Summary
The antigenic proteins of Mycobacterium tuberculosis (Mtb) have been defined. We used synthetic peptides of secreted antigens, early secreted antigenic target 6 (ESAT-6) and cultural filtrate protein-10 (CFP-10), of Mtb and characterized the immune response in context of HLA genes. Humanized mice lacking endogenous class II molecules but expressing various human DR and DQ HLA transgenes singly or as a haplotype were used to study the HLA-mediated immune response to peptides. Our observations showed that the overall response to the promiscuous ESAT-6 31-45 peptide may be dependent on the HLA haplotype rather than a single DR or DQ molecule. Further, our data showed that HLA transgenes generated a highly variable TH response to this promiscuous peptide. This provides an explanation for the variability of the bacillus Calmette-Guerin (BCG) vaccine. Our data highlights the use of HLA transgenic mice for determining the pathogenicity or therapeutic nature of a peptide in the context of HLA alleles.
Keywords: HLA, humanized mice ESAT-6, CFP-10, Tuberculosis
Introduction
Major histocompatibility Complex (MHC) encodes for the HLA class II genes, the most polymorphic genes in the human genome. HLA class II molecules on antigen presenting cells are responsible for antigen presentation to CD4+ T cells and generate an immune response to pathogens. Recently it was suggested that CD4 T cells, positively selected on the basis of class II alleles, are predetermined to generate a specific immune response (1). Accordingly, HLA class II alleles may also determine variability in generating a response to infectious agents. We are exposed to many microbes, some beneficial and other pathogenic. Evolutionarily, class II alleles that confer resistance to infections or clear infections efficiently are selected. Thus HLA polymorphism has been associated with susceptibility to various infectious agents.
Mycobacterium tuberculosis (Mtb) is a pathogen that has been able to evade the immune system and cause Tuberculosis (TB) in some individuals. Tuberculosis (TB) is a leading health problem in the world, specially developing countries. Despite availability of the vaccine, approximately 2 million deaths occur every year (http://www.who.int/tb/en/) making it one of the leading causes of death due to infectious agent. Thus there is a need to understand the host-pathogen interaction and resulting immune response for vaccine development. The host factors that determine the susceptibility or resistance to TB are not clear. There are studies showing the HLA-DR2/DQ6 haplotype with susceptibility to develop tuberculosis in many populations while other have shown the presence of DRB1*04/DQB1*03 and DRB1*14, DRB1*16 (2-5). One study has suggested that HLA-DQ alleles with homozygous Aspartic (Asp) acid at codon 57 are associated with pulmonary tuberculosis (6). Even though no specific HLA allele indicative of disease resistance or susceptibility in all ethnic populations has emerged, the studies suggest a role of HLA class II alleles in pathogenesis.
Mycobacterium tuberculosis secretes proteins that have been suggested to be involved in generating an immune response. These proteins have been tested for potential vaccine candidates. The major secretory antigens of Mtb are Ag-85, early secreted antigenic target 6 (ESAT-6) and cultural filtrate protein-10 (CFP-10). Among the major antigens, ESAT-6 and CFP-10 show the most reactivity and are involved in T cell activation as well as macrophage inhibition (7), suggesting epitopes from these proteins may be promiscuous in HLA binding and presentation. Immunodominant epitopes of ESAT-6 and CFP-10 and their HLA restriction has recently been defined (8). Response to CFP-10 has been significantly associated with the presence of DR15 (9) while DQ polymorphism at codon 57 is associated with susceptibility to pulmonary TB in a population (6). In humans it is difficult to determine the role of HLA molecules in vivo due to genetic complexity. We have previously shown that these HLA transgenic (Tg) mice recognize the same epitopes of Antigen85 as human cell lines, making them valuable for the determination of epitopes for generating vaccines (10, 11). In this study we have used humanized mice expressing specific HLA transgenes to determine reactivity by various HLA molecules to EAST-6 and CFP-10 epitopes.
METHODS
Transgenic mice
The generation of mice carrying human HLA transgenes, DQ8 (DQA1*0301/DQB1*0302), DQ6 (DQA1*0103/DQB1*0601), DR4 (DRB1*0401 and *0402), and DR3 (DRB1*0301) mice have been described previously and all mice have been characterized and published (12, 13). All mice are endogenous class II knockout (AEo). In addition to single Tg mice, double transgenic mice expressing DR and DQ genes, DR3/DQ8, DR4/DQ8, and DR3/DQ6 were also used. Double Tg mice were generated by mating single Tg mice and selecting for the presence of both alleles. Thus all strains have the same background except for the HLA gene construct. Mice of both sexes (8-12 weeks of age) used in this study were bred and maintained in the pathogen-free Immunogenetics Mouse Colony at the Mayo Clinic, Rochester, MN in accordance with the Animal Use and Care Committee. All the experiments included littermate controls and were carried out with the approval of the Institute's Animal use and care committee.
Peptides
Peptides were synthesized and purified at Mayo Clinic Peptide Facility ESAT-6 amino acid (aa) 31-45, EGKQSLTKLAAAWGG and 46-60, SGSEAYQGVQQKWDA CFP-10 aa 16-30, GNFERISGDLKTQID and 41-55 GQWRGAAGTAAQAAV
T cell proliferation Assay
Mice were immunized with 200 μg of peptide emulsified 1:1 in CFA (Difco) intradermally at the base of the tail and one hind footpad. Ten days post-immunization, draining lymph nodes/ spleen were removed and cultured in vitro. Lymph node cells (LNCs) (1×106) were cultured in HEPES-buffered RPMI 1640 containing 5% heat inactivated horse serum and the antibiotics streptomycin and penicillin in 96-well flat bottom tissue culture plates. Cells were challenged by adding 100μl of RPMI medium (negative control), Con A (20 μg/ml, positive control) and native collagen (50 μg/ml). The cells were incubated for 48 h at 37° C. During the last 18 h the cells were pulsed with 3H-thymidine (1μCi/well). At the end of the assay, the cells were harvested using a plate harvester and incorporated radioactivity was determined using an automated counter (Micrebeta, Perkin Elmer Wallac). Results are depicted as stimulation index (S.I.). A S.I. value of 2 was taken as cut off for positive response.
In other experiments, splenic cells from mice primed with ESAT-6 31-45 were also cultured with LPS (10ug/ml) and supernatants were collected for measuring cytokines.
Measurement of cytokines
Capture ELISA was done for measuring cytokines IFN-γ (TH1), IL-10 TH2), IL-17 and IL-12 (p40) (TH17) using kits according to manufacturer's instructions (BD Pharmingen).
Statistical analysis
All significances were calculated using Student's t test or T test with unequal variance. A P value of <0.05 was considered significant.
RESULTS AND DISCUSSION
Bacillus Calmette Guerin (BCG) has been used for vaccination against TB for more than 50 years but its poor efficacy in some populations has generated a need for developing new vaccines. Antigenic proteins of Mycobacterium tuberculosis, ESAT-6 and CFP-10, have been shown to elicit an immune response in tuberculosis patients (14). The TH1 dependent production of IFN-γ has been suggested to protect against TB. A screening of ESAT-6 derived overlapping peptides showed that human CD4+ T cell lines produced IFN-γ in response to challenge with peptides encompassing aa 22-65(15). However, the proliferation data was not consistent, as not all patients sharing a HLA haplotype generated response to the ESAT-6 peptides. For a vaccine to be successful, the antigen has to be a promiscuous binder and immunogenic. In humans, due to linkage disequilibrium between HLA-DR and DQ alleles, it is difficult to determine the role of the HLA genes. To overcome this issue, we used mice expressing a single HLA transgene or HLA haplotype to determine the immune response to ESAT-6 and CFP-10 derived peptides. Our previous study has shown that transgenic mice generate a similar response to BCG-derived antigen85 suggesting transgenic mice to be a good model to study epitopes for vaccines for TB.
Presence of Asp57 reduces response to the immunodominant peptides of ESAT-6 and CFP-10
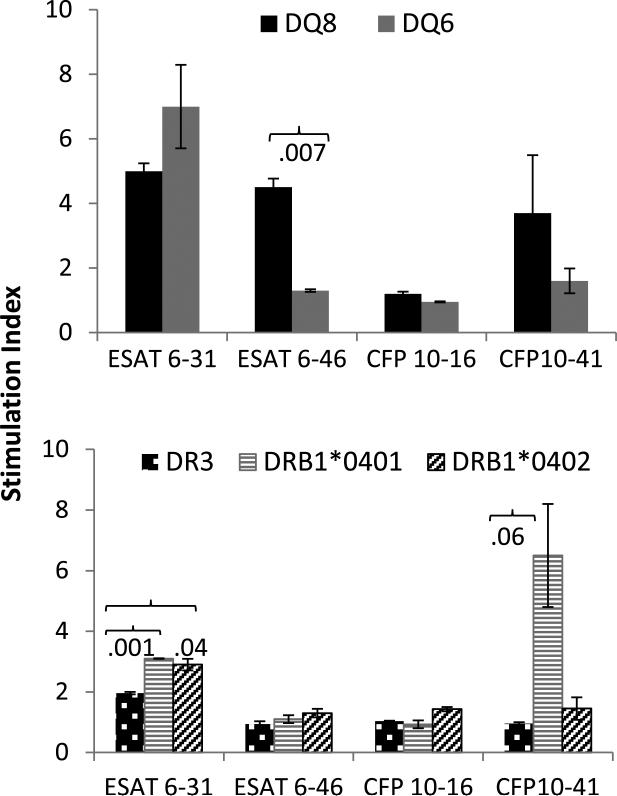
First we tested if DQ molecule carrying Asp 57, DQ6, or not , DQ8, show any difference in reactivity to ESAT-6 and CFP-10 peptides. Binding of ESAT-6 and CFP-10 peptides has been described (6). Mice were immunized with individual peptides and splenic cells were harvested and challenged in vitro with the peptides. DQ8 mice mounted a strong response in a recall challenge to the 2 immunodominant peptides ESAT-6 encoding aa 31-45 and 46-60 in vitro while DQ6 mice showed a response to ESAT-6- 31-45 only (Figure 1). Our studies are in confirmation of the previous report showing β57-Asp HLA-DQ molecules have reduced binding to ESAT-6-46-60 (6). Interestingly, the CFP-10 peptides encompassing aa 16-30 and 41-55 did not generate any response by DQ6 mice although DQ8 mice did mount a strong response to the latter peptide suggesting it is not a promiscuous peptide.
Fig 1.
ESAT-6 31-45 is a promiscuous peptide. Lymph node cell proliferation to ESAT-6 and CFP-10 derived peptides in a recall response in vitro. Mice were primed with individual peptides, N=3/strain. Data are presented as Mean±SEM. S.I.= Stimulation Index.
HLA-DR alleles can present ESAT-6 immunodominant epitope
Response to CFP-10 has been associated with DR alleles and ESAT-6 can be presented by both DQ and DR molecules (16). Since in humans DQ8 occurs in linkage with DR4, we tested the T cell response in DRB1*0401 and DRB1*0402 mice. In addition, DR3 mice were used as a control. Interestingly, both DR4 subtypes presented ESAT-6 derived 31-45 peptide while DR3 mice generated a very mild response with significant differences with *0401 and *0402 mice, P<0.001 and P<0.04 respectively (Figure 1). The other peptides were not presented by any DR Tg mice except CFP-10 derived 41-60 peptide, that was presented by the DRB1*0401 mice. However, due to variability in proliferation data, we did not find any significant difference. ESAT-6 51-70 has been previously shown to be immunogenic for DRB1*04 individuals (17). Based on our data it would appear that this immunogenic peptide may lay in the region spanning aa 60-70. This data suggests that some ESAT-6 and CFP-10 derived peptides may be promiscuous for presentation by various HLA alleles.
Cytokine response to ESAT-6 is HLA dependent
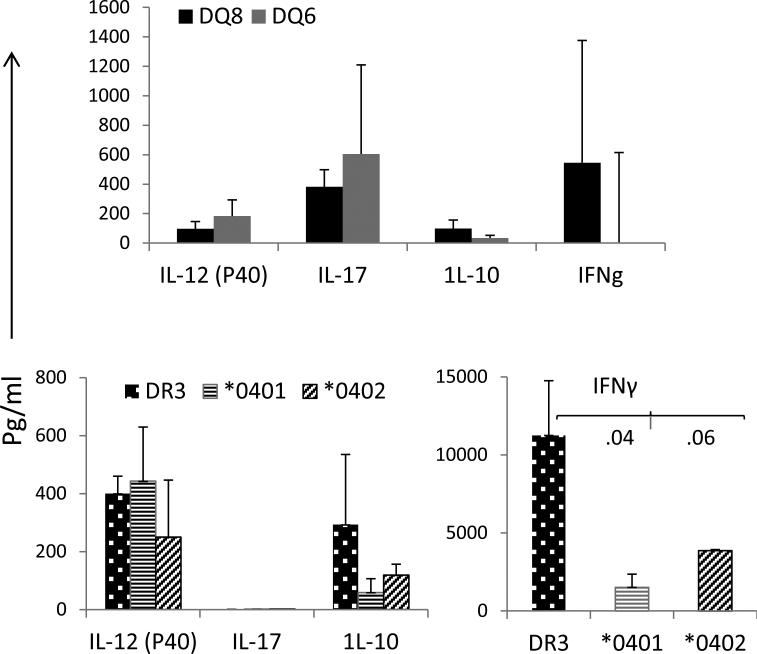
As ESAT-6- 31 was the one peptide that was promiscuous in generating an immune response, we chose this peptide to further explore the type of response, TH1 (IFN-γ), TH2 (IL-10) and TH17 (IL-12(p40) and IL-17), generated by transgenic mice (Figure 2). Even though ESAT-6-31-45 was presented by all the HLA transgenes, the cytokine response varied among the transgenic mice. DQ6 mice generated a TH17 response with very little IFN-γ while DQ8 mice produced more of a mixed response; both IFN-γ as well as IL-17 were produced by DQ8 mice. Both strains produced low levels of IL-10.
Fig 2.
TH response to ESAT-6- 31-45 peptide is HLA dependent. Supernatants from the cultures with ESAT-6-31-45 were used for measuring IFN-γ (TH1), IL-10 (TH2) and IL-17 and IL-12(p40) (TH17) by ELISA. Data are presented as Mean±SEM
Among the DR transgenic mice, DR3 mice produced more of a mixed response with production of all cytokines, TH1/TH17/TH2, though IFN-γ levels produced by DR3 were significantly higher than both *0401 and *0402 mice, P<0.04 and P<0.06 respectively. On the other hand, both strains of DR4 (*0401 and *0402) mice produced more of a TH17 response with much lower of IL-10 and IFN-γ as compared to DR3 mice. IFN-γ levels produced by DR4 mice were 3 to 7 times lower than DR3 mice. However, due to variability in production of cytokines by individual mouse, differences in other cytokines were significant among the strains. ESAT-6-specific IFN-γ secreting CD4 T cells have been correlated to clinical phenotype in humans and they decline following a successful therapeutic outcome (18). This data suggests that ESAT-6-31-45 peptide may be a target for vaccination. All mice produced IFN-γ in response to the immunodominant ESAT-6-31-45 except DQ6, the latter may be able to resolve infection via production of TH17.
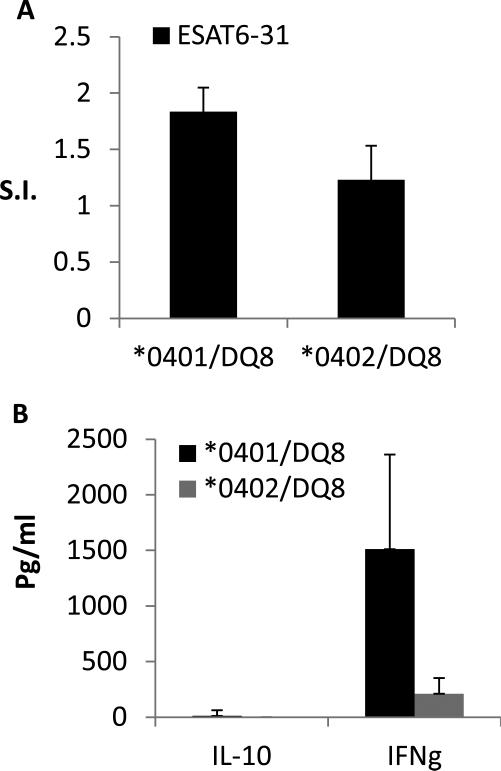
Complementation between HLA-DQ and DR modulates immune response to peptides
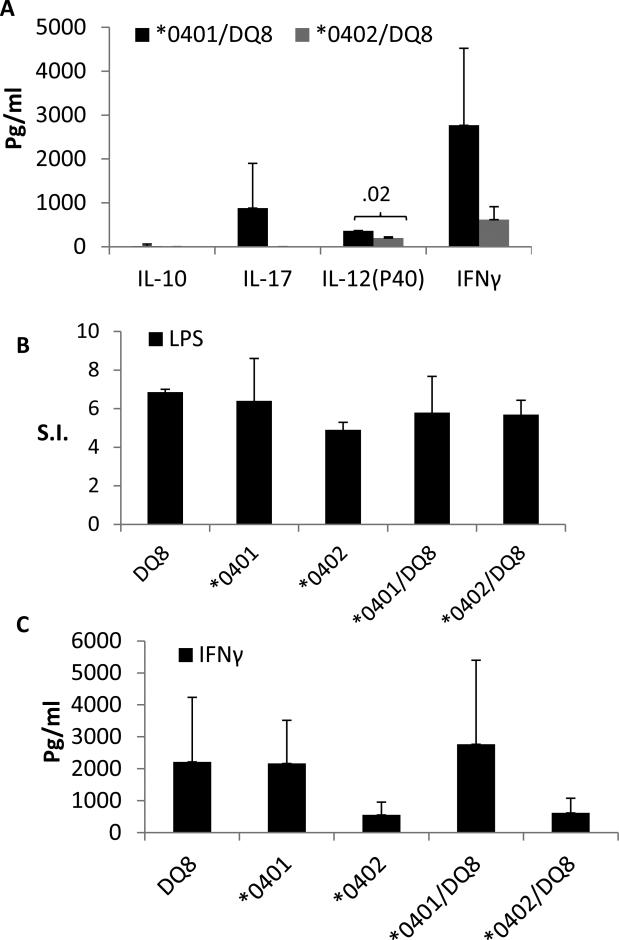
We have previously shown that the interaction between HLA-DQ and DR genes can modulate immune response and phenotype of disease (19). Since in humans DR and DQ are expressed as a haplotype, we used Tg mice expressing this same haplotype and determined immune response to ESAT-6-31-45. DR*0401 and *0402 occur in linkage with DQ8. To understand the role of DR polymorphism we measured the response to the immunodominant ESAT-6 peptide in *0401/DQ8 and *0402/DQ8 mice. The haplotype positive *0401/DQ8 mice generated a lower response compared to the single transgene positive parental strains (Figure 3A). Surprisingly, *0402/DQ8 mice did not generate any response to in vitro recall challenge with ESAT-6-31 peptide. This data suggested that the immune response to the immunodominant ESAT-6 derived peptide 31-45 is dependent on a haplotype rather than the individual DR or DQ alleles confirming that complementation between DR and DQ genes determines immune response. Next we determined if cytokine production is also modulated to this peptide. Both haplotype positive strains did not produce any TH17 cytokines (Figure 3B). *0401/DQ8 mice produced high IFN-γ with very low levels of IL-10 while *0402/DQ8 mice produced very low levels of IFN-γ with no IL-10. This data suggests that HLA haplotype needs to be considered for the immune response rather than a single HLA gene.
Fig 3.
Interaction between HLA-DR and DQ molecules in Haplotype transgenic mice modulates immune response to the immunodominant ESAT-6 31-45 peptide. A) Lymph node cellproliferation to ESAT-6-31-45 peptide in a recall response in vitro, N=2/strain. B) Supernatants from culture from were used to measure cytokine response. No Th17 response was detected in both strains. Data are presented as Mean±SEM. S.I. =Stimulation Index
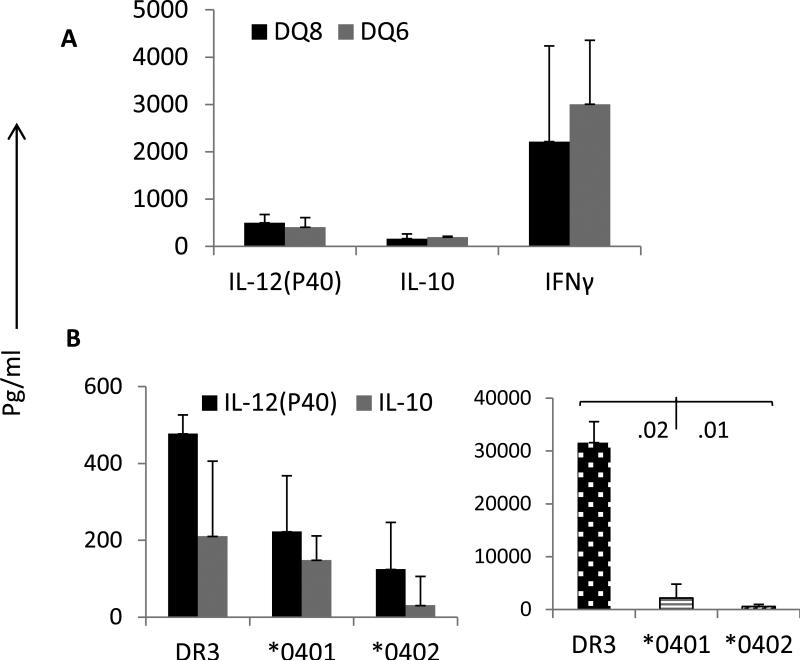
Mice immunized with ESAT-6 can generate TLR-4 mediated response
Toll-like receptor 4 (TLR4) is a ligand for bacterial protein lipopolysaccharide (LPS). We tested if transgenic mice immunized with ESAT-6 31-45 peptide still have the capability of generating a TLR4-mediated response. Splenic cells isolated from ESAT-6-primed mice were cultured with LPS and tested the supernatants for cytokines (Figure 4). Interestingly, even though DQ6 mice did not produce IFN-γ in response to ESAT-6 peptide, the TLR4-mediated response was intact with high IFN-γ production. Both strains, DQ8 and DQ6, produced similar profile of cytokines. Similarly, all 3 DR transgenic mice tested produced all cytokines, however DR3 mice produced much higher IFN-γ and IL-12(p40) compared to both strains of DR4 mice.
Fig 4.
TLR4-mediated cytokine response in ESAT-6-31-45 primed mice. Splenic cells isolated from peptide primed mice were challenged in vitro with LPS and supernatants were used to measure cytokines by ELISA in A) DQ mice and B) DR mice. Data are presented as Mean±SEM
An interaction between DR and DQ genes led to a change in TLR4-mediated cytokine profile as *0401/DQ8 and *0402/DQ8 mice produced much higher levels of IL-17, IL-12(p40) and IFN-γ while no IL-10 was produced in both strains (Figure 5). *0401/DQ8 mice produced significantly higher levels of pro-inflammatory IL-12 (P40) compared to *0402/DQ8 in response to LPS (Figure 5A). Interestingly, the presence of *0401/DQ8 haplotype is associated with susceptibility to rheumatoid arthritis in humans and collagen-induced arthritis in mice (12, 20) suggesting an infection may lead to heightened pro-inflammatory conditions in *0401/DQ8 mice but in *0402/DQ8 mice. Even though all transgenics primed with ESAT6-31-45 peptide generated a similar response to LPS in vitro. We have recently shown that T cells from *0402 and *0401 mice can be driven to produce IFNγ (21) but kinetics of cytokine responses differ between the two molecules. In this study, ESAT-6-31 primed *0402 mice produced lower amounts of IFNg compared to *0401 though the difference was not statistically different due to variability in individual mouse. The variability in response may be similar to what is observed in humans. A comparison of IFN-γ production in single and haplotype transgenic mice confirmed that DR molecules can modulate DQ-restricted response as *0402/DQ8 mice had much lower levels of IFN-γ as compared to DQ8 mice. A previous study has shown no difference in LPS-induced chemokine production in severe compared to limited TB patients (22).
Fig 5.
TLR4 mediated cytokine production is modulated by complementation between DR and DQ molecules in haplotype positive humanized mice. Splenic cells isolated from peptide primed mice were challenged in vitro with LPS and proliferation was measured. Supernatants were used to measure cytokines by ELISA. A) *0401/DQ8 and *0402/DQ8 mice produce IFN-γ in response to TLR4-mediated activation of cells. B) In vitro proliferation to LPS in ESAT-6-31 primed mice and C) Comparison of IFN-γ in single HLA transgene and haplotype positive mice. Data are presented as Mean±SEM.
Conclusion
While all the transgenic mice tested here generated a response to ESAT-6 31-45, only *0401 and DQ8 mice generated a response to CFP-10 derived peptide 41-60. None of the mice generated response to CFP-10, suggesting it to be a weaker immunogenic peptide than ESAT-6. All mice produced IFN-γ in response to LPS although *0402 and *0402/DQ8 mice generated a much lower cytokine response. Our studies are in confirmation of the previous report that showed β57-Asp HLA-DQ molecules have reduced binding to ESAT-6-46-60 and harbor a lower number of IFN-γ secreting T cells compared to β57Ala (6). Even though the TLR4-mediated response is generated via the innate immune system, it is indirectly under the control of class II molecules. In a comparison of sequences identified previously (15) and the present results, the common sequences in peptides generating a response are 42-45 “AWGG”. Tryptophan is a strong binder for peptide pocket 1 of DR molecules (23) providing an explanation why this peptide is promiscuous and suggesting this peptide to be a good option for vaccine. Our data suggests that variability in efficacy to BCG, in part, may be due to the host genetic factors. HLA transgenic mice provide a powerful means to study the epitope that may generate protective immunity as well as define the pathogenic epitopes of Mtb. The humanized mice may also be a good tool to study the efficacy of various available and new drugs for tuberculosis.
ACKNOWLEDGEMENTS
Authors thank Julie Hanson for the generation and maintenance of the transgenic mice. VT is supported by the NIH grant AR30752.
References
- 1.Mangalam AK, Taneja V, David CS. HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. Journal of immunology. 2013;190:513–518. doi: 10.4049/jimmunol.1201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duarte R, Carvalho C, Pereira C, Bettencourt A, Carvalho A, Villar M, Domingos A, Barros H, Marques J, Pinho Costa P, Mendonca D, Martins B. HLA class II alleles as markers of tuberculosis susceptibility and resistance. Rev Port Pneumol. 2011;17:15–19. doi: 10.1016/s0873-2159(11)70005-8. [DOI] [PubMed] [Google Scholar]
- 3.Mehra NK, Rajalingam R, Mitra DK, Taneja V, Giphart MJ. Variants of HLADR2/DR51 group haplotypes and susceptibility to tuberculoid leprosy and pulmonary tuberculosis in Asian Indians. Int J Lepr Other Mycobact Dis. 1995;63:241–248. [PubMed] [Google Scholar]
- 4.Dubaniewicz A, Moszkowska G, Szczerkowska Z. Frequency of DRB1-DQB1 two-locus haplotypes in tuberculosis: preliminary report. Tuberculosis (Edinb) 2005;85:259–267. doi: 10.1016/j.tube.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Park MH, Song EY, Park H, Kwon SY, Han SK, Shim YS. Association of HLA-DR and HLA-DQ genes with susceptibility to pulmonary tuberculosis in Koreans: preliminary evidence of associations with drug resistance, disease severity, and disease recurrence. Hum Immunol. 2005;66:1074–1081. doi: 10.1016/j.humimm.2005.08.242. [DOI] [PubMed] [Google Scholar]
- 6.Delgado JC, Baena A, Thim S, Goldfeld AE. Aspartic acid homozygosity at codon 57 of HLA-DQ beta is associated with susceptibility to pulmonary tuberculosis in Cambodia. J Immunol. 2006;176:1090–1097. doi: 10.4049/jimmunol.176.2.1090. [DOI] [PubMed] [Google Scholar]
- 7.Ganguly N, Siddiqui I, Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis (Edinb) 2008;88:510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Arlehamn CS, Sidney J, Henderson R, Greenbaum JA, James EA, Moutaftsi M, Coler R, McKinney DM, Park D, Taplitz R, Kwok WW, Grey H, Peters B, Sette A. Dissecting mechanisms of immunodominance to the common tuberculosis antigens ESAT-6, CFP10, Rv2031c (hspX), Rv2654c (TB7.7), and Rv1038c (EsxJ). J Immunol. 2012;188:5020–5031. doi: 10.4049/jimmunol.1103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arend SM, Geluk A, van Meijgaarden KE, van Dissel JT, Theisen M, Andersen P, Ottenhoff TH. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect Immun. 2000;68:3314–3321. doi: 10.1128/iai.68.6.3314-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geluk A, Taneja V, van Meijgaarden KE, de Vries RR, David CS, Ottenhoff TH. HLA-DR/DQ transgenic, class II deficient mice as a novel model to select for HSP T cell epitopes with immunotherapeutic or preventative vaccine potential. Biotherapy. 1998;10:191–196. doi: 10.1007/BF02678296. [DOI] [PubMed] [Google Scholar]
- 11.Geluk A, Taneja V, van Meijgaarden KE, Zanelli E, Abou-Zeid C, Thole JE, de Vries RR, David CS, Ottenhoff TH. Identification of HLA class II-restricted determinants of Mycobacterium tuberculosis-derived proteins by using HLA-transgenic, class II-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10797–10802. doi: 10.1073/pnas.95.18.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taneja V, David CS. Role of HLA class II genes in susceptibility/resistance to inflammatory arthritis: studies with humanized mice. Immunol Rev. 2010;233:62–78. doi: 10.1111/j.0105-2896.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 13.Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA class II transgenic mice mimic human inflammatory diseases. Adv Immunol. 2008;97:65–147. doi: 10.1016/S0065-2776(08)00002-3. [DOI] [PubMed] [Google Scholar]
- 14.Arend SM, Andersen P, van Meijgaarden KE, Skjot RL, Subronto YW, van Dissel JT, Ottenhoff TH. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181:1850–1854. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- 15.Mustafa AS, Shaban FA, Al-Attiyah R, Abal AT, El-Shamy AM, Andersen P, Oftung F. Human Th1 cell lines recognize the Mycobacterium tuberculosis ESAT-6 antigen and its peptides in association with frequently expressed HLA class II molecules. Scand J Immunol. 2003;57:125–134. doi: 10.1046/j.1365-3083.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar M, Sundaramurthi JC, Mehra NK, Kaur G, Raja A. Cellular immune response to Mycobacterium tuberculosis-specific antigen culture filtrate protein-10 in south India. Med Microbiol Immunol. 2010;199:11–25. doi: 10.1007/s00430-009-0129-2. [DOI] [PubMed] [Google Scholar]
- 17.Kumar M, Meenakshi N, Sundaramurthi JC, Kaur G, Mehra NK, Raja A. Immune response to Mycobacterium tuberculosis specific antigen ESAT-6 among south Indians. Tuberculosis (Edinb) 2010;90:60–69. doi: 10.1016/j.tube.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AV, Lalvani A. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–5225. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 19.Taneja V, Griffiths MM, Luthra H, David CS. Modulation of HLA-DQ-restricted collagen-induced arthritis by HLA-DRB1 polymorphism. Int Immunol. 1998;10:1449–1457. doi: 10.1093/intimm/10.10.1449. [DOI] [PubMed] [Google Scholar]
- 20.Taneja V, Mehra NK, Chandershekaran AN, Ahuja RK, Singh YN, Malaviya AN. HLA-DR4-DQw8, but not DR4-DQw7 haplotypes occur in Indian patients with rheumatoid arthritis. Rheumatol Int. 1992;11:251–255. doi: 10.1007/BF00301502. [DOI] [PubMed] [Google Scholar]
- 21.Luckey D, Behrens M, Smart M, Luthra H, David CS, Taneja V. DRB1*0402 may influence arthritis by promoting naive CD4 T-cell differentiation in to regulatory T cells. Eur J Immunol. 2014 doi: 10.1002/eji.201344424. doi: 10.1002/eji.201344424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan Z, Jamil B, Ashraf M, Islam M, Yusuf MS, Khan JA, Hussain R. ESAT6-induced IFNgamma and CXCL9 can differentiate severity of tuberculosis. PLoS One. 2009;4:e5158. doi: 10.1371/journal.pone.0005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Androulakis IP, Nayak NN, Ierapetritou MG, Monos DS, Floudas CA. A predictive method for the evaluation of peptide binding in pocket 1 of HLA-DRB1 via global minimization of energy interactions. Proteins. 1997;29:87–102. [PubMed] [Google Scholar]