After peripheral nerve injury, the process of Wallerian degeneration is initiated in the distal stump of injured nerves. Wallerian degeneration in peripheral nerves involves axonal degeneration and degradation of the myelin sheath in Schwann cells. This provides the necessary conditions for axonal regeneration and remyelination. After nerve injury, macrophages are also recruited to the distal nerve stump and, together with Schwann cells, play a role in the clearance of myelin debris. Thus, a series of processes help to promote peripheral nerve regeneration, which includes axonal regeneration and remyelination. This is in contrast to injuries within the adult central nervous system, in which successful regeneration encounters several significant barriers: myelin-associated inhibition (Neuman et al., 2002), diminished axonal growth capacity (Ruff et al., 2008) and glial scarring (Yiu and He, 2006). Because the successful regeneration of injured peripheral nerves relies on a harmonious degenerating process, it is essential to identify a molecular mechanism that regulates axonal degeneration or myelin fragmentation during Wallerian degeneration to foster the conditions allowing efficient peripheral nerve regeneration. We have recently shown that hydrogen sulfide (H2S) is important for axonal degradation and demyelination. We focus here on the effects of H2S on axonal degradation and on understanding the underlying mechanisms of H2S-associated demyelination, dedifferentiation and proliferation in Schwann cells during Wallerian degeneration. In addition, we discuss a novel strategy for nerve regeneration in the injured perip heral nerve or peripheral neuropathy.
The synthesis and regulation of H2S in the nervous system: H2S, the most recently described gas signaling molecule, performs a variety of physiological functions (Kimura, 2013). H2S is produced from pyridoxal-5′-phospate (PLP)-dependent enzymes [cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE)] and 3-mercaptopyruvate sulfurtransferase (MST), along with cysteine aminotransferase (CAT). These enzymes play physiological roles in a variety of human tissues in the body. In the central nervous system (CNS), the synthesis of H2S is regulated by CBS activity, and the imbalance in H2S production is linked to several CNS diseases including Alzheimer's disease (Beard and Bearden, 2011). We found that the peripheral nervous system (PNS) shows a very different pattern of enzymatic activity for H2S production. In the PNS, CSE and MST/CAT, but not CBS, are expressed in the normal nerves. There is reason to believe that H2S may play a significant role in the degeneration of peripheral nerves following injury, based on comparisons with nitric oxide (NO) and carbon monoxide (CO). Like H2S, NO and CO are gas transmitters used in a variety of signaling pathways. After nerve injury, inducible NO synthase (iNOS) is up-regulated in the distal stump of peripheral nerves, and iNOS knockout mice exhibit delayed demyelination during Wallerian degeneration (Levy et al., 2001; Campuzano et al., 2008). Previous studies suggest that NO is linked to delayed Wallerian degeneration after peripheral nerve injury and also point to the possibility that the other gasotransmitters CO or H2S may be related to nerve degeneration and regeneration. Of the three aforementioned gasotransmitters, the physiological functions of H2S are similar to those of NO. In other words, H2S dynamics are likely similar to NO dynamics during Wallerian degeneration in peripheral nerves. We have gathered enough evidence to support the hypothesis of a relationship between H2S dynamics and peripheral nerve degeneration/regeneration. After peripheral nerve injury, CSE is up-regulated, and its up-regulation occurs in Schwann cells, but not in axons, in mouse tissue in vivo. CSE activity is distributed diffusely in the cytoplasm of Schwann cells after nerve injury, whereas in uninjured Schwann cells, the localization of CSE has yet to be identified. These in vivo findings indicate a relationship between H2S and Wallerian degeneration, especially that mediated by CSE activity.
H2S dynamics during Wallerian degeneration: Demyelination, which results in the degradation of the myelin sheath, is one of the pathological phenotypes observed during Wallerian degeneration. During demyelination, the myelin sheath is fragmented and the myelin debris is engulfed and removed by Schwann cells and macrophages. The successful removal of myelin debris does not interrupt axonal regeneration. In our laboratory, we employed N-ethylmaleimide (NEM, inhibitor of all cysteine peptidases) to inhibit H2S production in Schwann cells during Wallerian degeneration. Through the blockage of all cysteine peptidases, the prevented increase in H2S production in Schwann cells during Wallerian degeneration regulates myelin ovoid fragmentation and influences axonal degradation (Figure 1). We propose that during Wallerian degeneration, activated H2S production in Schwann cells breaks down myelin sheaths mechanically, leading to myelin ovoid fragmentation. Because the activation of H2S production does not occur in the peripheral axons, the effect of the inhibitor on H2S production is restricted to Schwann cells. This implies that mechanical forces related to H2S production in myelin fragmentation during Wallerian degeneration may be sufficient for axonal degradation. However, we cannot exclude the possibility that H2S-mediated extracellular signaling affects axonal degradation directly. In addition, the inhibition of H2S production in the injured peripheral nerve blocks the recruitment of macrophages (unpublished observation). Although Wallerian degeneration is a condition that results when the peripheral nerve is injured, a related process is relevant to many neurodegenerative diseases and it is termed ‘Wallerian-like degeneration’ (Coleman and Freeman, 2010). Thus, effective control of H2S production in the injured peripheral nerve is important for myelin sheath dynamics or myelin debris clearance. Furthermore, the effective regulation of H2S production through the inhibition of CSE expression may contribute to a novel therapeutic strategy for demyelinating diseases, such as Guillain-Barré syndrome and Charcot-Marie Tooth Type 1 disease.
Figure 1.
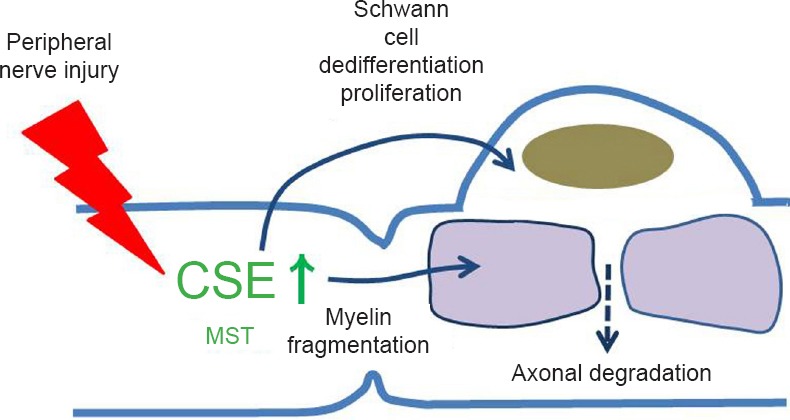
Hydrogen sulfide (H2S) is essential for Wallerian degeneration.
H2S functions as a physiological gas transmitter in both normal and pathophysiological cellular events. H2S is produced from cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase/cysteine aminotransferase in normal peripheral nerves. Injured static nerves in vivo up-regulate CSE in Schwann cells during Wallerian degeneration, influencing Schwann cell dedifferentiation/proliferation and demyelination. However, CSE was not up-regulated in peripheral axons.
During Wallerian degeneration after peripheral nerve injury, Schwann cell dedifferentiation, which refers to the denervated state of Schwann cell (Jessen and Mirsky, 2008) and proliferation (Siironen et al., 1994) are essential for axonal regenerative processes and subsequent successful nerve regeneration. In our laboratory, we have shown that the inhibition of H2S production in sciatic explants suppressed Schwann cell dedifferentiation and proliferation during Wallerian degeneration (Figure 1). The expression of several Schwann cell dedifferentiation or immaturity markers, LAMP1, p75NTR, c-Jun and p-ERK1/2, was inhibited by the H2S inhibitor in sciatic nerve explants in vitro. This indicates that H2S signaling broadly affects the processes of Schwann cell dedifferentiation through lysosomal protein degradation, neurotropin receptors, the MAPK pathway, and transcriptional regulation. In addition, expression of the proliferation marker ki67 was inhibited by the H2S inhibitor in vitro. Intriguingly, the activity of krox20, a marker for differentiation, myelination or maturity, was maintained after treatment with H2S inhibitors during Wallerian degeneration. Transcriptional regulation through several antagonistic interactions between transcriptional factors, such as c-Jun and Krox20, affects the denervated state (Jessen and Mirsky, 2008). We suggest the possibility that H2S regulates the delay or progression of Schwann cell differentiation, myelination or maturity through Krox20 and c-jun transcriptional regulation.
Unsolved questions in peripheral nerve regeneration: One of the remaining issues regarding the role of H2S is the possible link with peripheral nerve regeneration. For successful regeneration, the important issue centers on the responses of Schwann cells to H2S production. Because peripheral axons do not show altered CSE-associated H2S production during Wallerian degeneration, H2S-associated repair of peripheral axons is likely dependent on Schwann cell dynamics, but not axons, during the regeneration process. Thus, the key processes pertaining to Schwann cell responses are Schwann cell dedifferentiation, proliferation and remyelination. H2S broadly affects various events occurring in Schwann cells during Wallerian degeneration. First, H2S production is involved in Schwann cell dedifferentiation. After nerve injury, myelinated Schwann cells dedifferentiate into immature Schwann cells resembling undeveloped cells (Jessen and Mirsky, 2008). The inhibition of H2S production results in the down-regulation of several Schwann cell dedifferentiation markers such as LAMP1, p75NTR, c-Jun and p-ERK1/2. Therefore, at the endpoint of Wallerian degeneration, the efficient decrease of H2S production in Schwann cells may contribute to improved Schwann cell guidance for growth of regenerating axons or for remyelination through the suppression of the dedifferentiation-related molecules (Figure 2). Second, H2S production is involved in Schwann cell proliferation. After peripheral nerve injury, the immature Schwann cells proliferate in the presence of the endoneurium (Siironen et al., 1994). The proliferated Schwann cells guide an axonal sprout from the injured site to reach the target organ. Thus, the effective regulation of H2S production during Wallerian degeneration may enhance Schwann cell proliferation (Figure 2). Third, H2S dynamics may be involved in Schwann cell remyelination. Even when the growing axon terminals correctly reach the ending organ, if Schwann cell remyelination is not performed efficiently, nerve regeneration is incomplete. Because H2S production affects transcriptional regulation through krox20 and c-jun, effective transcriptional regulation through the inhibition of H2S production may influence the improvement in Schwann cell remyelination ability (Figure 2). Thus, we believe that H2S is a key modulator for peripheral nerve regeneration. In addition, many questions still remain regarding the role of H2S in peripheral nerve regeneration. It is important to assess the relationship between H2S production and various factors that have been implicated previously in the regulation of Schwann cell responses during peripheral regeneration. For example, it is necessary to evaluate the relationship between H2S production and remyelination-associated ECM proteins and related molecules (laminins, dystroglycan, L-periaxin, tPA/plasminogen and fibrin; Chen et al., 2007). Neurotrophic factors may be relevant, and receptors such as BDNF, FGF-2, and TGF-β, along with p75NTR, may be affected by H2S production. Future research should also address intracellular regulators (PI3-kinase/Akt signaling, cyclin D1 and ski; Chen et al., 2007), several hormones (progesterone and thyroid hormones; Chen et al., 2007) or transcriptional regulators (Oct-6, Sox-10, Brn2, NF-κB, Notch, Sox-2, Pax-3 and Id2; Jessen and Mirsky, 2008) (Figure 2). Further studies along these lines would provide important insight into peripheral nerve regeneration and contribute to the development of a novel therapeutic strategy for peripheral demyelinating diseases or nerve degenerative diseases.
Figure 2.
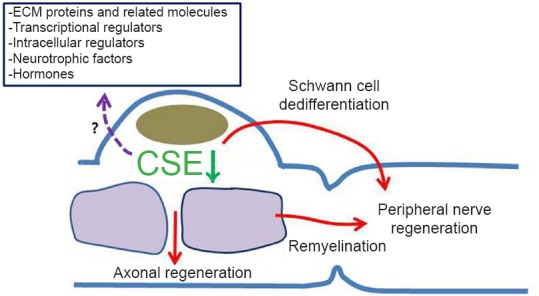
Hydrogen sulfide (H2S) is a key modulator for peripheral nerve regeneration.
A model of H2S dynamics in Schwann cells during peripheral nerve regeneration. Schwann cells may be regulated by H2S production, potentially an important aspect of successful regeneration. Schwann cell dedifferentiation/proliferation and remyelination are key processes potentially involving Schwann cell responses to improve the regenerative environment.
This work was supported by Dong-A University research fund. The authors have no conflicts of interest to disclose.
References
- 1.Beard RS, Jr, Bearden SE. Vascular complications of cystathionine β-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am J Physiol Heart Circ Physiol. 2011;300:H13–26. doi: 10.1152/ajpheart.00598.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B. Distinct pattern of microglial response, cyclooxygenase-2, and inducible nitric oxide synthase expression in the aged rat brain after excitotoxic damage. J Neurosci Res. 2008;86:3170–3183. doi: 10.1002/jnr.21751. [DOI] [PubMed] [Google Scholar]
- 3.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 4.Coleman MP, Freeman MR. Wallerian Degeneration, Wlds, and Nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int. 2013;63:492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Kubes P, Zochodne DW. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J Neuropathol Exp Neurol. 2001;60:411–421. doi: 10.1093/jnen/60.5.411. [DOI] [PubMed] [Google Scholar]
- 8.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 9.Ruff RL, McKerracher L, Selzer ME. Repair and neurorehabilitation strategies for spinal cord injury. Ann N Y Acad Sci. 2008;1142:1–20. doi: 10.1196/annals.1444.004. [DOI] [PubMed] [Google Scholar]
- 10.Siironen J, Collan Y, Röyttä M. Axonal reinnervation does not influence Schwann cell proliferation after rat sciatic nerve transection. Brain Res. 1994;654:303–311. doi: 10.1016/0006-8993(94)90492-8. [DOI] [PubMed] [Google Scholar]
- 11.Sumi-Ichinose C, Urano F, Shimomura A, Sato T, Ikemoto K, Shiraishi H, Senda T, Ichinose H, Nomura T. Genetically rescued tetrahydrobiopterin-depleted mice survive with hyperphenylalaninemia and region-specific monoaminergic abnormalities. J Neurochem. 2005;95:703–714. doi: 10.1111/j.1471-4159.2005.03402.x. [DOI] [PubMed] [Google Scholar]
- 12.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]


