Retinal regeneration: The retina is a part of the central nervous system (CNS) and has long attracted neurobiologists as an excellent model organ for the study of CNS regeneration. In classical studies using urodele amphibians like the salamander newt, it has been shown that the retina regenerates after the removal of the whole tissue even in the adulthood. This type of regeneration is considered as an example of “transdifferentiation”, since the source of the regenerating retina is the retinal pigmented epithelial cells (RPE cells) (Okada, 1991; DelRio-Tsonis and Tsonis, 2003; Fisher, 2005). In the newt, soon after retinal removal, the RPE cells lose their differentiation phenotypes and become retinal progenitor cells, which then undergo histogenesis of retinal structure. In further studies, chick embryonic RPE cells have been shown to transdifferentiate into retinal cells but only in the early period of development. Beside the RPE cells, several different cell sources are known to be involved in retinal regeneration. Intraretinal glial cells (Müller cells) can play a role in tissue repair in the chick and fish, but only when the damage is limited to a small area (Fisher, 2005). Ciliary epithelial cells also have a role in the regeneration of the peripheral retina in fish, amphibians and birds. In mammals, however, retinal regeneration does not occur after retinal injury, although there is a potential cell source in the ciliary epithelium. In a culture condition, mammalian ciliary cells including the human have been shown to manifest retinal stem/progenitor properties (Wohl et al., 2012). These studies suggest that by means of the potential ciliary stem/progenitor, a therapeutic approach to ocular diseases accompanied with retinal degeneration could be possible without transplantation of exogenous stem/progenitor cells. For clinical application of the potent stem cells, it is indispensable to understand the basic underlying cell and molecular mechanisms of regeneration. The amphibian regeneration models are superior, because there are several different animal models that are comparable to each other and well-defined culture models are also established that enable stem cells to form in vitro retinal stratification (Araki, 2014). In the present review, I will discuss our recent finding on retina regeneration of X. tropicalis as a new animal model and consider the possibility for the application to clinical medicine.
Retina regeneration involves several different cell sources and mechanisms, depending on the animal species. Although many vertebrates can repair more or less the damaged retina, complete regeneration of the retina in adult animals occurs only in amphibians when the whole retinal tissue is removed. This was once thought to occur only in the newt and has long been studied since the first description in the late 19th century (see a review by Okada, 1991). Contrary to this generally accepted notion, our research group recently found that adult Xenopus laevis are able to regenerate the whole retina when it is surgically removed (Yoshii et al., 2007). Since then, the Xenopus model for retinal regeneration has been intensively studied and we recently developed a new animal model of X. tropicalis (Miyake and Araki, 2014). Interestingly, X. tropicalis provides a novel mode of retinal regeneration, demonstrating a new approach to the regeneration study. In X. tropicalis, the ciliary marginal epithelial cells become active in cell proliferation immediately after the retina is removed and they regenerate the whole retina. No other tissue cells including the RPE play roles in retina regeneration as progenitor cells. Since the mammalian ciliary epithelium is also a potent tissue for regeneration, this new mode of retinal regeneration may possibly provide us with leads for developing application for therapeutic approach to ocular diseases.
Different cell sources for retinal regeneration and RPE transdifferentiation: One of the characteristic properties of Xenopus models for retina regeneration is the multi sources for the regenerating retina (DelRio-Tsonis and Tsonis, 2003; Araki, 2014). For local small damage, intraretinal progenitor cells are recruited to produce new retinal cells. Similarly to other vertebrates, Müller glial cells are considered to play a role in this type of regeneration as an intraretinal progenitor (Lenkowski and Raymond, 2014), while in a large-scale regeneration other stem/progenitor cells play a central role. When the whole retina is removed, the RPE cells and/or epithelial cells in the ciliary marginal zone (CMZ) are activated as retinal stem cells. Which cell type is employed mainly, the RPE or the CMZ cells, depends on the animal species. In X. laevis, the peripheral retina is regenerated from stem cells in the CMZ, while the central retina is from the RPE cells through transdifferentiation (Yoshii et al., 2007). In X. tropicalis, however, the whole retina regenerates solely from the CMZ stem cells and the RPE cells seem not to play any role as progenitor cells for regeneration (Figure 1). In urodele amphibians like the newt, however, regenerating retina is mostly from the RPE and the CMZ seems to regenerate only the peripheral part (Chiba and Mitashov, 2007).
Figure 1.
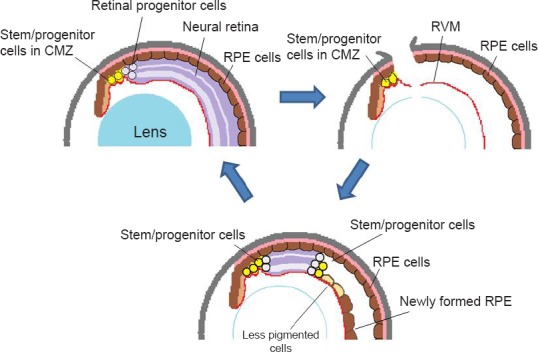
A schema for retinal regeneration in X. tropicalis: stem/progenitor cells in the ciliary marginal zone regenerate the whole retina.
(A) Normal eye. During the growth of the eye, stem/progenitor cells in the ciliary marginal zone (CMZ) produce retinal progenitor cells, which are incorporated into the retina at the periphery. (B) An eye immediately after retinal removal. An incision is made at the dorsal periphery of the eye, and the lens fibers are removed, leaving the retinal vascular membrane (RVM) in the posterior chamber. Some of the retinal pigmented epithelial (RPE) cells begin to be detached and migrate to the RVM, where they form a new RPE layer (as seen in Figure C). (C) At the later stage of regeneration (days 15–20), the regenerating retina extends further to the posterior. At the posterior edge of the regenerating retina, a proliferating zone (progenitor zone) is observed (consisting of actively proliferating cells). At the peripheral end of the regenerating retina (closer to the iris) there still remain progenitor cells that are intensely labeled for BrdU. The regenerating retina is sandwiched by progenitor cell zones at both ends. In X. tropicalis, RPE cells in the newly formed epithelium do not appear to transdifferentiate into neuronal cells, although some cells at the boundary to the regenerating retina become less pigmented. These cells are gradually lost as the regenerating retina extends more posteriorly. Contrary to X. tropicalis, the RPE cells on the RVM transdifferentiate into the retina in X. laevis. The schema is modified from the original figure (Miyake and Araki, 2014).
During Xenopus retinal regeneration, the retinal vascular membrane (RVM) plays a profound role, because retina regeneration does not occur when the RVM is also removed at the same time (Yoshii et al., 2007). This may partly explain why previous studies have been describing that adult Xenopus are unable to regenerate the retina: previous researchers may have removed the RVM together with the retina due to their surgical procedure. The RVM is basically the basement membrane of the retina facing the vitreous fluid and containing various extracellular matrices and blood capillaries. The RVM appears to play its role as a substrate for the stem/progenitor cells. In vitro study that shows 3-D retinal regeneration under Matrigel embedding culture condition, suggests that Matrigel, an artificial basement-like substance can substitute for the RVM. The RVM should have a permissive role as substratum for the stem/progenitor cells to develop a neuroepithelium. Soon after retinal removal, normally within 24 hours, a certain number of RPE cells are detached from the Brüch's membrane and migrate to the RVM, where they settle and form a new pigmented epithelium. RPE cells that are not detached from the Brüch's membrane remain there and re-form the RPE at their original site. Only those that migrate to the RVM become retinal stem/progenitor cells and develop a new retina in X. laevis. The RPE cells staying at the Bruch's membrane do not undergo transdifferentiation. These observations suggest that detachment and reattachment to the basement membrane is the necessary step for the RPE cells to transdifferentiate into retinal cells (Araki, 2014). In X. tropicalis the RPE cells also become detached from the Brüch's membrane, migrate and attach to the RVM, although they do not transdifferentiate (Figure 1). This indicates that there should be some additional condition for RPE cells to initiate transdifferentiation. The cell-cell communication and/or cell-substratum interaction will be the most probable condition, since neither N-cadherin nor connexin43 was localized in X. tropicalis RPE, formed on the RVM, while in X. laevis RPE these molecules were observed soon after they re-attached to the RVM. Cadherin- and connexin-dependent cell communications will play essential roles in cell differentiation. Studies in the in vitro model also suggest that X. tropicalis RPE cells do not transdifferentiate into neurons under the same culture condition used for X. laevis (Miyake and Araki, 2014). In another culture model of regeneration, in which X. laevis RPE cells can regenerate retinal 3-D structure, again no regeneration occurs in case of X. tropicalis (Araki, 2014). Further studies to reveal and compare the molecular natures between the two Xenopus will disclose the molecular mechanism for transdifferentiation. These results indicate that Xenopus model is superior for the analysis of cell and molecular mechanism involved in RPE transdifferentiation.
The above observations suggest an idea that for the initiation of retinal regeneration from the RPE cells they must lose the interaction with the substratum (the basement membrane) and recover it once again after a certain time of period. We have been attempting to examine this hypothesis under a tissue culture condition, and the importance of cell adhesion molecules was confirmed. For Pax6 upregulation, cell-cell interaction through cadherin and/or Gap junction is critical. The most important question is why and how the RPE cells become detached when the retina is removed. Our preliminary experiments indicate that matrix metalloproteinases (Mmps) are upregulated immediately after the retinal removal and inhibition of the enzyme activity suppresses neuronal differentiation from RPE cells (manuscript in preparation). These results suggest activation of Mmps is the primary event leading to the initiation of transdifferentiation or RPE cells. Mmp upregulation was also observed in limb regeneration, suggesting a common step for tissue regeneration.
The ciliary epithelial cells can regenerate the whole retina in X. tropicalis: a novel mode of retina regeneration: Another characteristic and most interesting feature of retina regeneration in Xenopus is that the stem/progenitor cells in the marginal zone of the ciliary epithelium (CMZ) are activated and regenerate the new retina in the eye. This is particularly apparent in X. tropicalis, because the whole retina regenerates only from the ciliary epithelial cells located in the CMZ. This is a novel mode of retinal regeneration, because there has been no report describing that stem cells in the CMZ actually regenerate the retina in the eye. This is particularly significant when we consider that ciliary epithelial cells possess stem-like properties under culture conditions in many vertebrate species including human (Wohl et al., 2012), suggesting a possibility of clinical application for retinal diseases. On the contrary, RPE cells in higher vertebrates (birds and mammals) do not show retinal stem cell-like properties both in vivo and in vitro conditions and are not most suitable candidates for clinical approach.
In Xenopus, soon after the retina is removed, cells in the CMZ start proliferation as shown by BrdU labeling (Miyake and Araki, 2014). Most of the cells in this area, referred as a proliferating zone, are labeled with BrdU (Figure 2A, B). These cells extend posteriorly onto the RVM, indicating that the RVM is an important structure as a substratum for progenitor cells. On days 10–14 after retinal removal, a well-organized retinal structure is formed at the periphery of the eye. BrdU labeling experiments show that most cells in both ends of the regenerating retina, one located at the ciliary body and the other at the more centrally located growing end of the regenerating retina, are intensely labeled with BrdU, indicating that newly born retinal cells are added from both sides (Figure 2C, D); the regenerating retina is sandwiched by two stem/progenitor cell zones. A pulse-chase experiment shows these BrdU-labeled cells in the proliferating zones actually produce cells in the regenerating retina (Miyake and Araki, 2014). It is interesting how the two proliferating zones are formed. In the developing eye, stem cells in the CMZ produce retinal cells, which are then added to the retina. Wnt signal controls proliferation of retinal stem cells in the ciliary zone and inhibits differentiation of retinal progenitor cells, while Shh in the retina has an opposite effect on the stem cells (Borday et al., 2012). In retinal regeneration of X. tropicalis, stem cells in the CMZ proliferate robustly first, then start to differentiate into retinal cells. Retinal removal will alter the Wnt-Shh balance and may activate proliferation of the ciliary epithelial cells through the Wnt signal pathway. The RPE layer, juxtaposed to the proliferating zone, may play a role to suppress cell differentiation and keep their progenitor properties probably by certain signals like Wnt (Perron et al., 2003). This may explain the localization of two proliferation zones at both ends of the regenerating retina. However, all these stories must be evaluated at a molecular level in further studies.
Figure 2.
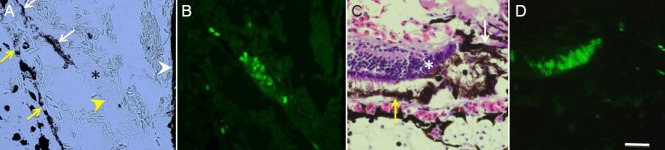
BrdU labeling of stem/progenitor cells at the early stage of regeneration (day 5) (A, B) and late stage (day 20) (C, D).
(A, B) At the early stage, there is an actively proliferating cell zone (asterisk in A) juxtaposed to the presumable ciliary marginal zone (CMZ) (yellow arrowhead) and the iris (white arrowhead). This proliferating cell zone (progenitor cells) appear continuous to the newly formed retinal pigmented epithelial (RPE) layer (white arrow). The original RPE layer is shown by yellow arrows. A is a phase contrast micrograph image and is identical to B. (C, D) At the later stage, there is another progenitor cell clusters (asterisk) at the posterior end of regenerating retina. There still remain some RPE cells (white arrow). The original RPE is shown by a yellow arrow. C is a micrograph of a section stained with hematoxylin and eosin, and is identical to D. Scale bar in D is 45 μm and applied to A–C.
Conclusion and future perspectives: We have shown that Xenopus is an excellent animal model for retina regeneration for several reasons; First, retinal tissue can regenerate after any type of injuries in adult animals, making it possible to investigate various aspects of regeneration in one model. Complete retinal regeneration occurs only in amphibians, although we must emphasize the importance of the retinal vascular membrane (RVM). Without this structure no regeneration occurs. Second, there are multi sources of stem/progenitor cells in the ocular tissues. Third, different animal models, X. laevis and X. tropicalis, genetically close to each other, are available and show different modes of regeneration. Comparison at cell and molecular levels can reveal the environmental cues for regeneration to occur. Fourth, several different culture models are available for different purposes, and fifthly, X. tropicalis, in particular, is suitable for genetic study of molecular mechanism. In the present article, the author briefly described these aspects of Xenopus models and particularly put emphasis on the ciliary epithelial cells, since this cell type in human also retains a property of retinal stem/progenitor cells, which has been shown in vitro. The retinal vascular membrane, which is lacking in mammalian eyes, appears to have a crucial role in vivo regeneration. Its chemical nature as extracellular matrices is not yet well understood, and it is still unknown whether it is a source of certain growth factors. Like the in vitro studies of the RPE tissues, future studies are needed to characterize the ciliary epithelial cells under a culture condition. In our recent study we have applied the culture method to the chick and porcine tissues (Matsushita et al., 2014) and have found it works for the retinal cell differentiation from ocular stem/progenitor cells. The potential for in vivo retinal regeneration from the ciliary epithelial cells will bring in new therapies for retinal diseases.
Still now many organs of the Xenopus are considered to regenerate only in the embryonic stages (such as brain tissue, limb, digestive organs and so on). Like the history of retinal regeneration study in Xenopus, which was described here only briefly, it is possible to say that Xenopus may retain a regenerative potency in the adult stage in organs other than the retina, and that we only do not know how to realize it in an experimental way. The way, if we can realize, should provide us with a new way of regeneration study.
This study was supported by Grant-in-Aid (Scientific Research on Innovative Area: MEXT KAKENHI Grant Number 23124506) and a Grant-in-Aid (Kiban-C: JSPS KAKENHI Grant Number 23570255).
References
- 1.Araki M. A model for retinal regeneration in Xenopus. In ‘Xenopus Development’. In: Kloc M, Kubiak J Z, editors. John Wiley & Sons, Inc; 2014. pp. 346–367. [Google Scholar]
- 2.Borday C, Cabochette P, Parain K, Mazurier N, Janssens S, Tran HT, Sekkali B, Bronchain O, Vleminckx K, Locker M, Perron M. Antagonistic cross-regulation between Wnt and Hedgehog signalling pathways controls post-embryonic retinal proliferation. Development. 2012;139:3499–509. doi: 10.1242/dev.079582. [DOI] [PubMed] [Google Scholar]
- 3.Chiba C, Mitashov VI. Cellular and molecular events in the adult newt retinal regeneration. In: Chiba C, editor. “Strategies for retinal tissue repair and regeneration in vertebrantes: From fish to human”. Kerala, India: Research Signpost; 2007. pp. 16–37. [Google Scholar]
- 4.DelRio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- 5.Fisher AJ. Neural regeneration in the chick retina. Prog Retina Eye Res. 2005;24:161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Lenkowski JR, Raymond PA. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retina Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita T, Fujihara A, Royall Lars, Kagiwada S, Kosaka M, Araki M. Immediate differentiation of neuronal cells from stem/progenitor-like cells in the avian iris tissues. Exp Eye Res. 2014;123:16–26. doi: 10.1016/j.exer.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Miyake A, Araki M. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: A study of retinal regeneration in a novel animal model. Dev Neurobiol. 2014;74:739–756. doi: 10.1002/dneu.22169. [DOI] [PubMed] [Google Scholar]
- 9.Okada TS. New York: Oxford University Press; 1991. Transdifferentiation. [Google Scholar]
- 10.Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Pieler T, Harris WA. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development. 2003;130:1565–1577. doi: 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- 11.Wohl SG, Schmeer CW, Isenmann S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog Retina Eye Res. 2012;31:213–242. doi: 10.1016/j.preteyeres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol. 2007;303:45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]


