Abstract
Claudin 14 has been shown to promote nerve repair and regeneration in the early stages of Wallerian degeneration (0–4 days) in rats with sciatic nerve injury, but the mechanism underlying this process remains poorly understood. This study reported the effects of claudin 14 on nerve degeneration and regeneration during early Wallerian degeneration. Claudin 14 expression was up-regulated in sciatic nerve 4 days after Wallerian degeneration. The altered expression of claudin 14 in Schwann cells resulted in expression changes of cytokines in vitro. Expression of claudin 14 affected c-Jun, but not Akt and ERK1/2 pathways. Further studies revealed that enhanced expression of claudin 14 could promote Schwann cell proliferation and migration. Silencing of claudin 14 expression resulted in Schwann cell apoptosis and reduction in Schwann cell proliferation. Our data revealed the role of claudin 14 in early Wallerian degeneration, which may provide new insights into the molecular mechanisms of Wallerian degeneration.
Keywords: nerve regeneration, peripheral nerve injury, Wallerian degeneration, sciatic nerve injury, Claudin 14, rat, Schwann cell, Signal pathways, c-Jun, Akt, ERK1/2, NSFC grant, neural regeneration
Introduction
Wallerian degeneration is the process of degeneration that occurs in a nerve fiber when it is crushed or cut. Wallerian degeneration occurs in both the central nervous system and peripheral nervous system, and usually begins within 24–36 hours in the axon stump distal to a site of injury. Wallerian degeneration comprises cellular and molecular changes, and requires effective Schwann cell and macrophage responses (Waller, 1850; Ohri et al., 2011; Munzel et al., 2012; Ohata et al., 2012). During Wallerian degeneration, Schwann cells may play a central role in the function of degeneration and regeneration after nerve injury. The Schwann cells release a wide variety of cytokines and chemokines during the first few days post injury, and participate in the clearance of myelin debris from the degenerative nerves (Kirsch et al., 2003; Navarvo et al., 2007; Martini, et al., 2008; Hu et al., 2013). Schwann cells also proliferate, dedifferentiate, and provide growth substrates to regenerating axons which are regulated by the activation of transcription factor signaling pathways, such as c-Jun, Akt and extracellular signal-regulated kinase (ERK) (Hirakawa et al., 2003; Yang et al., 2007; Feltri, et al., 2008; Chattopadhyay et al., 2009; Arthur et al., 2012; Li et al., 2013, 2014).
Claudins are tight-junction membrane proteins that are key regulators of the paracellular pathway, consisting of a family of 27 members. As tight-junction proteins, claudins are crucial for the maintenance of cellular polarity and paracellular transportation of molecules. Claudin 14 is an important tight-junction molecule, and is capable of self assembly into tight junction, and integrates into claudin 16 and claudin 19 channels to form a higher oligomeric complex (Miyamoto et al., 2005; Hewitt et al., 2006; Kominsky et al., 2006; Angelow et al., 2008; Elkouby et al., 2008; Lal Nag et al., 2009; Shiozaki et al., 2012).
In our previous study, we have reported that claudin 14 and claudin 15 are the two key regulatory factors which may perform a crucial role in early Wallerian degeneration after sciatic nerve injury (Li et al., 2013). Claudin 14 and claudin 15 are two tight-junction membrane proteins that exert different functions in early Wallerian degeneration, and can regulate cytokine up or down expression, neural networks, signal pathways and signal flow (Li et al., 2013). Here, we have found a mechanistic role for claudin 14 in nerve degeneration and regeneration during early Wallerian degeneration. Our findings may provide insights into the molecular mechanisms of other factors that regulate nerve degeneration and/or regeneration during Wallerian degeneration.
Materials and Methods
Animal models of Wallerian degeneration
For further analysis of the expression of claudin 14 in injured sciatic nerve at 0, 4, 7, 14, and 21 days, 180 male Sprague-Dawley rats weighing 180–220 g were selected. Of them, 30 rats were used for real-time PCR assay and 30 for western blot assay. All assays were performed in triplicate. All rats were provided by the Experimental Animal Center of Nantong University in China (License No. SCXK (Su) 2014-0001). The protocols were conducted in accordance with “NIH Guidelines for the Care and Use of Laboratory Animals”.
Experimental groups
The 30 rats were equally and randomly divided into 5 groups: 0, 4, 7, 14, and 21 days. Rats underwent sciatic neurectomy and were anesthetized with complex narcotics. The sciatic nerve on the lateral aspect of the mid-thigh was lifted from the right hind limb and a 1-cm segment was excised. Rats were killed immediately and at 4, 7, 14, and 21 days following sciatic nerve surgery (Hirakawa et al., 2003; Li et al., 2013, 2014). The rats at 0 day were subjected to sham operations. Under the same condition, the skin was incised, muscle was dissociated, and sciatic nerve was exposed and sutured in the sham surgery group.
Schwann cell culture and transfection
Schwann cells were obtained from the sciatic nerves of 1-day-old rats as previously described (Barretta et al., 2008; Mantuano et al., 2008; Peltonen et al., 2013). Cells were cultured in Dulbecco's Minimum Eagle's Medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal calf serum (Invitrogen, Carlsbad, CA, USA) and 100 IU/mL penicillin, and 100 g/mL streptomycin (Sigma Aldrich, St Louis, MO, USA) at 37°C with 5% CO2. Cells were selected from fibroblasts using monoclonal mouse anti-Thy1.1 antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The final cells consisted of 98% Schwann cells, as determined by immunofluorescence for mouse anti-S100 monoclonal antibody (1:500 dilution; Santa Cruz Biotechnology), which is a specific Schwann cell marker. Schwann cells were transfected with siRNAs for 2 days (Ribobio, Guangzhou, Guangdong Province, China) and pCMV6-claudin 14 plasmid using Lipofectamine RNAi MAX transfection reagent (Invitrogen), according to the manufacturer's instructions.
Immunofluorescence
Schwann cells were examined by immunofluorescence as previously described. Cells were fixed with 4% paraformaldehyde in Tris-buffered saline, washed with Tris-buffered saline, and permeabilized with 0.1% Triton. Nonspecific binding was blocked with 10% goat serum. The cells were incubated with primary antibodies for 2 hours (1:1,000; Santa Cruz Biotechnology), and then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies for 1 hour. 4′,6-Diamidino-2-phenylindole (DAPI) was applied. Replacement of primary antibody with respective normal IgG occurred to control signal specificity. Imaging was performed using a Leica DMR bright-light and fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Immunofluorescence staining was utilized for cultured Schwann cells, immunostaining for S100, claudin 14 and their overlay.
RNA isolation and real-time quantitative PCR assay
Total RNA was isolated from distal nerve stumps and Schwann cells according to the manufacturer's protocols. The distal nerve stumps were dissected from rats that were killed at 0, 4, 7, 14, and 21 days following injury. Real-time quantitative PCR was performed with the SYBR Green PCR Master Mix (Qiagen, Valencia, CA, USA). The primers used in the real-time quantitative PCR assays and RNAi are provided in Table 1. The relative expression values of each mRNA were calculated using comparative Ct and were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mature mRNA for each data.
Table 1.
Primers used in real-time quantitative PCR assays

Western blot assay
The distal nerve stumps were obtained from rats killed at 0, 4, 7, 14, and 21 days after injury and Schwann cells were directly lysed. Protein expression was analyzed with mouse anti-claudin 14, Akt, p-Akt, ERK1/2, p-ERK1/2, c-Jun, p-c-Jun, beta-actin, and beta-catenin monoclonal antibodies conjugated affinity purified goat anti-mouse IgG (1:500 dilution; Santa Cruz Biotechnology). The image was scanned with a GS800 Densitometer Scanner (Bio-Rad, Hercules, CA, USA). The data of optical density were analyzed using PDQuest 7.2.0 software (Bio-Rad). Beta-actin and beta-catenin were used as an internal control.
Flow cytometric analysis
After 48 and 72 hours, cell apoptosis was analyzed using a Flow Collect Annexin Red Kit (Millipore, Bedford, MA, USA). Cells were trypsinized, centrifuged, and resuspended in assay buffer. Annexin V solution was added to each sample for 15 minutes. Cells were resuspended. 7-AAD reagent was added to sample and then incubated in the dark. Cells were analyzed by a BD LSRII Flow Cytometer (BD Biosciences, San Jose, CA, USA). Cell number in different quadrants was analyzed using quadrant statistics. Cells in the lower right quadrant represented apoptosis, and those in left lower quadrant represented survival.
Cell migration assay
Cell migration assays were performed in Transwell cell-culture chambers (Costar, Cambridge, MA, USA) as described previously (Mantuano et al., 2008). The lower surface of each membrane was coated with fibronectin. Schwann cells were transferred to the top chambers of each transwell and allowed to migrate. The complete medium was injected into the lower chambers. The non-migrated cells were cleaned with a cotton swab at the indicated time point. Migrated cells were fixed with methanol and then stained with crystal violet solution. Cell migration was defined by images of randomly selected fields obtained on fluorescence. Cell migration imaging and counting occurred using a DMR inverted microscope (Leica Microsystems).
Cell proliferation assay
Schwann cells were resuspended in fresh complete medium, counted and plated on poly-L-lysine-coated 96 well plates. EdU was applied and the cells were cultured after cell transfection for 48 hours. The cells were fixed with formaldehyde in PBS and assayed using a Cell-Light EdU DNA Cell Proliferation Assay Kit (Ribobio) following the manufacturer's protocol. Cell proliferation was expressed as the ratio of EdU-positive cells to total cells. Schwann cell proliferation analysis was performed using images of randomly selected fields obtained on a DMR fluorescence microscope (Leica Microsystems). All samples were analyzed in three independent experiments.
Statistical analysis
All data were expressed as the mean ± SD and were analyzed by Student's t-test, one-way analysis of variance and Scheffe's post-hoc test with the SPSS 13.0 software package (SPSS, Chicago, IL, USA). All values of P < 0.05 were considered statistically significant.
Results
Claudin 14 expression in injured sciatic nerve and Schwann cells during Wallerian degeneration
Real-time quantitative PCR and western blot assay were applied to investigate claudin 14 expression at 0, 4, 7, 14, and 21 days after injury. These tests revealed that claudin 14 expression was increased at 4 days, and then decreased (P < 0.05; Figure 1). Western blot assay findings were in agreement with the real-time quantitative PCR data. We also examined the expression of claudin 14 in cultured Schwann cells to determine its function. Our data indicate that claudin 14 is present in Schwann cells. Experiments were repeated in triplicate.
Figure 1.
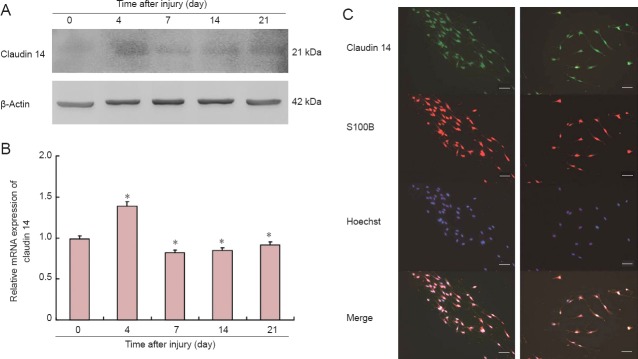
Claudin 14 expression in injured sciatic nerve and cultured Schwann cells.
(A) Western blot assay of claudin 14 expression in injured sciatic nerve at 0, 4, 7, 14, and 21 days. Beta-actin levels were used as a control. (B) Real-time quantitative PCR analysis of sciatic nerve at 0, 4, 7, 14, and 21 days after injury using GAPDH as control. *P < 0.05, vs. day 0. All data were expressed as the mean ± SD and were analyzed by one-way analysis of variance and Scheffe's post-hoc test. Experiments were performed in triplicate. (C) Claudin 14 (green) and Hoechst (blue) expression in cultured Schwann cells after purification (left) and before purification (right). S100 (red) was used as a Schwann cell specific marker. Scale bars: 20 μm. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Knockdown of claudin 14 resulted in cytokine expression change
To explore the function of claudin 14 in Schwann cells, three specific small interfering RNAs (siRNAs) against claudin 14 were synthesized to transfect Schwann cells. As shown in Figure 2A and 3A, siRNA-1-3 greatly reduced the expression of claudin 14 mRNA and protein (P < 0.05). Claudin 14 siRNA-2 was selected for transfection Schwann cells for the following studies. Real-time quantitative PCR results showed that the expression of bcl-2, krox20, NT3, bFGF and PKCα was significantly down-regulated after claudin 14 siRNA-2 was transfected for 1 and 2 days. By contrast, bax and nf2 were up-regulated (P < 0.05; Figure 2B). The data indicated that claudin 14 siRNA transfection was able to silence claudin 14 expression.
Figure 2.
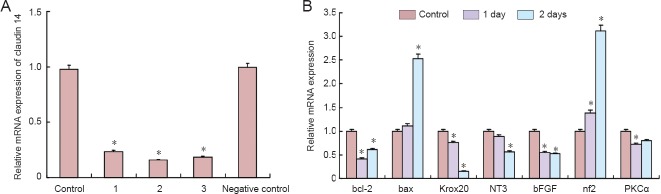
Cytokine expression changes in claudin 14 siRNA knockdown Schwann cells.
(A) Real-time quantitative PCR of mRNA relative expression of claudin 14 in siRNA-1, siRNA-2 and siRNA-3 transfected Schwann cells compared with negative control. Control represents non-interfered normal cells; 1, 2, 3: claudin 14 siRNA-1, siRNA-2 and siRNA-3. Negative control: without DNA interference. (B) Real-time quantitative PCR analysis of bcl-2, bax, krox20, NT3, bFGF, nf2 and PKCα after claudin 14 siRNA-2 transfection into Schwann cells for 1 and 2 days. GAPDH was used as a normalizer. (A, B) *P < 0.05, vs. control. All data are expressed as the mean ± SD (n = 3) and are analyzed by one-way analysis of variance and Scheffe's post-hoc test. All reactions were run in triplicate. NT3: Neurotrophin 3; bFGF: basic fibroblast growth factor; nf2: neurofibromin 2; PKC: protein kinase C.
Figure 3.
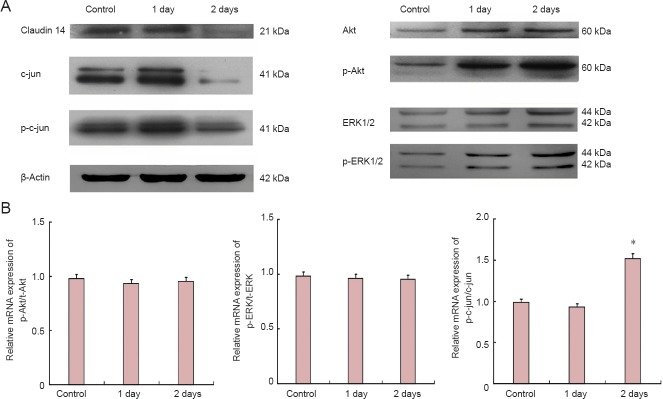
Silencing of claudin 14 up-regulated c-Jun but not Akt or ERK1/2.
(A) Western blot assay of claudin 14, Akt, p-Akt, ERK1/2, p-ERK1/2, c-Jun and p-c-Jun protein expression in claudin 14 siRNA-2 transfected Schwann cells. Beta-actin was used as a loading control. (B) Relative protein expressions of p-Akt/Akt, p-ERK1/2/ERK1/2 and p-c-Jun/c-Jun in claudin 14 siRNA-2 transfected Schwann cells. *P < 0.05, vs. control and 1 day. All data are expressed as the mean ± SD (n = 3) and are analyzed by one-way analysis of variance and Scheffe's post-hoc test. All reactions were run in triplicate. ERK: Extracellular signal-regulated kinase.
Expression of claudin 14 activated c-Jun but not Akt and ERK1/2 pathways
As we previously reported, claudin 14 and 15 are the key factors which initiated signal flow and pathways during early Wallerian degeneration (Li et al., 2013, 2014). To identify the effect of claudin 14 expression in Schwann cells, the signaling pathways and their phosphorylated (activated) ratios were investigated. The results showed that c-Jun and p-c-Jun expressions were significantly changed when the changes in Akt and ERK1/2 were observed (P < 0.05). Their phosphorylated (activated) p-Akt, and p-ERK1/2 were not significantly changed in both claudin 14 knockdown and claudin 14 over-expression in Schwann cells (Figures 3, 4). These data suggest that altered expressions of claudin 14 activated c-Jun, but not Akt and ERK1/2 signaling pathways.
Figure 4.
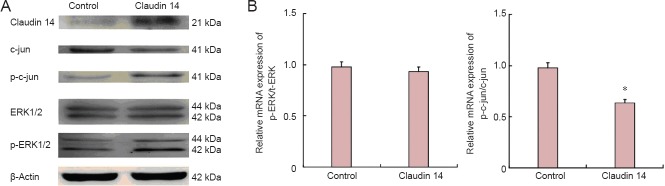
Enhanced expression of claudin 14 (2 days) down-regulated c-Jun but not ERK1/2 expression.
(A) Western blot assay of claudin 14, c-Jun and p-c-Jun, ERK1/2, p-ERK1/2 protein expression in claudin 14 siRNA-2 transfected Schwann cells for 2 days. Beta-actin levels were used as a control. (B) Relative protein expression of p-ERK1/2/ERK1/2 and p-c-Jun/c-Jun in claudin 14 siRNA-2 transfected Schwann cells. *P < 0.05, vs. control. All data are expressed as the mean ± SD (n = 3) and are analyzed by Student's t-test. All reactions were run in triplicate. ERK: Extracellular signal-regulated kinase.
Silencing of claudin 14 resulted in Schwann cell apoptosis
To evaluate the effect of claudin 14 on Schwann cell apoptosis, cells were transfected with claudin 14 siRNA-2 for 48 and 72 hours. The apoptosis was detected and quantified by flow cytometry after FITC-conjugated annexin-V and propidium iodide staining. Results indicated that Schwann cell apoptosis increased significantly in cell population after claudin 14 siRNA-2 transfection compared with control siRNA transfected cells (Figure 5). These data suggested that silencing of claudin 14 expression may induce Schwann cell apoptosis.
Figure 5.
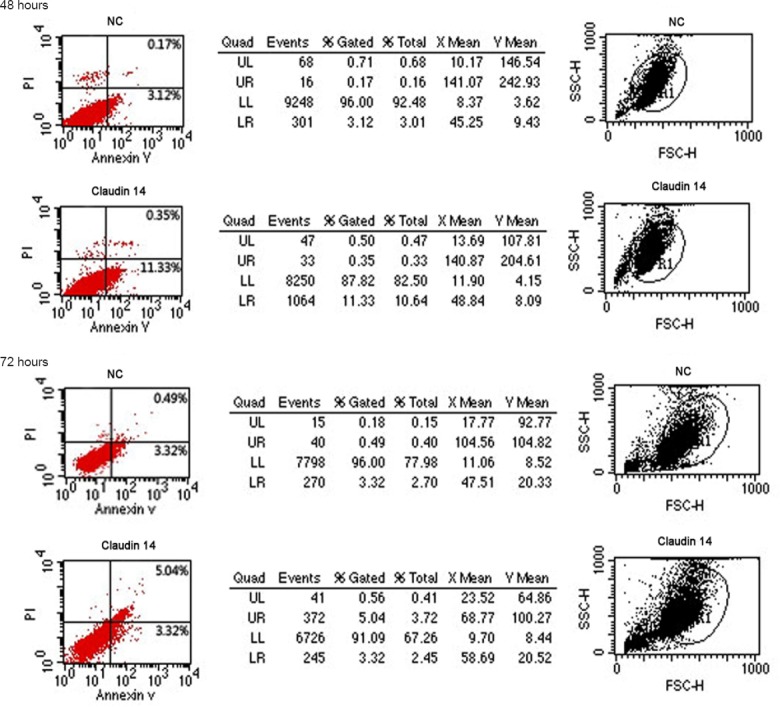
Silencing of claudin 14 resulted in Schwann cell apoptosis.
Apoptosis analysis of claudin 14 siRNA-2 transfected Schwann cells for 48 hours and 72 hours compared with the control siRNA transfected cells. The apoptosis was detected and quantified by flow cytometry after FITC-conjugated annexin-V and propidium iodide (PI) staining. All samples were analyzed in three independent experiments. All reactions were run in triplicate. FITC: Fluorescein isothiocyanate.
Silencing of claudin 14 reduced Schwann cell migration
Following the hypothesis that silencing of claudin 14 might inhibit Schwann cell migration, we knocked down the expression of claudin 14 in Schwann cells and detected Schwann cell migration. The number of migrated Schwann cells was significantly lower when Schwann cells were transfected with claudin 14 siRNA-2 compared with the normal control (Figure 6). The results indicated that claudin 14 knockdown can decrease Schwann cell migration, which suggested a functional association with sciatic nerve regeneration.
Figure 6.
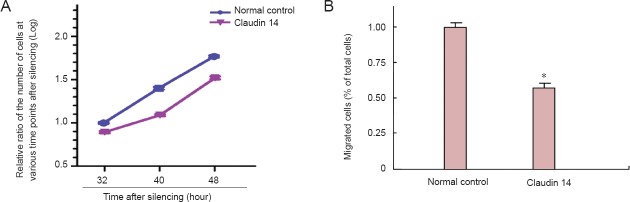
Silencing of claudin 14 reduced Schwann cell migration.
(A) Silencing of claudin 14 expression inhibits cell migration in claudin 14 siRNA-2 transfected Schwann cell. (B) The number of migrated Schwann cells was significantly lower when Schwann cells were transfected with claudin 14 siRNA-2 compared with the normal control. *P < 0.05, vs. normal control. All data are expressed as the mean ± SD (n = 3) and are analyzed by Student's t-test.
Enhanced claudin 14 expression promoted Schwann cell proliferation
Cell migration assay showed that claudin 14 siRNA-2 significantly facilitated the migration of Schwann cells. Furthermore, we investigated the effect of claudin 14 on Schwann cell proliferation. Because claudins are up-regulated in early Wallerian degeneration after sciatic nerve injury, we hypothesized that enhanced claudin 14 can increase Schwann cell proliferation and migration. To further address this issue, we over-expressed claudin 14 in cultured Schwann cells. We observed a significant increase in cell proliferation (Figure 7). Taken together, these findings show that claudin 14 could induce Schwann cell proliferation and migration, suggesting that claudin 14 is an important functional factor for Schwann cell regeneration.
Figure 7.
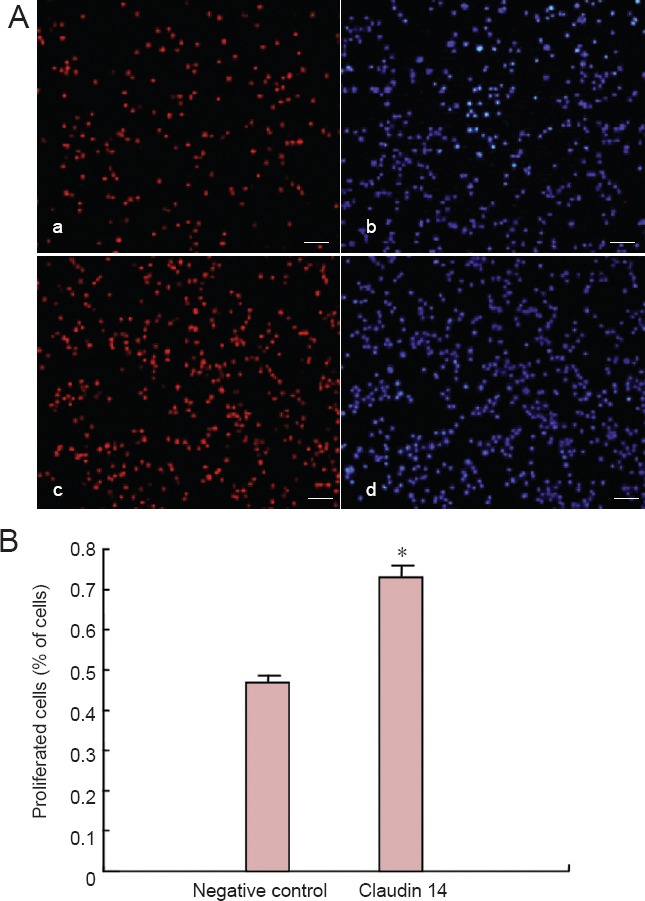
Enhanced expression of claudin 14 promoted Schwann cell proliferation (2 days).
(A) Over-expression of claudin 14 for 2 days promoted Schwann cell (Red) proliferation in pCMV6-claudin 14 plasmid transfected Schwann cells. (a, b) Negative control Schwann cells were stained with Apollo (Red; a) or Hoechst33342 (blue; b). (c, d) Schwann cells transfected with pCMV6-claudin 14 plasmid were stained with Apollo (red; c) or Hoechst 33342 (blue; d). (B) The number of proliferated Schwann cells was significantly higher when Schwann cells were transfected with pCMV6-claudin 14 plasmid compared with the negative control. *P < 0.05, vs. negative control. All data are expressed as the mean ± SD (n = 3) and analyzed using Student's t-test. Scale bars: 100 μm.
Discussion
Wallerian degeneration is a process that occurs when a nerve fiber is cut or crushed and essentially prepares the distal stump for reinnervation. This process requires that the distal portion of the damaged peripheral nerves was well underway in early Wallerian degeneration (De et al., 2003; Boivin et al., 2007; Feltri et al., 2008; Barrette et al., 2010; Girolami et al., 2010). Nerve injury following the disconnection interferes with the retrograde flow and may lead to the emergence of a negative denervation signal. Retrograde transport is reduced for 24 hours until the onset of regeneration in the injured sciatic nerve. Peripheral nerve degeneration is not a typical cell death mechanism, since neurons undergoing the process remain alive. Peripheral nerve repair is a result of reactivated regeneration mechanisms in combination with newly activated injury-dependent reactions (Hanz et al., 2003; Lindwall et al., 2005; Lee et al., 2007; Martini et al., 2008; Kim et al., 2009; Kirsch et al., 2009). Wallerian degeneration occurs several hours after injury and is followed by a long-lasting regeneration (4–28 days) in which immunohistochemical, functional and neurophysiological results all support the slow extension of nerve fibers in the distal direction. Thus, early activation is of great importance in nerve degeneration and regeneration during the Wallerian degeneration process (Raivich et al., 2004; Navarro et al., 2007; Chattopadhyay et al., 2009; Hu et al., 2013).
Claudins are a family of small transmembrane proteins that are the most important components of the tight junction, where they establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of the epithelium. They share sequence homology and functional similarity, and are often known as the classic tight junction. Claudin 14 is observed to interact with claudin 16, but not claudin 19. Claudin 14 relays the extracellular Ca2+ signal to claudin 16 and effects Ca2+ transport through direct functional modulation of their permeabilities (Ben et al., 2003; Hewitt et al., 2006; Elkouby et al., 2008; Lal et al., 2009; Kuscha et al., 2012). Claudin 14 diminished the cation permeability of the claudin 16 channel, suggesting that claudin 14 might physiologically bind to the claudin complex to act as a negative regulator of divalent cation reabsorption (Ben et al., 2003; Huang et al., 2009; Lu et al., 2011; Arthur et al., 2012). Thus, understanding the function of claudins in peripheral nerve injury may provide insights into the molecular mechanisms of Wallerian degeneration.
In the peripheral nerve, Schwann cells play a critical role in degeneration and regeneration by secreting trophic cytokines, migrating into the injury sites and guiding the regenerating axons to the target. Here, we found that claudin 14 is up-regulated in early Wallerian degeneration until 4 days and also expressed in Schwann cells. Claudin 14 induced expression changes in cytokines and c-Jun, but not Akt and ERK1/2. Enhanced claudin 14 promotes Schwann cell proliferation and migration. Silencing of claudin 14 resulted in Schwann cell apoptosis and reduced Schwann cell proliferation. Claudin 14 is a tight-junction protein, expresses in different types of cells, which have different functions in early Wallerian degeneration after sciatic nerve injury (Li et al., 2013). Therefore, we speculate that claudin 14 plays an important role in Wallerian degeneration. Although we provide evidence that claudin 14 affects Wallerian degeneration after nerve injury, the role of other claudins in peripheral nerves is still poorly understood. Further studies into the functions of claudin 14 and 15 in vivo may open new possibilities for providing insight into the mechanism of nerve degeneration and/or regeneration.
Acknowledgments:
We thank Ian Haigler from Nantong University in China for editorial assistance and suggestions relating to the manuscript.
Footnotes
Funding: This research was supported by grants from the National Natural Science Foundation of China, Grant No. 81370982, 31170946, Key Program, Grant No. 81130080; the Scientific Research Foundation for Returned Scholars, Ministry of Education of China; the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interest: None declared.
Copyedited by Apricò K, Robens J, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrette B, Calvo E, Vallies N, Lacroix S. Transcriptional profiling of the injured sciatic nerve carrying the Wld(S) mutant gene: identification of genes involved in neuroprotection neuroinflammation and nerve regeneration. Brain Behav Immun. 2010;24:1254–1267. doi: 10.1016/j.bbi.2010.07.249. [DOI] [PubMed] [Google Scholar]
- 4.Ben Yosef T, Belyantseva IA, Saunders TL, Hughes E, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER. Claudin 14 knockout mice a model for autosomal recessive deafness DFNB29 are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 5.Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chattopadhyay S, Shubayev V. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ErbB receptor-mediated activation of MEK/ERKpathway. Glia. 2009;57:1316–1125. doi: 10.1002/glia.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De S, Trigueros MA, Kalyvas A, David S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24:753–765. doi: 10.1016/s1044-7431(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 8.Elkouby Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res. 2008;333:427–438. doi: 10.1007/s00441-008-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen K. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girolami EI, Bouhy D, Haber M, Johnson H, David S. Differential expression and potential role of SOCS1 and SOCS3 in Wallerian degeneration in injured peripheral nerve. Exp Neurol. 2010;223:173–182. doi: 10.1016/j.expneurol.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirakawa H, Okajima S, Nagaoka T, Takamatsu T, Oyamada M. Loss and recovery of the blood-nerve barrier in the rat sciatic nerve after crush injury are associated with expression of intercellular junctional proteins. Exp Cell Res. 2003;284:196–210. doi: 10.1016/s0014-4827(02)00035-6. [DOI] [PubMed] [Google Scholar]
- 14.Hu CH, Xiao K, Luan ZS, Song J. Early weaning increases intestinal permeability alters expression of cytokine and tight junction proteins and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- 15.Huang YH, Bao Y, Peng W, Goldberg M, Love K, Bumcrot DA, Cole G, Langer R, Anderson DG, Sawicki JA. Claudin-3 gene silencing with siRNA suppresses ovarian tumor growth and metastasis. Proc Natl Acad Sci USA. 2009;106:3426–3430. doi: 10.1073/pnas.0813348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Lee S, Lee SJ. Toll-like receptors in peripheral nerve injury and neuropathic pain. Curr Top Microbiol Immunol. 2009;336:169–186. doi: 10.1007/978-3-642-00549-7_10. [DOI] [PubMed] [Google Scholar]
- 17.Kirsch M, Terheggen U, Hofmann HD. Ciliary neurotrophic factor is an early lesion-induced retrograde signal for axotomized facial motoneurons. Mol Cel Neurosci. 2003;24:130–138. doi: 10.1016/s1044-7431(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Kominsky SL. Claudins: emerging targets for cancer therapy. Expert Rev Mol Med. 2006;8:1–11. doi: 10.1017/S1462399406000056. [DOI] [PubMed] [Google Scholar]
- 19.Lal Nag M, Morin PJ. The claudins. Genome Biol. 2009;10 doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 2007;102:686–698. doi: 10.1111/j.1471-4159.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 21.Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci. 2005;29:269–282. doi: 10.1016/j.mcn.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Ding L, Hong H, Hoggard J, Lu Q, Chen YH. Claudin-7 inhibits human lung cancer cell migration and invasion through ERK/MAPK signaling pathway. Exp Cell Res. 2011;317:1935–1946. doi: 10.1016/j.yexcr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28:11571–11582. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martini R, Fischer S, Lopez-Vales R, David S. Interactions between schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- 25.Meiyuan L, Weimin G, Pingan Z, Huaiqin L, Xiaosong G, Dengbing Y. Signal flow and pathways in response to early Wallerian degeneration after rat sciatic nerve injury. Neurosci Lett. 2013;536:56–63. doi: 10.1016/j.neulet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Meiyuan L, Pingan Z, Weimin G, Huaiqin L, Xiaosong G, Dengbing Y. Protein expression profiling during wallerian degeneration after rat sciatic nerve injury. Muscle Nerve. 2014;50:73–78. doi: 10.1002/mus.24082. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, Okamura Y, Sasaki H, Miyachi Y. Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J Cell Biol. 2005;169:527–538. doi: 10.1083/jcb.200501154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Münzel EJ, Schaefer K, Obirei B, Kremmer E, Burton EA, Kuscha V, Becker CG, Brösamle C, Williams A, Becker T. Claudin k is specifically expressed in cells that form myelin during development of the nervous system and regeneration of the optic nerve in adult zebrafish. Glia. 2012;60:253–270. doi: 10.1002/glia.21260. [DOI] [PubMed] [Google Scholar]
- 29.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Ohata C, Imai N, Hinogami H, Akamatsu K, Sumimura Y. Hybrid schwannoma/perineurioma: a report of two cases including a possible radiation-induced case. J Cutan Pathol. 2012;39:56–62. doi: 10.1111/j.1600-0560.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peltonen S, Alanne M, Peltonen J. Barriers of the peripheral nerve. (1-6).Tissue Barriers. 2013:e24956. doi: 10.4161/tisb.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Shiozaki A, Bai XH, Shen-Tu G, Moodley S, Takeshita H, Fung SY, Wang Y, Keshavjee S. Claudin 1 mediates TNFalpha-induced gene expression and cell migration in human lung carcinoma cells. PLoS One. 2012;7:e38049. doi: 10.1371/journal.pone.0038049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog and observations of the alterations produced thereby in the structure of their primitive fibres. Phil Transact Royal Soc London. 1850;140:423–429. [Google Scholar]
- 36.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]


