as a data source. Semin Perinatol. 2002;26:12–16. doi: 10.1053/sper.2002.29835. [DOI] [PubMed] [Google Scholar]
- 19.Martin JA, Hoyert DL. The national fetal death file. Semin Perinatol. 2002;26:3–11. doi: 10.1053/sper:2002.29834. [DOI] [PubMed] [Google Scholar]
- 20.Altshuler G, Hyde SR. Clinicopathologic implications of placental pathology. Clin Obstet Gynecol. 1996;39:549–570. doi: 10.1097/00003081-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Smith GC, Crossley JA, Aitken DA, et al. First-trimester placentation and the risk of antepartum stillbirth. JAMA. 2004;292:2249–2254. doi: 10.1001/jama.292.18.2249. [DOI] [PubMed] [Google Scholar]
- 22.Stallmach T, Hebisch G. Placental pathology: its impact on explaining prenatal and perinatal death. Virchows Arch. 2004;445:9–16. doi: 10.1007/s00428-004-1032-2. [DOI] [PubMed] [Google Scholar]
- 23.Magee JF. Investigation of stillbirth. Pediatr Dev Pathol. 2001;4:1–22. doi: 10.1007/s100240010121. [DOI] [PubMed] [Google Scholar]
- 24.Horn LC, Langner A, Stiehl P, Wittekind C, Faber R. Identification of the causes of intrauterine death during 310 consecutive autopsies. Eur J Obstet Gynecol Reprod Biol. 2004;113:134–138. doi: 10.1016/S0301-2115(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 25.Zanconato G, Piazzola E, Caloi E, Iacovella C, Ruffo R, Franchi M. Clinicopathological evaluation of 59 cases of fetal death. Arch Gynecol Obstet. 2007;276:619–623. doi: 10.1007/s00404-007-0391-8. [DOI] [PubMed] [Google Scholar]
- 26.Bendon RW. Review of some causes of stillbirth. Pediatr Dev Pathol. 2001;4:517–531. doi: 10.1007/s10024001-0084-4. [DOI] [PubMed] [Google Scholar]
- 27.Naeye RL. Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum Pathol. 1987;18:680–691. doi: 10.1016/s0046-8177(87)80239-3. [DOI] [PubMed] [Google Scholar]
- 28.Kidron D, Bernheim J, Aviram R. Placental findings contributing to fetal death, a study of 120 stillbirths between 23 and 40 weeks gestation. Placenta. 2009;30:700–704. doi: 10.1016/j.placenta.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16:901–907. doi: 10.1080/15513819609168713. [DOI] [PubMed] [Google Scholar]
- 30.Pinar H, Stephens M, Singer DB, et al. Triplet placentas: reference values for weights. Pediatr Dev Pathol. 2002;5:495–498. doi: 10.1007/s10024-002-0014-0. [DOI] [PubMed] [Google Scholar]
- 31.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Mayhew TM. Stereology and the placenta: where's the point?—A review. Placenta. 2006;27(Suppl A):S17–S25. doi: 10.1016/j.placenta.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 2008;29:1–14. doi: 10.1016/j.placenta.2007.05.010. [DOI] [PubMed] [Google Scholar]