Accurately documenting and quantifying peripheral nerve axonal degeneration and regeneration is critically important for clinical research in peripheral nerve disorders such as nerve trauma, peripheral neuropathy and amyotrophic lateral sclerosis (ALS). Current strategies include clinical assessments and neurophysiological studies (including nerve conduction studies and electromyography (EMG)). However, the information provided by these strategies is limited in a number of ways.
In neurodegenerative diseases such as ALS, substantial peripheral nerve motor axon loss has already occurred before the patient develops clinical weakness (Simon et al., 2014a). In addition, both motor nerve conduction studies and electromyography reflect changes of axonal loss as well as reinnervation processes occurring by terminal branching of remaining motor neurons, thus imprecisely measuring the number of remaining or degenerate axons. Additional neurophysiological techniques such as motor unit number estimation (MUNE) have been developed to more directly measure motor axonal loss, but there are issues with reproducibility and there is no consensus on the optimum methodology (Simon et al., 2014c).
There are similar concerns when attempting to measure peripheral nerve regeneration following peripheral nerve trauma. Following severe nerve injury, there may be disruption of axons but preservation of nerve connective tissue elements that act as ‘highways’ for axonal regeneration (axonotmetic injury) or disruption of both axons and connective tissue elements (neurotmetic injury). In severe axonotmetic injuries, axonal regeneration proceeds from the proximal stump through the distal nerve at a rate between 1 and 3 mm per day. Neurotmetic injuries do not spontaneously recovery due to intrusion of scar tissue at the site of nerve trauma preventing regenerating axons from advancing into the distal nerve. It is in the early phase of nerve regeneration that current measurement strategies are ineffective. EMG will detect successful nerve regeneration, however, it requires regenerating nerve fibres to reach and reinnervate the target muscle. Clinical measurements are less sensitive than EMG to detect early reinnervation. It is critically important to determine which nerve injuries are likely to regenerate spontaneously as this will determine the need for surgical intervention.
Because of the limitations of clinical and neurophysiological measurement methods, novel approaches to detect and quantify peripheral nerve degeneration and regeneration are needed to provide practical tools in the management of peripheral nerve disorders as well as more powerful instruments in clinical trials of novel therapeutic approaches.
Diffusion weighted MRI is poised to provide potentially useful surrogate markers of peripheral nerve axonal injury and regeneration. Diffusion weighted MRI provides a measure of the movement of water molecules in tissues. Free water demonstrates Brownian (random) motion of mobile hydrogen nuclei. In nerve tracts, water diffuses relatively freely along the long axis of the tract, but is restricted by the myelin sheath in the perpendicular plane. In this instance, diffusion is anisotropic (directional). Obtaining meaningful diffusion measures requires scans to be performed using multiple b values. A baseline scan (b value 0) is performed which provides a T2-weighted echo planar image. Calculating diffusion gradients requires scans to be performed with different b values, which range up to approximately 1,000 for clinical scanning protocols. The higher the b value the greater the diffusion weighting, and higher b values are associated with reduced signal-to-noise ratios. The difference between scans with higher b values and the baseline scan (b = 0) is used to calculate apparent diffusion coefficient (ADC) values, which reflect the extent of water diffusion. ADC values are of some relevance to peripheral nerve disease, in particular in the evaluation of nerve trauma and compression, and distinguishing malignant from benign peripheral nerve sheath tumors.
Standard diffusion weighted MRI does not provide any information regarding directionality of diffusion. When diffusion-sensitizing gradients are applied from multiple directions (at least 6), the water diffusion in a voxel can be modeled as an ellipsoid by estimating the diffusion tensor, which is the basis of diffusion tensor imaging (DTI). The ellipsoid is comprised of three principal eigenvectors e1, e2, and e3 (Figure 1A). The diffusion tensor model can be used to calculate fractional anisotropy (FA) maps as well as other parameters based on eigenvalues (λ1, λ2, λ3), such as axial (λ1), radial (λ2, λ3) and mean diffusivity, which provide a measure of the integrity of nerve fiber tracts (Basser et al., 1994).
Figure 1.
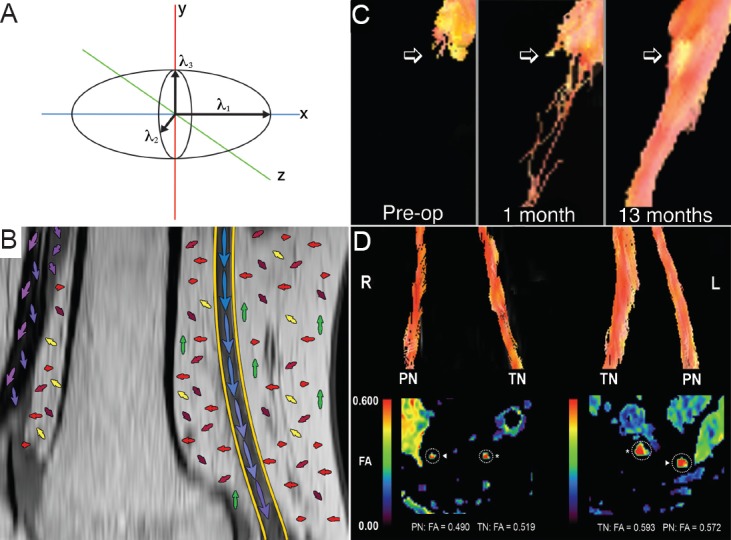
Diffusion tensor imaging (DTI) relies on analysis of the diffusion tensor ellipsoid.
(A) The direction and extent of water diffusion is modeled on three axes (x, y and z), with eigenvalues calculated in each of these directions (λ1, λ2, λ3). The direction (long axis) of the ellipsoid is determined by the largest of the three eigenvalues. (B) The distal sciatic nerve (yellow outline) at the knee, with a stylized representation of the principal eigenvalues in each tissue. Peripheral nerve demonstrates relatively pronounced, uniform anisotropic water diffusion while other tissues such as fat and connective tissue may demonstrate less uniform and pronounced directionality of water diffusion. This forms the basis of tractography, where the principal eigenvalues of adjacent voxels are linked in order to track nerve tissue. (C) The use of tractography to monitor axon regeneration following peroneal nerve graft repair. Pre-operatively there were no axons passing the point of transection. One month after grafting a few axons were seen extending into the grafts, although there was no evidence of clinical or neurophysiological improvement at that stage. At 13 months after the graft procedure, a compact nerve tract can be seen extending through the grafts, and the patient demonstrated marked clinical improvement. Figure C is adapted from Simon et al. (2014d) with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health. (D) The use of DTI and tractography to monitor the progression of neurodegeneration. The images were acquired from a patient with amyotrophic lateral sclerosis with severe right lower limb weakness and mild left lower limb weakness. Tractography (upper images) demonstrated attenuation of the tibial nerve (TN) and peroneal nerve (PN) on the right relative to the left side. Fractional anisotropy (FA) maps (lower images) demonstrated reduced right-sided values.
Using post-processing algorithms, tractography images can be produced by linking the direction of maximum diffusivity in adjacent voxels. Tractography may demonstrate axon bundles and hence provide a visual depiction of nerve integrity (Figure 1B).
DTI and tractography are well established in imaging of the central nervous system and have been applied to a myriad of disease processes involving the brain, and more recently the spinal cord. In the brain, FA values are reduced in the context of axonal injury or demyelination, while mean diffusivity increases (Werring et al., 1999). Tractography has been used to demonstrate individual white matter tracts, and to depict connectivity of different brain regions. Similarly, in peripheral nerve injury, FA values decrease with axonal degeneration and increase with nerve regeneration (Takagi et al., 2009; Simon et al., 2014d), while changes in other derived parameters are less well described.
While standard MRI has been used to document changes with nerve regeneration (Dailey et al., 1997; Tseng et al., 2014), there have been relatively few studies exploring peripheral nerve DTI and tractography and most studies to date have focused on entrapment neuropathy. However, this deficit may be overcome with further exploration of the technique, as diffusion tensor tractography can effectively delineate normal peripheral nerve fascicles (Simon et al., 2014b).
There have been isolated reports in humans on the use of tractography to visualize nerve regeneration following peripheral nerve trauma (Meek et al., 2006; Simon et al., 2014d). In an early report (Meek et al., 2006), tractography was performed on a patient with median nerve transection. At 1 month after surgical repair, nerve fibres extended to the site of surgical repair but not beyond. Two months post-operatively, nerve fibres could be tracked more distally, although clinical and electrodiagnostic evidence of recovery was not reported. In a recent report, tractography findings and FA values were reported in a patient with peroneal nerve transection repaired with sural nerve grafts (Simon et al., 2014d). Preoperatively, trackable nerve fibres terminated at the proximal stump. At 1 month after surgical repair, a few fibres were seen extending into the grafts, and at 13 months after injury, a compact bundle of nerve fibres was identified extending through the grafts (Figure 1C). FA values in the grafts increased over serial scans consistent with axonal regeneration. Nerve regeneration represented by tractography findings correlated with clinical and electrodiagnostic evidence of recovery.
There is also scarce data available on the use of DTI and tractography in nerve degeneration (Figure 1D). Following sciatic nerve crush in the rat, Wallerian degeneration manifests as reduced FA and axial diffusivity, associated with a corresponding attenuation of trackable fibres on tractography (Takagi et al., 2009). A study of tibial nerve DTI in 10 patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) demonstrated that FA values were reduced in patients relative to healthy subjects (Kakuda et al., 2011). The lowest FA values were obtained in those patients in whom a compound muscle action potential (CMAP) was not recordable, and FA values correlated with CMAP size recorded on nerve conduction studies but not nerve conduction velocity, suggesting that axonal loss was the most likely cause of reduced FA values. In a separate study, DTI of the sciatic nerve in 11 patients with peripheral neuropathy (CIDP, Guillain-Barre Syndrome with residual deficits, or idiopathic neuropathy) demonstrated reduced FA values (Mathys et al., 2013). Similar to the previous study, FA values correlated with tibial and peroneal CMAP amplitudes, as well as clinical disability.
There are important caveats to performing and interpreting peripheral nerve DTI and tractography. Image acquisition must balance satisfactory image resolution and scan time. Current DTI sequences have relatively low spatial and contrast resolution. Reducing voxel size may dramatically increase scan time due to the need to perform an exponentially increasing number of excitations for each reduction in slice thickness in order to maintain a satisfactory signal-to-noise ratio. From a practical perspective, performing peripheral nerve DTI on lower limb nerves may be straightforward, while scanning the brachial plexus is complicated by respiration movement artifact, and gating scans with the respiratory cycle increases scan time further. Reproducibility of peripheral nerve DTI parameters has not yet been established, which will be a necessary step before the technique is applied as a surrogate marker of axon loss.
Interpreting tractography images must also be undertaken with caution because these images represent the nerve fibers that have been reconstructed by a processing algorithm but which may not necessarily represent the exact number or distribution of fibers. DTI and tractography can be considered to be an estimate of the integrity of nerve fiber tracts rather than arriving at a ‘fiber count’. Tractography may be considered less robust when there are crossing fiber tracts, although peripheral nerves are typically longitudinally oriented and hence this issue may be considered of lesser relevance.
Despite these limitations there is great potential for peripheral nerve DTI to become a powerful clinical and research tool. Clinical applications include localization of nerve injury, where conventional imaging or electrodiagnostic studies have been unable to identify the precise site of nerve injury. Assessment of ongoing axonal loss may be an important clinical indicator of treatment efficacy in a patient with peripheral neuropathy. Similarly, identification of axonal regeneration may provide support for non-surgical management of peripheral nerve injury. From a research perspective, development of a robust surrogate marker of peripheral nerve axonal loss and regeneration would have widespread applications, including clinical trials of novel treatments in ALS, peripheral neuropathy, and peripheral nerve injury (Simon et al., 2014c).
Dr. Simon gratefully acknowledges funding from the National Health and Medical Research Council of Australia and the Motor Neurone Disease Research Institute of Australia (grant No. 1039520).
References
- 1.Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dailey AT, Tsuruda JS, Filler AG, Maravilla KR, Goodkin R, Kliot M. Magnetic resonance neurography of peripheral nerve degeneration and regeneration. Lancet. 350:1221–1222. doi: 10.1016/S0140-6736(97)24043-2. [DOI] [PubMed] [Google Scholar]
- 3.Kakuda T, Fukuda H, Tanitame K, Takasu M, Date S, Ochi K, Ohshita T, Kohriyama T, Ito K, Matsumoto M, Awai K. Diffusion tensor imaging of peripheral nerve in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a feasibility study. Neuroradiology. 2011;53:955–960. doi: 10.1007/s00234-010-0833-z. [DOI] [PubMed] [Google Scholar]
- 4.Mathys C, Aissa J, Meyer Z, Horste G, Reichelt DC, Antoch G, Turowski B, Hartung HP, Sheikh KA, Lehmann HC. Peripheral neuropathy: assessment of proximal nerve integrity by diffusion tensor imaging. Muscle Nerve. 2013;48:889–896. doi: 10.1002/mus.23855. [DOI] [PubMed] [Google Scholar]
- 5.Meek MF, Stenekes MW, Hoogduin HM, Nicolai JP. In vivo three-dimensional reconstruction of human median nerves by diffusion tensor imaging. Exp Neurol. 2006;198:479–482. doi: 10.1016/j.expneurol.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Simon NG, Lomen-Hoerth C, Kiernan MC. Patterns of clinical and electrodiagnostic abnormalities in early amyotrophic lateral sclerosis. Muscle Nerve. 2014a doi: 10.1002/mus.24244. doi: 10.1002/mus.24244. [DOI] [PubMed] [Google Scholar]
- 7.Simon NG, Cage T, Narvid J, Noss R, Chin C, Kliot M. High-resolution ultrasonography and diffusion tensor tractography map normal nerve fascicles in relation to schwannoma tissue prior to resection. J Neurosurg. 2014b;120:1113–1117. doi: 10.3171/2014.2.JNS131975. [DOI] [PubMed] [Google Scholar]
- 8.Simon NG, Turner MR, Vucic S, Al-Chalabi A, Shefner J, Lomen-Hoerth C, Kiernan MC. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014c;76:643–57. doi: 10.1002/ana.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon NG, Narvid J, Cage T, Banerjee S, Ralph JW, Engstrom JW, Kliot M, Chin C. Visualizing axon regeneration after peripheral nerve injury with magnetic resonance tractography. Neurology. 2014d;83:1382–1384. doi: 10.1212/WNL.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takagi T, Nakamura M, Yamada M, Hikishima K, Momoshima S, Fujiyoshi K, Shibata S, Okano HJ, Toyama Y, Okano H. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. NeuroImage. 2009;44:884–892. doi: 10.1016/j.neuroimage.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Tseng TC, Yen CT, Hsu SH. Visualization of peripheral nerve regeneration. Neural Regen Res. 2014;9:997–999. doi: 10.4103/1673-5374.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]


