Abstract
Background
Relatively little contemporary data are available that describe differences in acute heart failure (AHF) hospitalization expenditures as a function of patient and hospital characteristics, especially from a population-based investigation. This study aimed to evaluate factors associated with variations in hospital expenditures for AHF in the United States.
Methods
A cross-sectional analysis using discharge data from the 2011 Nationwide Inpatient Sample, Healthcare Cost and Utilization Project, was conducted. Discharges with primary International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis codes for AHF in adults were included. Costs were estimated by converting Nationwide Inpatient Sample charge data using the Healthcare Cost and Utilization Project Cost-to-Charge Ratio File. Discharges with highest (≥80th percentile) versus lowest (≤20th percentile) costs were compared for patient characteristics, hospital characteristics, utilization of procedures, and outcomes.
Results
Of the estimated 1 million AHF hospital discharges, the mean cost estimates were $10,775 per episode. Younger age, higher percentage of obesity, atrial fibrillation, pulmonary disease, fluid/electrolyte disturbances, renal insufficiency, and greater number of cardiac/noncardiac procedures were observed in stays with highest versus lowest costs. Highest-cost discharges were more likely to be observed in urban and teaching hospitals. Highest-cost AHF discharges also had 5 times longer length of stay, were 9 times more costly, and had higher in-hospital mortality (5.6% vs 3.5%) compared with discharges with lowest costs (all P < .001).
Conclusions
Acute heart failure hospitalizations are costly. Expenditures vary markedly among AHF hospitalizations in the United States, with substantial differences in patient and hospital characteristics, procedures, and in-hospital outcomes among discharges with highest compared with lowest costs.
Heart failure (HF) is a growing health and economic burden, and patients with HF are at high risk for hospital admission, morbidity, and mortality.1 Approximately 1 million acute HF (AHF) discharges occur in the United States per year.1 In 2012, an estimated 5.8 million American adults had HF, with a prevalence of 2.4%.1 Projected total medical costs for HF medical care are expected to increase from $20.9 billion in 2012 to $53.1 billion in 2030 with 80% of expenditures attributed to hospitalization.1 Despite the magnitude and impact of HF in the United States, there has been limited examination of the factors associated with inpatient resource utilization and expenditures for hospitalization for AHF. Understanding patient and health system factors associated with higher expenditure hospitalizations would aid medical providers, health service researchers, and policy makers in developing strategies for providing high-quality, value-driven care.
This study examined the demographic, clinical, and hospital factors of AHF patients associated with variations in hospital expenditures nationally. The analysis used discharge data from the 2011 Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, the largest all-payer acute care hospitalization database in the United States.2
Methods
Data sources
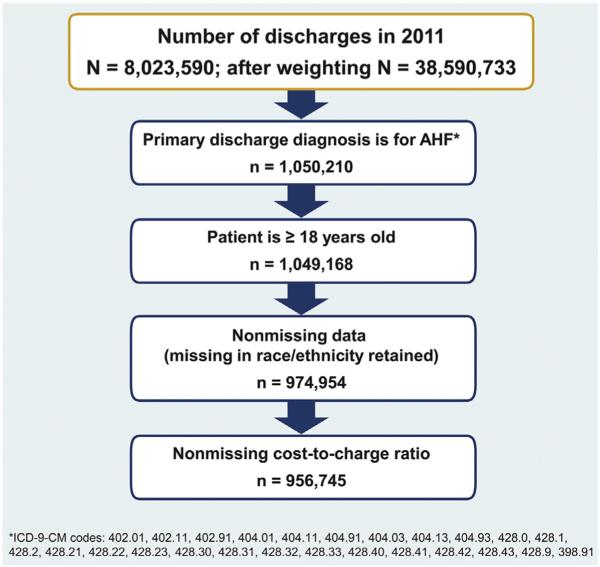
The NIS is sponsored by the Agency for Healthcare Research and Quality through HCUP and is the largest all- payer inpatient database available publically in the United States containing discharge data from about 1,000 hospitals across 46 states in 2011. The database includes charge information regardless of payer or insurance status, as well as clinical and resource use information included in a typical discharge abstract. Approximately 8 million hospitalizations per year are selected from a 20% stratified random sample of community hospitals representing over 97% of the American population. All discharges from sampled hospitals are included in the NIS database.2 We used the 2011 NIS to study AHF discharges and their costs in the United States. All hospital stays with a primary discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), code for AHF for patients ≥18 years of age were included; patients<18 years of age were excluded. The unit of analysis in NIS is a discharge; therefore, readmissions are not identified.2
Statistical analysis
The NIS provides hospital and discharge weights to calculate national estimates for variables of interest. Patient hospitalizations were organized into nationally representative quintiles by hospital cost estimates. The NIS provides total charges, which reflect the amount a hospital billed for services, rather than actual costs or the amount a hospital received in payment. In this study, we used the HCUP Cost-to-Charge Ratio (CCR) File developed by the Agency for Healthcare Research and Quality to translate total charges into cost estimates.3 This file provides hospital-specific CCR for 88% of HCUP hospitals in states that give permission to participate in CCR. The remainder of hospitals are imputed from the weighted average in a group defined by state, urban/rural, investor-owned/other, and bed size.3 We reweighted all discharges to account for cases where CCR values were missing as suggested by HCUP and Mach to gain national estimates.3,4 Studies show that hospital-specific CCRs alone do not account for cost variation observed among hospital service departments.5 We further adjusted expenditures from the CCR (hospital-specific or weighted group average) by multiplying by the appropriate adjustment factor for the discharge's Medicare Severity Diagnosis Related Groups or Clinical Classifications Software (CCS) category to obtain the more accurate final hospitalization cost estimates.
Discharges in the highest 80th percentile (highest quintile) for hospital costs were compared with the lowest 20th percentile (lowest quintile). Patient variables of interest included demographic (age, sex, race, median income by ZIP code), primary payer (Medicare, Medicaid, private, uninsured, other), source of admission (ie, emergency room), comorbidities present on admission, and common hospital procedures. The top 10 prevalent comorbidities and procedures in the full HF sample were screened for inclusion in the model. Procedures were collated into clinical meaning groups using the HCUP Clinical Classification Software for ICD-9-CM procedures (available at www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp). Hospital variables included region of the country, rural versus urban density, hospital ownership, teaching status, and bed size.
The complex sampling design and sample discharge weights were taken into account in all analyses.2 After appropriate weighting, continuous variables were described using mean and standard error and categorical variables using frequency and percentages. Bivariate analyses of differences in characteristics between the highest and lowest quintiles were evaluated using Pearson χ2 test for categorical variables and the adjusted Wald test for continuous variables. Hospital and patient variables were evaluated in multivariable logistic regression models to identify factors associated with the highest quintile of AHF hospitalizations in comparison with the lowest quintile. All data management and analysis were done using SAS 9.3 (Cary, NC) and Stata 13 (College Station, TX) programs. Novartis Pharmaceuticals (East Hanover, NJ) provided funding and review of manuscript prior to submission. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
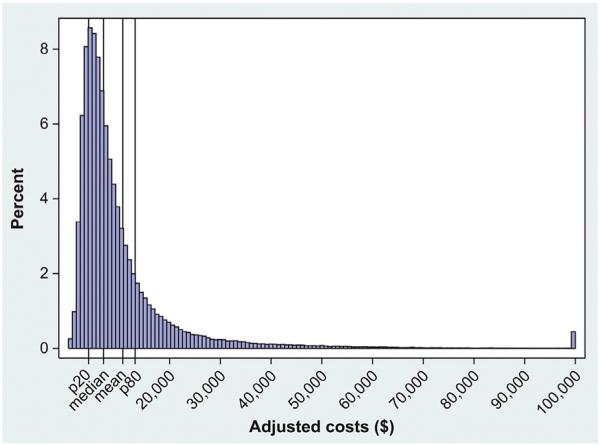
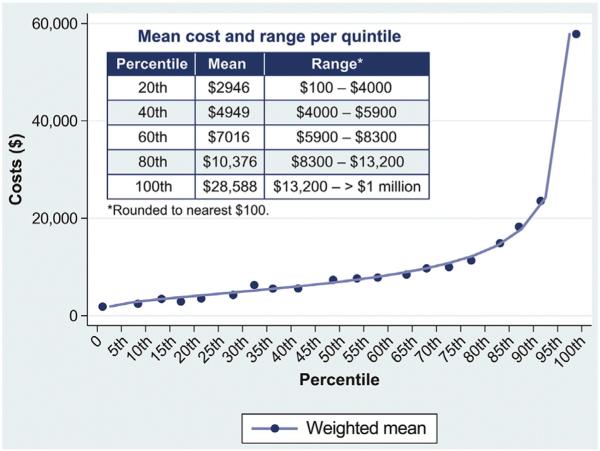
The NIS data set for 2011 includes 8 million discharges. There were 217,449 discharges with a primary diagnosis of AHF for patients <18 years of age. After weighting, we estimated that there were approximately 1 million AHF discharges in the United States in 2011 (Figure 1). The mean national cost estimates for AHF were $10,775 per AHF hospitalization episode, which was about one-third the amount of mean hospital charges. Inpatient costs for 2011 AHF hospitalizations were right-skewed, with a median cost of $7,000 (Figure 2). The mean inpatient costs by percentile and by quintile are shown in Figure 3. When stratified into quintiles of hospitalization-level costs, the mean cost for the lowest 20th percentile was $2,946 (range $100 to $4,000) and for the highest 80th percentile was $28,588 (range $13,200 to <$1 million; ranges rounded to nearest $100).
Figure 1.
Acute heart failure hospitalization study selection.
Figure 2.
Distribution of inpatient cost estimates among weighted AHF hospitalizations. Top-coded costs at $100,000; p20 = the value of the 20th percentile = $4,000; median = $7,000; p80 = the value of the 80th percentile = $13,200; rounded to nearest $100.
Figure 3.
Weighted mean inpatient cost estimates for AHF by percentile.
With regard to patient characteristics, slightly more than one-half of the AHF cohort was ≥75 years of age (Table I). Patients were 50.8% women and primarily white (60.4%), with 76.0% of AHF hospitalizations covered by Medicare. Comorbid conditions were frequent: 68.3% with hypertension, 44.4% with diabetes, 41.9% with renal insufficiency, and 38.4% with atrial fibrillation. Hospital characteristics of the weighted sample classified 62.6% as large by bed size, 84.2% as urban, and 41.3% as teaching hospitals. In-hospital mortality averaged 3.1%, and a U-shaped relationship was noted with the highest rate of mortality in the lowest and highest hospital cost groups (online Appendix Supplementary material).
Table I.
Patient and hospitalcharacteristics among AHF discharges overalland for the lowest- and highest-cost quintiles
| Characteristics | Total sample (N = 956745*) | ≤ 20th percentile ($100-4000) (n = 191350*) | ≥ 80th percentile ($13200- > 1000000) (n = 191350*) |
|---|---|---|---|
| Length of stay, d, mean (SE) | 5.2 (0.1) | 2.1 (0.03) | 10.9(0.2) |
| Total costs, US $, mean (SE) | 10775 (311) | 2946 (14) | 28588 (853) |
| Demographics | |||
| Age group, y | |||
| 18-44 | 4.0% | 4.3% | 3.9% |
| 45-54 | 8.2% | 8.3% | 8.6% |
| 55-64 | 14.7% | 13.7% | 17.1% |
| 65-74 | 20.3% | 19.0% | 23.6% |
| 75-84 | 27.7% | 27.3% | 28.2% |
| 85+ | 25.0% | 27.4% | 18.6% |
| Gender | |||
| Female | 50.8% | 49.3% | 47.3% |
| Race | |||
| White | 60.4% | 62.0% | 59.2% |
| Black | 19.0% | 20.1% | 18.4% |
| Hispanic | 7.3% | 5.3% | 8.9% |
| Asian/Pacific Islander/Native American/other | 4.2% | 3.1% | 5.7% |
| Missing/invalid/NA | 9.1% | 9.5% | 7.8% |
| Median household income by ZIP code | |||
| First quartile (the poorest) | 33.0% | 38.6% | 28.8% |
| Second quartile | 25.4% | 26.4% | 23.2% |
| Third quartile | 24.3% | 21.8% | 25.7% |
| Fourth quartile | 17.4% | 13.2% | 22.3% |
| Emergency department admission | 75.9% | 71.6% | 71.2% |
| Primary expected payer | |||
| Medicare | 76.0% | 76.0% | 73.9% |
| Medicaid | 7.6% | 7.0% | 8.9% |
| Private insurance | 11.4% | 11.3% | 12.7% |
| Self-pay/no charge/other | 5.0% | 5.8% | 4.5% |
| Comorbidities | |||
| Hypertension | 68.3% | 70.3% | 64.0% |
| Diabetes | 44.4% | 40.9% | 46.5% |
| Renal insufficiency | 41.9% | 36.1% | 48.1% |
| Atrial fibrillation | 38.4% | 36.0% | 41.6% |
| Chronic pulmonary disease | 37.1% | 31.0% | 40.0% |
| Anemia | 31.2% | 23.1% | 36.2% |
| Fluid and electrolyte disorders | 29.4% | 19.5% | 42.1% |
| Obesity | 17.1% | 13.0% | 20.3% |
| Peripheral vascular disorders | 11.9% | 10.6% | 13.6% |
| Died in hospital | 3.1% | 3.5% | 5.6% |
| Hospital characteristics | |||
| Bed size | |||
| Small | 13.8% | 13.9% | 11.3% |
| Medium | 23.6% | 24.6% | 22.0% |
| Large | 62.6% | 61.5% | 66.7% |
| Control/ownership | |||
| Government, nonfederal (public) | 11.6% | 12.0% | 10.6% |
| Private, not-for-profit (voluntary) | 74.2% | 68.9% | 78.4% |
| Private, investor-owned (proprietary) | 14.2% | 19.1% | 11.0% |
| Location (urban/rural) of hospital | |||
| Rural | 15.8% | 20.3% | 9.4% |
| Urban | 84.2% | 79.7% | 90.6% |
| Region | |||
| 1: Northeast | 18.3% | 11.6% | 24.7% |
| 2: Midwest | 24.2% | 25.0% | 20.1% |
| 3: South | 43.2% | 54.3% | 36.8% |
| 4: West | 14.4% | 9.1% | 18.4% |
| Teaching status | |||
| Teaching | 41.3% | 36.1% | 51.0% |
National estimates based on NIS weighted samples; all differences at P < .001, except bed size (P = .065) and emergency department admission (P = .811).
After multivariable risk adjustment for patient and hospital characteristics, patients aged ≥65 years were less likely to have been in the highest-cost quintile, with an adjusted odds ratio (OR) of 0.88 and 95% CI of 0.81 to 0.96 (Table II). Patients of Hispanic origin (OR 1.36, 95% CI 1.05-1.76) and other minority status (OR 1.42, 95% CI 1.17-1.72) were more likely to have been in the highest cost cohort when compared with white patients. Being in the wealthiest median income quartile was predictive of higher costs (OR 1.65, 95% CI 1.35-2.03) when compared with the poorest median income quartile.
Table II.
Adjusted ORs of most expensive quintile hospitalization cost estimates (compared with least expensive quintile)
| Demographics | Unadjusted OR (95% CI) | P value (unadjusted OR) | Adjusted OR (95% CI) | P value (adjusted OR) |
|---|---|---|---|---|
| Age ≥65 y | 0.85 (0.78-0.92) | .0001 | 0.88 (0.81-0.96) | .0035 |
| Female | 0.92 (0.89-0.96) | .0002 | 0.91 (0.87-0.95) | <.0001 |
| White (ref.) | ||||
| Black | 0.96 (0.81-1.13) | .6052 | 1.04 (0.89-1.20) | .6378 |
| Hispanic | 1.76 (1.43-2.18) | <.0001 | 1.36 (1.05-1.76) | .0199 |
| Asian/Pacific Islander/Native American/other | 1.93 (1.42-2.62) | <.0001 | 1.42 (1.17-1.72) | .0004 |
| Missing | 0.86 (0.67-1.10) | .2284 | 1.03 (0.77-1.37) | .8463 |
| Medicare (ref.) | ||||
| Medicaid | 1.31 (1.15-1.50) | <.0001 | 1.04 (0.92-1.16) | .5546 |
| Private insurance | 1.16 (1.06-1.28) | .0022 | 1.12 (1.00-1.25) | .0496 |
| Self-pay/no charge/other | 0.80 (0.70-0.92) | .0017 | 0.91 (0.80-1.04) | .1562 |
| Median household income by ZIP code: 1st quartile (the poorest) (ref.) | ||||
| Median household income: 2nd quartile | 1.18 (1.04-1.34) | .0094 | 1.07 (0.95-1.21) | .2569 |
| Median household income: 3rd quartile | 1.58 (1.36-1.83) | <.0001 | 1.22 (1.05-1.40) | .0072 |
| Median household income: 4th quartile | 2.27 (1.87-2.74 | <.0001 | 1.65 (1.35-2.03) | <.0001 |
| Emergency department admission | 0.98 (0.85-1.14) | .8107 | 0.69 (0.60-0.80) | <.0001 |
| Comorbidities | ||||
| Hypertension | 0.75 (0.71-0.80) | <.0001 | 0.69 (0.66-0.73) | <.0001 |
| Renal insufficiency | 1.64 (1.54-1.74) | <.0001 | 1.17 (1.11-1.24) | <.0001 |
| Diabetes | 1.26 (1.19-1.33) | <.0001 | 1.14 (1.08-1.19) | <.0001 |
| Fluid and electrolyte disorders | 3.00 (2.78-3.24) | <.0001 | 2.52 (2.37-2.68) | <.0001 |
| Atrial fibrillation | 1.27 (1.20-1.33) | <.0001 | 1.22 (1.16-1.27) | <.0001 |
| Chronic pulmonary disease | 1.48 (1.38-1.58) | <.0001 | 1.52 (1.44-1.60) | <.0001 |
| Anemia | 1.89 (1.74-2.05) | <.0001 | 1.28 (1.2-1.37) | <.0001 |
| Obesity | 1.71 (1.59-1.83) | <.0001 | 1.69 (1.58-1.81) | <.0001 |
| Peripheral vascular disorders | 1.33 (1.24-1.42) | <.0001 | 1.23 (1.16-1.31) | <.0001 |
| Procedures | ||||
| Mechanical ventilation | 8.94 (7.86-10.16) | <.0001 | 5.87 (5.16-6.69) | <.0001 |
| Blood transfusion | 11.55 (10.13-13.17) | <.0001 | 8.57 (7.58-9.68) | <.0001 |
| Echocardiogram | 3.85 (2.72-5.45) | <.0001 | 2.89 (2.20-3.79) | <.0001 |
| Hemodialysis | 2.73 (2.45-3.04) | <.0001 | 1.75 (1.55-1.97) | <.0001 |
| Thoracentesis | 9.83 (8.52-11.34) | <.0001 | 8.46 (7.35-9.74) | <.0001 |
| Other therapeutic procedures | 5.19 (3.28-8.23) | <.0001 | 3.05 (1.99-4.66) | <.0001 |
| Hospital characteristics | ||||
| Bed size: small (ref.) | ||||
| Bed size: medium | 1.10 (0.84-1.45) | .4837 | 0.86 (0.64-1.15) | .3154 |
| Bed size: large | 1.34 (1.06-1.68) | .0146 | 1.08 (0.86-1.37) | .5004 |
| Government, nonfederal (public) (ref.) | ||||
| Private, not-for-profit (voluntary) | 1.29 (0.97-1.71) | .0796 | 0.79 (0.61-1.04) | .0881 |
| Private, investor-owned (proprietary) | 0.65 (0.47-0.90) | .0101 | 0.59 (0.43-0.82) | .0014 |
| Location of hospital: rural (ref.) | ||||
| Location of hospital: urban | 2.46 (1.96-3.08) | <.0001 | 1.46 (1.12-1.88) | .0044 |
| Northeast (ref.) | ||||
| Midwest | 0.38 (0.25-0.57) | <.0001 | 0.42 (0.28-0.62) | <.0001 |
| South | 0.32 (0.21-0.48) | <.0001 | 0.38 (0.25-0.57) | <.0001 |
| West | 0.94 (0.6-1.47) | .7941 | 1.02 (0.66-1.59) | .919 |
| Teaching status: teaching hospital | 1.84 (1.46-2.32) | <.0001 | 1.56 (1.23-1.98) | .0003 |
C-statistic = 0.82, 95% CI 0.80-0.83, P < .0001.
Unweighted sample size = 75986 discharges; weighted population = 382700.
Of the comorbid conditions examined, AHF hospitalizations of patients with comorbid fluid and electrolyte disorders (OR 2.52, 95% CI 2.37-2.68) or with obesity (OR 1.69, 95% CI 1.58-1.81) had larger odds of being in the highest-cost quintile (Table II). Several additional comorbid conditions (atrial fibrillation, anemia, renal insufficiency, diabetes, chronic pulmonary disease, and peripheral vascular disorders) had ORs in the range of 1.14 to 1.52. Hypertension, however, had smaller odds of being in the highest-cost quintile (OR 0.69, 95% CI 0.66-0.73). Procedures with larger odds of being in the highest cost hospitalizations included blood transfusions (OR 8.57, 95% CI 7.58-9.68), thoracentesis (OR 8.46, 95% CI 7.35-9.74), mechanical ventilation (OR 5.87, 95% CI 5.16-6.69), echocardiograms (OR 2.89, 95% CI 2.20-3.79), and hemodialysis (OR 1.75, 95% CI 1.55-1.97).
Differences in hospital size or private, nonprofit status were not significant when controlling for other factors (Table II). Treatment in private, investor-owned hospitals had a statistically significant lower odds of being in the highest-cost quintile (OR 0.59, 95% CI 0.43-0.82) when compared with treatment in public hospitals. Treatment in an urban center had higher odds of higher-cost hospitalizations (OR 1.46, 95% CI 1.12-1.88). Hospital stays in the Midwest and South had lower odds of highest-cost hospitalizations when compared with the Northeast.
The C-statistic for our final model was 0.82 (95% CI 0.80-0.83), which suggests that the model had good discrimination for distinguishing highest- and lowest-cost hospitalizations based on the included covariates.6 An analysis comparing the lowest 10th percentile and highest 10th percentile by hospital costs is presented in the online Appendix Supplementary material, with similar findings.
Discussion
Hospital expenditures varied substantially among patients in the United States hospitalized with AHF in 2011, with highest-cost AHF inpatient stays having approximately 9-fold higher expenditures and 5 times longer length of stay compared with lowest-cost stays. Substantial differences were found in patient and hospital characteristics, procedures, and in-hospital outcomes among AHF hospitalizations with highest versus lowest costs. In-hospital mortality was higher for highest-cost compared with lowest-cost hospitalizations (5.6% vs 3.5%). These findings provide important insights into those factors that are independently associated with AHF hospitalization expenditures and have important implications for providing value-driven care to patients hospitalized with AHF in the United States.
After controlling for multiple factors, we found that select demographic and comorbid factors were predictive of lowest- and highest-expenditure hospitalizations for AHF. Although certain racial/ethnic minorities, such as Hispanics, were associated with highest-expenditure hospitalizations, prior research suggests that Hispanic patients have better in-hospital survival rates compared with non-Hispanic whites.7 We did not find any strong associations between insurance status and AHF hospitalization expenditures, which may suggest that the level of care provided at hospitals across the country does not vary considerably based on type or lack of insurance. On the other hand, income was more strongly associated with highest expenditure hospitalizations. Patients in the highest quartile for median household income zip codes received care that was more costly when compared with patients in the lowest quartile. The strength of this relationship between income and medical expenditures has been described and attributed to ability to pay for services.8 Underlying expectations or cultural factors of both patients and medical providers may be driving forces in the relationship.
All comorbid conditions examined, with the exception of hypertension, were associated with the highest-cost AHF hospitalizations. Prior studies have shown that hospital length of stay and outcomes are influenced by comorbid conditions.9 The importance of fluid and electrolyte disturbances as AHF hospitalization cost drivers in the present analysis reflects that these are more likely in patients with worse cardiac systolic dysfunction and cardiorenal syndrome. Interestingly, we found that obesity was predictive of more costly hospitalizations. The obesity paradox is well described, wherein higher–body mass index (BMI) patients have a lower risk of in-hospital mortality.10 The relationship between BMI and mortality is U-shaped, with the lowest-risk group between a BMI of 30 and 35 kg/m2.11 Although mortality rates may be lower for obese patients, the observed higher expenditures may be a function of longer and more complicated hospitalizations, which increase costs. That hypertension is associated with less advanced HF and cardiac compensation is more readily achieved in hypertensive HF patients may explain the finding that hypertensive HF patients were more likely to be in the lowest quintile for AHF hospitalization costs.9,12
Certain cardiovascular and noncardiovascular procedures are clearly a direct correlate with higher-cost hospitalizations. Mechanical ventilation in AHF is a marker of severe life-threatening disease that is rarely an elective procedure in this population. However, other procedures performed may have been more discretionary. Blood transfusions and their frequency of use have been more controversial in AHF and other cardiovascular conditions. There are limited studies examining the use of blood transfusions in both stable and decompensated AHF, with insufficient evidence to direct recommendations.13
A prior study measuring annual cost variations among Medicare patients with HF found that comorbidities were associated with increased medical costs.14 Variations in AHF hospitalization expenditures were noted in an analysis with 1997 NIS data where comorbidities and hospital characteristics were also correlated with higher expenditures.15 Increasingly, HF patients have additional comorbidities that require hospital-based treatments. Research suggests that the bulk of costs incurred by HF patients overall is for non–HF-related conditions.16 Our approach was to characterize AHF hospitalizations specifically and not hospitalizations for other primary diagnoses among HF patients. A primary AHF hospitalization should be a cause for alarm, as it portends future adverse health effects and increased expenditures following the event.17,18
The most striking hospital characteristic predictive of hospitalization expenditures was region, with smaller odds of being in the highest-cost quintile for hospitals in the Midwest and South when compared with the Northeast as a reference. The western region of the United States was not considerably different from the Northeast. Our study attempted to control for patient characteristics that included demographics and comorbidities, as well as commonly used procedures that may be considered a surrogate for health care utilization. Although an unexplained difference in patient characteristics and health care utilization is possible, other factors outside of our model are likely driving the difference. Prior work on regional variations by hospital referral regions, most notably through the Dartmouth Atlas of Health Care, suggests that unknown regional differences may be driving the variation, with concern for differences in provider practices and incentives.19–21 More recent work using models with expanded patient characteristics has noted that most regional variation may be explained by patient characteristics and burden of disease.22,23 The recent Institute of Medicine report on variations in health care spending notes that differences in price markups between geographic regions are a larger factor in differential cost when compared to differences in utilization, specifically in relation to the commercial insurance market; however, unexplained differences persist.24 The regional differences we detected using only 4 national regions may reflect underlying assumptions in hospital charge calculations. Alternative methods quantifying expenditures using standardized costs may assist in understanding this issue further.25
Limitations
The NIS data set unit is based on hospitalizations and lacks individual patient identifiers; consequently, readmissions are not identified. Rehospitalization rates are estimated to approach 30% for HF.12 We are therefore not able to distinguish variation in costs between AHF hospitalizations and AHF rehospitalizations. We only included hospitalizations with a primary discharge diagnosis for AHF and not secondary diagnoses, and the degree of variation in expenditures and associated factors may differ in patients with AHF as secondary diagnosis. Because the NIS is limited to billing (charge) data for comorbid conditions, differences in underlying patient characteristics may not have been well captured. Residual measured and unmeasured confounding may have influenced these findings. Total charges reflect what a hospital billed for services and not what costs a hospital incurred or received in payment. The analysis is dependent upon CCR conversions to accurately make comparisons between specific hospitals and medical services. Data on organization and structural differences for hospitals were not available in the NIS, and the extent that these factors contributed to the observed variation could not be determined. The data do not include laboratory tests ordered or medications prescribed, which might be factors associated with the highest quintile of hospitalizations. Several states did not supply race/ethnicity data, with approximately 10% missing the information in 2011.
Conclusions
This study provides insights into the high cost and variation in hospital expenditures among AHF hospitalizations in the United States and identifies factors associated with higher and lower expenditures. Select demographic factors and comorbidities are independently associated with variations in hospital expenditures, as are certain in-hospital procedures. Expenditures also vary by hospital characteristics, including geographic location. These findings may assist in further understanding resource utilization in patients hospitalized with AHF and encourage further studies for improving the value of inpatient care provided for AHF.
Supplementary Material
Acknowledgements
The authors developed the manuscript, and all authors significantly edited the first draft for intellectual content.
All authors approved the final manuscript that is submitted for publication.
Financial support: Novartis Pharmaceuticals (East Hanover, NJ) provided funding and review of manuscript prior to submission.
Footnotes
Author disclosures
Boback Ziaeian: no relationships to disclose.
Puza P. Sharma: Novartis Pharmaceuticals Corporation employee and stockholder.
Tzy-Chyi Yu: Novartis Pharmaceuticals Corporation employee and stockholder.
Katherine Waltman Johnson: Novartis Pharmaceuticals Corporation employee and stockholder (significant).
Gregg C. Fonarow: Research AHRQ (significant), NHLBI (significant); Consultant Bayer (modest), Gambro (modest), Medtronic (modest), Novartis (significant), Boston Scientific (modest), Medicines Company (modest), Johnson & Johnson (modest) Takeda (modest)
All authors have approved the final article.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality [April 16, 2014];HCUP-NIS database information. 2013 [Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2011.pdf]
- 3.HCUP Databases [April 16, 2014];Cost-to-Charge Ratio Files: 2011 Nationwide Inpatient Sample (NIS) user guide. 2013 Available at: http://www.hcup-us.ahrq.gov/db/state/CCR2011NISUserGuide.pdf.
- 4.Mach L. Imputation vs reweighting for total nonresponse in a business survey. SSC Annual Meeting, July 1995Proceedings of the Survey Methods Section: 67-74; 1995. [Available at: http://www.ssc.ca/survey/documents/SSC1995_L_Mach.pdf]
- 5.Sun Y, Friedman B. Tools for more accurate inpatient cost estimates with HCUP databases, 2009. Errata added October 25, 2012. HCUP Methods Series Report # 2011–04. [April 16, 2014];Online October 29, 2012U.S. Agency for Healthcare Research and Quality; 2012. [Available at: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp.]
- 6.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 7.Vivo RP, Krim SR, Krim NR, et al. Care and outcomes of Hispanic patients admitted with heart failure with preserved or reduced ejection fraction: findings from get with the guidelines-heart failure. Circ Heart Fail. 2012;5:167–75. doi: 10.1161/CIRCHEARTFAILURE.111.963546. [DOI] [PubMed] [Google Scholar]
- 8.Chen AY, Escarce JJ. Quantifying income-related inequality in healthcare delivery in the United States. Med Care. 2004;42:38–47. doi: 10.1097/01.mlr.0000103526.13935.b5. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Srikanthan P, Costanzo MR, et al. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Padwal R, McAlister FA, McMurray JJ, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.203. http://dx.doi.org/10.1038/ijo.2013.203. [Epub ahead of print] [DOI] [PubMed]
- 12.Gheorghiade M, Vaduganathan M, Fonarow GC, et al. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Kansagara D, Dyer E, Englander H, et al. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159:746–57. doi: 10.7326/0003-4819-159-11-201312030-00007. [DOI] [PubMed] [Google Scholar]
- 14.Greiner MA, Hammill BG, Fonarow GC, et al. Predicting costs among medicare beneficiaries with heart failure. Am J Cardiol. 2012;109:705–11. doi: 10.1016/j.amjcard.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi AV, D'Souza AO, Madhavan SS. Differences in hospital length-of-stay, charges, and mortality in congestive heart failure patients. Congest Heart Fail. 2004;10:76–84. doi: 10.1111/j.1527-5299.2004.02008.x. [DOI] [PubMed] [Google Scholar]
- 16.Whellan DJ, Greiner MA, Schulman KA, et al. Costs of inpatient care among Medicare beneficiaries with heart failure, 2001 to 2004. Circ Cardiovasc Qual Outcomes. 2010;3:33–40. doi: 10.1161/CIRCOUTCOMES.109.854760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lissovoy G, Zodet M, Coyne K, et al. Treatment charges and resource use among patients with heart failure enrolled in an MCO. Manag Care Interface. 2002;15:46–52. [PubMed] [Google Scholar]
- 18.Bharmal M, Gemmen E, Zyczynski T, et al. Resource utilisation, charges and mortality following hospital inpatient admission for congestive heart failure among the elderly in the US. J Med Econ. 2008;11:397–414. doi: 10.3111/13696990802193081. [DOI] [PubMed] [Google Scholar]
- 19.Wennberg JE, McAndrew Cooper M. The Dartmouth Atlas of Health Care in the United States. The Center for the Evaluative Clinical Sciences, Dartmouth Medical School, American Hospital Publishing, Inc.; 1998. [April 16, 2014]. [Available at: http://www.dartmouthatlas.org/downloads/atlases/98Atlas.pdf.] [Google Scholar]
- 20.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 21.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman S, Waidmann T, Berenson R, et al. Clarifying sources of geographic differences in Medicare spending. N Engl J Med. 2010;363:54–62. doi: 10.1056/NEJMsa0909253. [DOI] [PubMed] [Google Scholar]
- 23.Reschovsky JD, Hadley J, Romano PS. Geographic variation in feefor-service medicare beneficiaries’ medical costs is largely explained by disease burden. Med Care Res Rev. 2013;70:542–63. doi: 10.1177/1077558713487771. [DOI] [PubMed] [Google Scholar]
- 24.Newhouse JP, Garber AM, Graham RP, et al. Variation in health care spending: target decision making, not geography. National Academies Press; Washington, D.C.: p. 2013206. [PubMed] [Google Scholar]
- 25.Lagu T, Krumholz HM, Dharmarajan K, et al. Spending more, doing more, or both? An alternative method for quantifying utilization during hospitalizations. J Hosp Med. 2013;8:373–9. doi: 10.1002/jhm.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.