Abstract
The spinal cord has the ability to regenerate but the microenvironment generated after trauma reduces that capacity. An increase in Src family kinase (SFK) activity has been implicated in neuropathological conditions associated with central nervous system trauma. Therefore, we hypothesized that a decrease in SFK activation by a long-term treatment with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine (PP2), a selective SFK inhibitor, after spinal cord contusion with the New York University (NYU) impactor device would generate a permissive environment that improves axonal sprouting and/or behavioral activity. Results demonstrated that long-term blockade of SFK activation with PP2 increases locomotor activity at 7, 14, 21 and 28 days post-injury in the Basso, Beattie, and Bresnahan open field test, round and square beam crossing tests. In addition, an increase in white matter spared tissue and serotonin fiber density was observed in animals treated with PP2. However, blockade of SFK activity did not change the astrocytic response or infiltration of cells from the immune system at 28 days post-injury. Moreover, a reduced SFK activity with PP2 diminished Ephexin (a guanine nucleotide exchange factor) phosphorylation in the acute phase (4 days post-injury) after trauma. Together, these findings suggest a potential role of SFK in the regulation of spared tissue and/or axonal outgrowth that may result in functional locomotor recovery during the pathophysiology generated after spinal cord injury. Our study also points out that ephexin1 phosphorylation (activation) by SFK action may be involved in the repulsive microenvironment generated after spinal cord injury.
Keywords: nerve regeneration, trauma, regeneration, Src family kinase, Eph receptors, ephexin, spared tissue, locomotor recovery, GFAP, ED1, serotonin fibers, neural regeneration
Introduction
Trauma to the central nervous system (CNS) triggers a series of events leading to loss of function and movement. Different strategies have been used to improve locomotor activity after spinal cord injury (SCI) including stimulation of axonal regeneration and cell survival, remyelination of spared axons, reduction of edema and decreasing the inflammatory response (Baptiste and Fehlings, 2006; Kwon et al., 2009; Oudega et al., 2012; Alluin et al., 2014). However, none of these strategies have been entirely effective in animal or human studies. In vertebrates, one of the main problems is the amount of inhibitory proteins that are normally present in the adult spinal cord (Fawcett, 2006; Niclou et al., 2006; Fitch and Silver, 2008; Young, 2014).
After SCI, inhibitory proteins such as NOGO, MAG, OMgp, Eph and Semaphorin (Grados-Munro and Fournier, 2003; McGee and Strittmatter, 2003; Goldshmit et al., 2006) are upregulated, promoting axonal growth cone retraction. Deactivation of any of these proteins individually results in only partial recovery (Kim et al., 2003; Simonen et al., 2003; Zheng et al., 2003; Goldshmit et al., 2004; Figueroa et al., 2006; Fabes et al., 2007; Lee et al., 2010). These repellent signals mediate growth cone collapse through the activation of intracellular kinases (Forgione and Fehlings, 2014; Lin et al., 2014; Wang et al., 2014). Phosphorylation of NOGO-A by Src points to the complex regulation of this protein in the repulsive environment for axonal outgrowth that could be generated after trauma (Yokoyama et al., 2006). However, the role of Src family kinases (SFK) in the pathophysiology generated after SCI has not been studied in detail in the chronic stage after spinal cord trauma.
Src proteins belong to the tyrosine kinases family, which regulate cell migration, survival, adhesion and differentiation (Thomas and Brugge, 1997). V-Src was initially identified as the transforming protein of the Rous sarcoma virus (Ohta et al., 1989). Several proteins are included in the Src kinases family: Src, Yes, Fyn, Fgr, Lyn, Hck, Lck, Blk and Yrk. Src kinases are proteins of approximately 52–62 kDa with similar structure, containing six domains: (a) myristylation domain, (b) unique region, (c) SH3 domain, (d) SH2 region, (e) kinase domain and (f) C-terminal region (Xu et al., 1997). The cellular counterpart of v-Src is c-Src that is a member of the non-receptor tyrosine kinases. c-Src is observed in nerve growth cones (Robles et al., 2005) and mediates the signal cascade that has been implicated in the regulation of neurite outgrowth (Lakshmi et al., 2006; Zhao et al., 2009), as well as microglial activation after transient forebrain ischemia (Choi et al., 2005). Blockade of SFK in the brain or in spinal cord injury results in some neuroprotective outcomes, suggesting a role of these kinases in the adverse environment generated after trauma (Akiyama et al., 2004; Lennmyr et al., 2004; Jadhav et al., 2007; Liang et al., 2009; Liu et al., 2014). However, its analysis is limited to the first few hours to days after injury (7 days post-injury), and to the best of our knowledge, chronic/long-term treatment (28 days post-injury) has not been evaluated.
We hypothesize that a long-term decrease in SFK activity after SCI would generate a permissive environment for axonal outgrowth/sprouting and/or functional locomotor recovery. The rationale behind this hypothesis is that blockade of SFK activity with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine (PP2) will prevent the activation of a cascade of intracellular events, result in the formation of growth cones, extension or sprouting of axons across the lesion epicenter, decrease inflammation process or gliotic response, and eventually increase in functional locomotor recovery. In this study, we analyzed the role of SFK in the pathophysiology generated after SCI at the behavior, anatomical and cellular levels.
Materials and Methods
Surgery and injection of PP2
Forty adult female Sprague-Dawley rats (Hilltop Lab Animals Inc, Scottdale, PA, USA), weighing 200–250 g were randomly divided into a sham group (n = 8) and an experimental group (n = 32). Rats were anesthetized with intraperitoneal injections of acepromazine (Vetus Animal Health, Rockville Center, NY, USA; 0.85 mg/kg), ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 87.7 mg/kg) and xylazine (Boehringer Ingelheim, Ridgefield, CT, USA; 4.2 mg/kg). Control (sham) rats received only a T10 laminectomy (n = 8), without contusion. Injured rats received a moderate contusion (12.5 mm height) at the T10 level with the New York University (NYU) Impactor device (Gruner, 1992; Miranda et al., 1999; Cruz-Orengo et al., 2006; Figueroa et al., 2006). The surgery was concluded with the skin, fascia and muscles sutured.
Ten minutes after contusion, PP2 (Tocris Biosciences, Missouri, USA; 1.5 mg/kg), an SFK inhibitor, was administered to the rats in the experimental group (n = 12) (Paul et al., 2001; Lennmyr et al., 2004; Hou et al., 2007). Injections of 4-amino-7-phenylpyrazolo[3,4-d]pyramidine (PP3; Tocris Biosciences, Ellisville, MO, USA), an inactive analog (n = 12), and dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) (n = 8) were used as controls (Gilmore et al., 2001). PP2, PP3 and DMSO (vehicle) injections were administered intraperitoneally every 3 days for 28 days, to ensure long-term blockade of SFK activity throughout the 4 weeks of behavioral analyses and end-point histochemical studies. After surgery, cefazolin (25 mg/kg; Bristol-Myers Squibb, New York, NY, USA) was subcutaneously administered for 7 consecutive days to all groups to reduce the possibility of bacterial infections. Finally, the bladders of injured rats were expressed three times per day until spontaneous voiding returned. In addition, Buprenex (buprenorphine; Reckitt & Colman Pharmaceuticals, Inc., Richmond, VA, USA; 0.05 mg/kg, subcutaneously) was used in all rats for 3 consecutive days as a post-operative analgesic (Santiago et al., 2009).
Animal experimentation followed NIH guidelines and was conducted with the approval of the University of Puerto Rico Institutional Animal Care and Use Committee. All efforts were made to reduce the number of animals used and their suffering.
Behavioral studies
Rats treated with PP2, PP3 and DMSO (n = 8 for each group) were analyzed with the Basso, Beattie, and Bresnahan (BBB) open field, grid walking and beam crossing tests to observe functional locomotor recovery. The BBB open field test was used to assess hindlimb movement as reported previously (Basso et al., 1995). Briefly, the score range from 0 to 21, each number has a description of movement improvement. All rats were previously tested (each one reflected a score of 21). After surgery, each group was tested at 7, 14, 21 and 28 days post-injury. Two investigators observed the rats for 4 minutes without knowing which treatment was administered (double blinded), and assigned a score depending on the movement of the rat hindlimbs (Santiago et al., 2009).
The beam-crossing test was used to examine the ability of the rat to control its balance (Merkler et al., 2001). The rat crossed two different beam shapes: a 1-meter square (2 cm × 2 cm) and a round (2 cm diameter) bar. The score ranged from 0 to 2. Briefly, crossing half bar without legs equals 0.5 points and crossing the whole bar equals 1.0 point. If the rat used one leg, 0.5 was added and if the rat used both, 1.0 was added. The time course of the study was exactly the same as previous tests (i.e., at 7, 14, 21 and 28 days post-injury).
Immunohistochemistry
Single labeling studies were performed to identify fibers associated with 5-hydroxytryptamine (5-HT), reactive astrocytes and macrophages in spinal cord of injured rats (n = 3) treated with PP2, PP3 and DMSO. To euthanize the rats, pentobarbital (40–50 mg/kg) was injected intraperitoneally. This was followed by a transcardial perfusion, initiated with ice-cold phosphate buffered solution (PBS), pH 7.4 (Sigma-Aldrich), followed by ice-cold 4% paraformaldehyde to fix the tissue as described by Rosas et al. (2011). The spinal cords were sectioned at a thickness of 40 μm using a Leica crystat cryocut 1800 (Nussloch, Germany), either transversally or longitudinally.
Sections were washed with 0.01 M PBS at room temperature and post-fixed with 4% paraformaldehyde at 4°C. Also, 0.1% Triton x-100 was added to permeate cell membranes and sections were blocked with 0.01 M PBS/3% bovine serum albumin to reduce non-specific background. To analyze supraspinal axonal outgrowth, a rabbit anti-5-HT polyclonal antibody (1:1,000; Immunostar Inc., Hudson, WI, USA), to study reactive gliosis, a mouse anti-GFAP (1:100; BD Biosciences, Franklin Lakes, NJ, USA) and to determine macrophages at the lesion epicenter, a mouse anti-ED-1 (1:500; Serotec, Raleigh, NC, USA) were used to incubate spinal cord sections (overnight at 4°C). On the following day, slides were washed and then incubated with rhodamine-red donkey anti-rabbit (1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) or ALEXA-488 donkey anti-mouse IgG (1:250; Carlsbad, CA, USA) secondary antibodies for 2 hours at room temperature to visualize axonal or neurite fibers, reactive astrocytes and macrophages. Sections incubated with control IgG1 (Cell Signaling, Danver, MA, USA) as well as other sections treated with saline instead of the primary antibody were used as negative controls. Finally, sections were mounted with ProLong ® Antifade Kit (Molecular Probe Invitrogen, Eugene, OR, USA) and the cover slips were fixed with nail polish (Rosas et al., 2011). Images for the immunoreactivity of 5-HT fibers were captured with a Zeiss LSM 5 PASCAL Confocal Microscope system (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA) from areas located 2 mm rostral and caudal to the lesion epicenter. GFAP or ED1 immunoreactive images were obtained with an Axio Observer Z1 Zeiss Fluorescence microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA). At least three sections per animal (n = 3), rostral and caudal within the lesion epicenter (5 mm), were analyzed with Adobe Photoshop® CS5 (Adobe system Inc., San Jose, CA, USA) to determine the intensity of the signal. The images, taken at the same magnification (40×) and illumination parameters, were divided with a standard grid of 8 × 10 and each block/quadrant with immunofluorescence was counted. GFAP and ED1 immunofluorescence were determined with ImageJ 1.43u® software (Wayne Rashband, NIH, USA) to quantify the intensity of the signal. Figures were prepared and assembled with Adobe Photoshop and the photographs in each figure were edited equally.
Luxol fast blue/cresyl violet histochemistry
Several sections (3–5) that cover an area of 5 mm within the lesion epicenter, from each animal (n = 3), were stained with cresyl violet (Sigma-Aldrich) to visualize nuclei and with luxol fast blue (Luxol Fast Blue, Alfa Aesar, Heysham, Lancashire, UK) for myelin as previously described (Figueroa et al., 2006; Santiago et al., 2009). The selection of sections in this range of 5 mm within the lesion epicenter provides an overview of the area and any effect of PP2 in white matter spared tissue. Once the counter-stain assay was done, the sections were visualized using a Digital Microscope (Fisher Scientific, Pittsburgh, PA, USA) and photomicrographs were taken using the Motic version 1.2 professional software. The stained spinal cord sections were morphometrically analyzed with ImageJ 1.43u® software (Wayne Rashband) to determine the extent of white matter spared tissue. To calculate the area of remnant tissue, the outer border of the spinal cord and the inner border of the lesion cavity, including the remainder of the grey matter, were delineated and the redundant area was substracted from the total (outer border) area.
Protein extraction, immunoprecipitation and western blot assays
Immunoprecipitation and western blots assays were used to determine the effect of PP2 on ephexin1 phosphorylation (activation). Pentobarbital (40–50 mg/kg) was injected intraperitoneally to euthanize the rats (n = 4) at 4 days post-injury because this time point is the peak of ephexin activation after SCI (Rosas et al., 2011). Then, transcardial perfusion was made with 0.01 M ice-cold PBS, pH 7.4 (Sigma-Aldrich). The spinal cords containing approximately 5 mm of the lesion epicenter were dissected and removed. Protein extraction and immunoprecipitation protocols were conducted as described by Rosas et al. (2011). Briefly, 86.5 μg of protein extract was mixed overnight with 40 μL of protein G sepharose™ beads (Amersham Biosciences, Uppsia, Sweden) and 5 μL of antibody against phospho-ephexin1 (GLLY(PO4)QEYRDKST – NCBI References Sequence: 001104784; GenScript, Piscataway, NJ, USA). The immunocomplex (G-bead/Antibody/Phospho-ephexin1) was centrifuged and the pellets were resuspended in Lammeli Sample Buffer (Bio-Rad, Hercules, KY, USA). A 10% SDS-PAGE, followed by western blot assay was used to analyze the effect of PP2 and PP3 on Ephexin phosphorylation from the immunoprecipitated extracts. Antibody against ephexin1 (rabbit anti-ephexin polyclonal antiserum (1:1,000; ECM Biosciences, Versailles, KY, USA)) was used to identify the amount of ephexin from the immunoprecipitate complex. HRP signal was enhanced with SuperSignal West Dura extended version (Pierce, Rockford, IL, USA). The development and analysis of the nitrocellulose membranes were performed in the Versadoc™ Imaging System and Quantity One Software (Bio-Rad). To standardize phospho-ephexin1 protein levels, GAPDH (monoclonal anti-GAPDH 1:5,000; Sigma-Aldrich) was utilized as a loading control to analyze the initial extract used for immunoprecipitation assay.
Statistical analysis
Two-way analysis of variance was used to determine the effect of PP2 on functional locomotor recovery at different time points after SCI. Pairwise comparison of the means was made using Bonferroni post hoc test to analyze the data and compare the values of PP2 treated rats versus the rats treated with PP3 or DMSO. Similar analyses were used for the other behavioral tests. Moreover, one-way analysis of variance followed by Bonferroni post hoc test was used to compare the effect of PP2 versus PP3 or DMSO at 28 days post-injury on the amount of spared tissue and serotonin-stained fibers to analyze axonal outgrowth, on the amount of glial fibrillary acidic protein expresion to study the gliotic response and on ED-1 immunoreactivity to determine the amount of macrophage cells. In addition, paired two-tailed t-test was used to demonstrate statistical difference for the effect of PP2 on ephexin phosphorylation (activation) versus the control group (PP3) through SFK. Error bars showed the standard error of the mean (SEM) and statistical tests were performed at a pre-set alpha of 0.05. All statistical tests were performed with the GraphPad Prism® Version 5.0a for MAC (GraphPad Software, San Diego, CA, USA).
Results
Functional locomotor recovery
The BBB open field test and the beam crossing test were used to assess the recovery of locomotor activity in injured rats after treatment with PP2, PP3 or DMSO. In the BBB open field test (Figure 1A), rats treated with PP2 (a Src family kinase inhibitor) achieved higher BBB scores than those treated with PP3 (an inactive analog used as a control) at 7 (P < 0.05), 14 (P < 0.05) and 28 (P < 0.01) days post-injury or DMSO (control) at 7 (P < 0.01), 14 (P < 0.001), 21 (P < 0.01) and 28 (P < 0.001) days post-injury. However, BBB scores of the PP2 treated group were lower than those of the sham group (at 7, 14, 21 and 28 (P < 0.001) days post-injury). Two-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(3,140) = 148.18; P < 0.0001)
Figure 1.
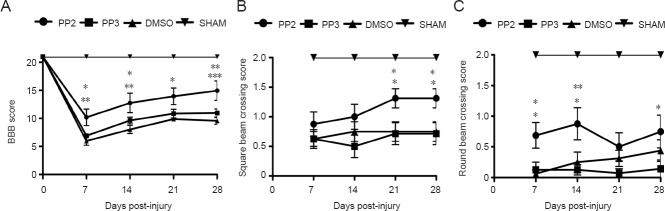
Behavioral tests demonstrating an increased locomotor activity of rats with spinal cord injury after treatment with PP2, a blocker of Src family kinases.
(A) Statistical analysis showed a significant increase in the Basso, Beattie, and Bresnahan (BBB) open field test score in PP2-treated rats compared to DMSO-treated rats (at 21 days post-injury, *P < 0.05; at 7 and 14 days post-injury, **P < 0.01; at 28 days post-injury, ***P < 0.001). Also an increase was observed between PP2-treated and PP3-treated rats (at 7 and 14 days post-injury, *P < 0.05; at 28 days post-injury, **P < 0.01). Two-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(3,140) = 148.18; P < 0.0001) (n = 8). (B) Spinal cord injury (SCI) rats treated with PP2 exhibit an improvement in balance agility. Statistical analysis in the square bar test showed an improvement in balance in rats treated with PP2 compared to those treated with PP3 and DMSO (at 21 and 28 days post-injury, *P < 0.05). (C) Statistical analysis in the round test showed significant difference (at 7 and 28 days post-injury, *P < 0.05; at 14 days post-injury, **P < 0.01) between PP2-treated and PP3-treated rats, and a significant difference between PP2-treated and DMSO-treated rats at 7 and 14 days post-injury (*P < 0.05) (n = 8). Two-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied in the square test (F(3,110) = 69.58, P < 0.0001) and the round test (F(3,110) = 126.87, P < 0.0001). Error bars show the standard error of the mean (SEM). PP2: 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine; PP3: 4-amino-7-phenylpyrazolo[3,4-d]pyramidine (the inactive analog of PP2); DMSO: dimethyl sulfoxide. SHAM: Sham group (rats received only T10 laminectomy, without contusion).
The beam crossing test was divided in two assays, using square and round bars. PP2-treated rats showed a higher capacity for crossing both bars than control (DMSO and PP3) groups. In the square bar test, rats treated with PP2 showed an increase in score compared with PP3 or DMSO groups at 21 and 28 days post-injury (P < 0.05; Figure 1B). In the round beam test, PP2 group also demonstrated an increase in score compared with PP3 (7 (P < 0.05), 14 (P < 0.01) and 28 (P < 0.05) days post-injury) or DMSO (7 and 14 (P < 0.05) days post-injury) (Figure 1C). Rats treated with PP2 were able to cross the round beam without paw positioning but maintaining some balance by the first 2 weeks. This pattern was also observed in the 4th week, where significant difference was observed in both beams, but more prominent with the square beam crossing test. Sham groups, as expected, achieved higher scores in both tests compared to PP2, PP3 and DMSO groups (P < 0.001). Two-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied in square (F(3,110) = 69.58; P < 0.0001) and round tests (F(3,110) = 126.87; P < 0.0001).
Spared tissue, axonal outgrowth, astrocytic response, and infiltration of cells
Histological studies of white matter spared tissue were done with luxol blue/cresyl violet staining. Stained spinal cord sections (within 5 mm of the lesion epicenter) treated with the SFK inhibitor, PP2, showed an elevated amount of spared tissue and reduced size of the lesion cavity, relative to DMSO and PP3 control groups (Figure 2A). Statistical analysis (Figure 2B) showed that PP2-treated group displayed more white matter spared tissue than PP3 (P < 0.05) and DMSO (P < 0.001) groups at 28 days post-injury (n = 3). One-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(2,6) = 31.72, P = 0.0006).
Figure 2.
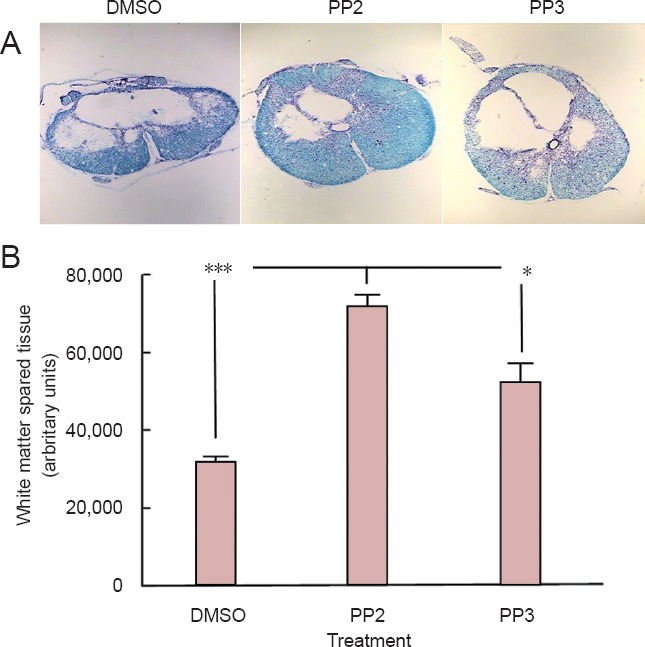
A blocker of Src family kinases PP2 treatment increased white matter spared tissue after spinal cord injury.
(A) Histological studies with luxol fast blue staining performed on representative spinal cord sections harvested within the 5 mm lesion epicenter in rats treated with PP2, PP3 and DMSO (top: dorsal region; bottom: ventral region). (B) Densitometric analysis demonstrated increased white matter spared tissue at 28 days post-injury in rats treated with PP2 compared to PP3 and DMSO treatment (*P < 0.05, ***P < 0.001) (n = 3). One-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(2,6) = 31.72, P = 0.0006). Error bars show the standard error of the mean (SEM). PP2: 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine; PP3: 4-amino-7-phenylpyrazolo[3,4-d]pyramidine (the inactive analog of PP2); DMSO: dimethyl sulfoxide.
Anatomical assays using an antibody against serotonin (5-HT) illustrated the presence of fibers caudal (approximately 2 mm) to the lesion epicenter (Figure 3A) in PP2 group (35.67 ± 0.8819) compared to PP3 (P < 0.01, 13.67 ± 2.333) and DMSO (P < 0.001, 10.33 ± 3.180 (Figure 3B)) groups at 28 days post-injury (n = 3). Control experiments performed with IgG1 or saline, instead of the primary antibody against 5-HT, did not show any immunoreactivity (data not shown). One-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(2,6) = 34.80, P = 0.0005).
Figure 3.
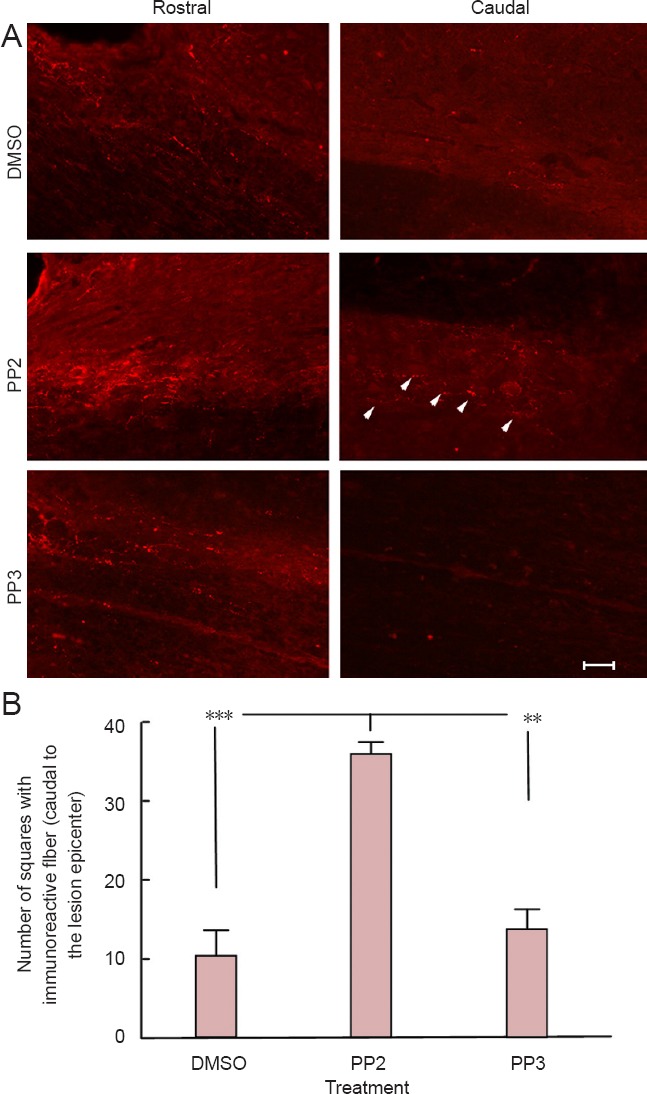
A blocker of Src family kinases PP2 increased serotonin immunoreactivity caudal to the lesion epicenter after spinal cord injury.
(A) Immunoreactive fibers for serotonin (5-hydroxytryptamine) (white arrowheads) were observed caudal (approximately 2 mm) to the lesion epicenter in PP2-treated rats but very few in PP3-treated and DMSO-treated rats. Scale bar: 30 μm. (B) Statistical analysis shows an increase in 5-hydroxytryptamine fiber density caudal to the lesion epicenter in PP2-treated rats (**P < 0.01 for PP3-treated rats and ***P < 0.001 for DMSO-treated rats) (n = 3). One-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(2,6) = 34.80, P = 0.0005). Error bars show the standard error of the mean (SEM). PP2: 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine; PP3: 4-amino-7-phenylpyrazolo[3,4-d]pyramidine (the inactive analog of PP2); DMSO: dimethyl sulfoxide.
Immunohistochemistry studies were used to identify reactive astrocytes (GFAP) and macrophages (ED1) infiltration to the lesion epicenter (within 5 mm of the lesion epicenter). In both cases, no significant differences were observed between groups at 28 days post-injury. For GFAP (Figure 4) and ED1 analyses (Figure 5), one-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied [GFAP: (F(2,9) = 0.1948, P = 0.864); ED1: (F(2,9) = 0.2224, P = 0.8048)]. Sections treated with control IgG1 or saline did not present any immunoreactive signal in the GFAP or ED1 experiments (data not shown), confirming the specificity of the signal obtained with the primary antibodies.
Figure 4.
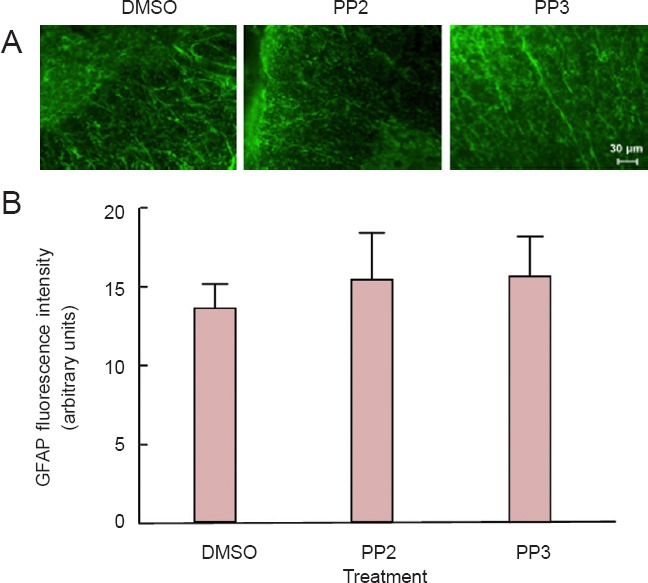
Astrogliosis immunoreactivity was not altered after a blocker of Src family kinases PP2 treatment in injured spinal cords.
(A) GFAP immunoreactivity was used to identify reactive astrocytes within the 5 mm lesion epicenter from PP2-, PP3- and DMSO-treated rats. Scale bar: 30 μm. (B) PP2 treatment for 28 days did not exert any differences in the amount of reactive astrocytes to the spinal cord after trauma. Densitometric analysis of confocal images demonstrated no significant differences between groups (n = 3). For GFAP analysis, one-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(2,9) = 0.1948, P = 0.864). Error bars show the standard error of the mean (SEM). GFAP: Glial fibrilary acidic protein; PP2: 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine; PP3: 4-amino-7-phenylpyrazolo[3,4-d]pyramidine (the inactive analog of PP2); DMSO: dimethyl sulfoxide.
Figure 5.
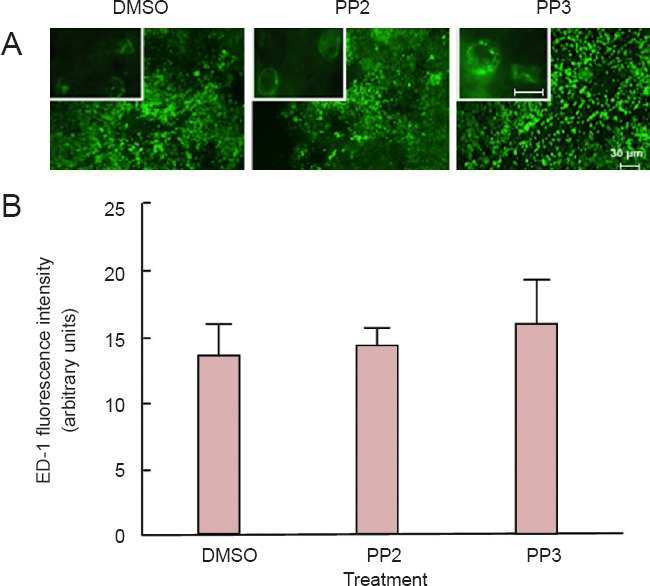
Macrophage immunoreactivity was not altered after a blocker of Src family kinases PP2 treatment in injured spinal cords.
(A) ED-1 immunoreactive cells were not affected in injured spinal cords treated with PP2 compared to PP3 or DMSO-treated spinal cords. Scale bar: 30 μm. Insert shows a high magnification picture (63×) of a microglia at the lesion epicenter in each treatment. Scale bar: 10 μm. (B) PP2 treatment for 28 days did not exert any differences in the amount of infiltrated macrophages in the injured spinal cord, compared to PP3 and DMSO treatment. Densitometric analysis of confocal images demonstrates no significant differences between groups (n = 3). For ED-1 analysis, one-way analysis of variance followed by Bonferroni post hoc test was used to determine the significant differences among samples studied (F(2,9) = 0.2224, P = 0.8048). Error bars show the standard error of the mean (SEM). PP2: 4-amino-5-(4-chlorophenyl)-7- (t-butyl)pyrazolo[3,4-d]pyramidine; PP3: 4-amino-7-phenylpyrazolo[3,4-d]pyramidine (the inactive analog of PP2); DMSO: dimethyl sulfoxide.
Ephexin phosphorylation
Ephexin1 was investigated as a possible target of SFK activation. Immunoprecipitation assays demostrated that PP2 treatment affected ephexin1 phosphorylation at 4 days post-injury. The 4 days post-injury time point was used since previous studies demonstrated a peak of ephexin phosphorylation at this stage (Rosas et al., 2011). The antibody against the ephexin1 phosphorylated at Tyr 87 showed a unique band at 80 kDa in the injured spinal cord treated with PP3 (Figure 6A). Densitometry analysis (Figure 6B) demonstrated a significant reduction at 4 days post-injury (P < 0.05) of ephexin1 phosphorylation in rats treated with PP2 versus those treated with PP3, confirming that ephexin1 is a substrate of Src family kinases. A t-test was used to determine the significant difference among the studied groups (P = 0.0465, t = 3.278, df = 3, paired two-tailed t-test, n = 4).
Figure 6.
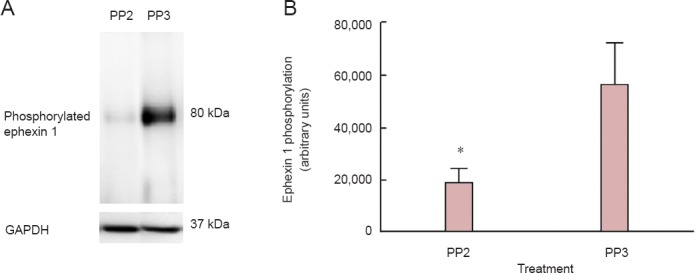
A blocker of Src family kinases PP2 treatment after spinal cord injury reduced ephexin 1 phosphorylation.
(A) Immunoprecipitated ephexin1 after spinal cord injury in rats treated with PP2 presented a reduced immunoreactive band without significant change in GAPDH levels representing a decrease in activity. (B) Densitometry analysis demonstrated a significant decrease at 4 days post-injury (*P < 0.05) between PP2- and PP3-treated (the inactive analog of PP2; control group) rats, confirming the blockade of ephexin activation by PP2 after spinal cord injury. Paired two-tailed t-test was used to determine the significant differences among samples studied (P = 0.0286, n = 4). Error bars show the standard error of the mean (SEM). PP2: 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine; PP3: 4-amino-7-phenylpyrazolo[3,4-d]pyramidine.
Discussion
In the present study, we demonstrated that blockade of SFK by PP2 after SCI resulted in an improved functional locomotor recovery using two behavioral assays. Moreover, the amount of spared tissue and serotonin fibers increased when PP2 was administered to injured rats. We also examined the levels of reactive gliosis and the amount of microglia at the lesion epicenter but failed to find significant changes. The results obtained also suggest that ephexin1 (a guanine nucleotide exchange factor) could be a substrate of this family of kinases, which may contribute to the repulsive environment generated after trauma. Taken together, these results support our hypothesis that SFK activation after SCI plays a role in the microenvironment for axonal outgrowth, sprouting, and/or cell survival.
We focused on altering SFK, which may modulate growth cone activity, neurite extension and synapse formation using an SFK inhibitor. PP2 is an inhibitor that effectively blocks SFK activity, in vivo and in vitro, but recent data suggest that it could be classified as a non-selective blocker (Brandvold et al., 2012). Published reports established that PP2 is an inhibitor for the SFK that blocks Src, Lck, Fyn and Hck (Hanke et al., 1996; Bain et al., 2007), with some inhibitory activity on kinases like CK1, RIP2, GAK and the c-terminal Src kinase. Permeation of PP2 into the spinal cord was not investigated in this study, but other investigators published that PP1 (a similar hydrophobic compound) penetrates well the central nervous system (Paul et al., 2001). Studies on SCI, brain trauma, cerebral ischemia (transient or permanent) and/or a stroke utilized intraperitoneal injections of PP1 or PP2 and obtained positive results, suggesting that these compounds have the ability to cross the blood-brain barrier (Paul et al., 2001; Akiyama et al., 2004; Lennmyr et al., 2004; Jadhav et al., 2007; Liu et al., 2014). However, we could not discard the possibility that PP2 also enters the spinal cord because a compression injury to this tissue may generate some damage to the blood-brain barrier permitting its entrance.
Long-term administration of PP2 enhanced locomotor activity (Figure 1), spared tissue (Figure 2) and axonal outgrowth and/or sprouting (Figure 3). An improvement in functional locomotor activity of rats treated with PP2 was confirmed by increased BBB score (Figure 1A) and beam crossing-tests (square and round (Figure 1B and 1C)) when compared to control groups (PP3 and DMSO). Long-term neurological recovery after closed head injury was also observed when male mice were treated with PP2 (Schumann et. al., 2008). Anatomical and histological results support the improvement seen in hindlimb locomotion activity of the behavioral analysis, because rats treated with PP2 for 28 days (long-term treatment) presented more white matter spared tissue (Figure 2) than PP3 or DMSO groups. Also, we observed more 5-HT fibers (Figure 3) caudal to the lesion epicenter in the PP2 treated group after 4 weeks of SFK blockade suggesting axonal outgrowth and/or sprouting. Similar results were observed in the acute phase after trauma when PP1 (another SFK inhibitor) was administered to rats with mild spinal cord compression injury (Akiyama et al., 2003). They observed some motor function improvement and reduction in the area of edema within the first 2 weeks.
In the BBB open field test, we observed a significant improvement in locomotor function at 7 days post-injury and the differences of scores (rate of change) between PP2-treated group and DMSO-treated or PP3-treated group were similar between 7 and 21 days. However, the rate of change in motor function changed between 21 and 28 days post-injury when rats treated with PP2 were compared to control groups (DMSO or PP3), suggesting a chronic effect on locomotor recovery in this stage after treatment. The beam crossing test results suggest that long-term blockade of SFK significantly improves equilibrium and body balance in the acute and chronic phases after SCI. Considerably, higher scores were obtained by rats treated with PP2 in the round beam test, without falling (with partial paw placement or without paw placement) compared to control groups at the beginning (7–14 days after SCI). These results were also observed at the 4th week with the square beam test and suggest that inhibition of SFK could have an effect on the vestibulospinal and propioception related tracts, improving body posture, balance, and equilibrium. Therefore, our results confirmed those findings obtained by Akiyama et al. (2003) in the acute phase after SCI. Moreover, our results confirmed a role of SFK activity in the chronic phase of the pathophysiology generated after SCI in adult rats since long-term blockade of this kinase (28 days) improved locomotor behavior, spared tissue and neurite outgrowth.
Activation of c-Src in reactive astrocytes has been observed after cortical lesions (Gangoso et al., 2012). The results obtained at 28 days post-injury were different than expected. Rats treated with PP2 at 28 days post-injury presented an increased spared tissue (Figure 2) without a change in reactive gliosis immunoreactivity (Figure 4) compared with those treated with PP3 and DMSO after injury. These results suggest that SFK activity is not involved in cyst formation or in the reactive gliosis differentiation.
Injury to the spinal cord promotes macrophage infiltration (Schwartz et al., 2006) as a part of the inflammatory responses to remove debris after the physical insult. We studied this cellular process to determine if SFK activity plays a role in macrophage infiltration to the spinal cord after trauma. However, immunohistochemistry analysis did not demonstrate significant change at 28 days post-injury (Figure 5). On the other hand, previous studies demonstrated a reduction in macrophage infiltration to the lesion epicenter after administration of PP1 at 3 and 7–8 days post-injury, which is important for neuroprotection (Akiyama et al., 2003, 2004). This discrepancy could be possible because macrophages mainly infiltrate the lesion epicenter between day 3 and day 8 (Popovich et al., 1997), and we studied the amount of macrophages at 28 days post-injury. Any change in the infiltration of macrophages by PP2 probably already occurred in the first week after trauma.
The immunoprecipitation analysis demonstrated that ephexin-1 is a possible target for Src family kinases. Moreover, previous studies demonstrated the presence of ephexin1 at the lesion epicenter and its activity (phosphorylation) altered at different time points after SCI (2, 4 and 14 days post-injury) (Rosas et al., 2011). In addition, in vitro reports demonstrated that ephexin1 phosphorylation retracts neurites (Shamah et al., 2001), promotes axonal growth cone collapse (Sahin et al., 2005), and regulates dendritic spine retraction (Fu et al., 2007a). Ephexin1 function is mediated when EphA receptors interact with any of its ligands (ephrinA1–A5 or ephrin B1–B3) (Klein, 2009) or after the activation of the fibroblast growth factor receptor-EphA4 heterocomplex (Zhang et al., 2007). Once the Eph-ephrin interaction takes place, EphA receptor dimerizes and an autophosphorylation occurs. Then, it recruits other proteins such as Src tyrosine kinase (Sahin et al., 2005), Cdk5 (Fu et al., 2007b) and ephexin1 (Sahin et al., 2005).
Immunoprecipitation studies were performed, as a first approach, to demonstrate that PP2 decreases ephexin1 phosphorylation after intraperitoneal injection. The result confirmed that PP2 is effective against ephexin1 phoshorylation/activation (Figure 6), as has been reported for the effect of PP2 on N-methyl-D-aspartarte receptor subunit NR2B in the hippocampus after closed head injury (Schumann et al., 2008). Phosphorylation of ephexin1 plays a critical role in the induction of axonal growth cone collapse because it activates preferentially RhoA, which causes cytoskeletal changes (Shamah et al., 2001; Knoll and Drescher, 2004; Sahin et al., 2005) and this cascade of intracellular events may inhibit axonal outgrowth and/or synapse formation. In our experimental model, long-term blockade of SFK activity after SCI with PP2 ensured a reduction in the level of ephexin1 activation (phosphorylation) for 28 days and this may result in a decrease in RhoA activity (Sahin et al., 2005). Consequently, supraspinal axons may sprout in our contusion model resulting in the formation of synapse that improved the locomotor activity of the injured animals. Therefore, a possible Eph-ephrin interaction is mediated through ephexin after SCI that triggers a cellular response, generating a non-permissive environment for functional locomotor recovery.
A previous report has demonstrated that ephexin1 is expressed in neurons and axons (Rosas et al., 2011) that contain EphA3, A4 and A7. Therefore, if ephexin1 phosphorylation (activation) is decreased by PP2 (a SFK inhibitor) for 28 days, more neurons and axons can extend their neurites and growth cone bulbs, suggesting fiber sprouting at the lesion epicenter. However, we could not discard the possibility of SFK and/or ephexin1 role in synapse remodeling after SCI (Frank et al., 2009; Shi et al., 2010a, b) or regulation of vascular permeability in the injured central nervous system (Eliceiri et al., 1999; Paul et al., 2001; Akiyama et al., 2003, 2004).
In conclusion, this study provides information regarding the behavioral, anatomical/cellular and biochemical effect of PP2 treatment after SCI. These findings suggest that SFK initiates a cascade of events that block locomotor recovery. Our study also point out that ephexin1 phosphorylation (activation) by SFK action may be involved in the repulsive microenvironment generated after SCI. The results obtained cannot exclude the possibility that SFK regulates axonal outgrowth and locomotor recovery through modulation of the phosphorylation of other substrates and/or intermediates that may phosphorylate ephexin1. While Bain et al. (2007) published in vitro studies reporting that other kinases (CK1, RIP2, GAK and the c-terminal Src kinase) may be blocked by PP2, published in vivo studies in models of trauma, focal or global brain ischemia point to the inactivation of Src-kinase family by PP2 (Lennmyr et al., 2004; Hou et al., 2007; Liu et al., 2014). Therefore, future experiments should combine a group of kinase inhibitors to dissect in detail the kinases involved in ephexin phosphorylation. The locomotor recovery observed after PP2 treatment is not complete due to additional inhibitory proteins and factors that block axonal regeneration. More studies are needed to elucidate the intracellular cascade of events initiated by these kinases.
Acknowledgments:
This research project is in partially fulfillment of Odrick R. Rosas doctoral thesis dissertation. We acknowledge Dr. Luz C. Arocho for her technical support during the surgeries. Also, we would like to recognize Dr. Martine Behra and Luis Colón for their support in the capture of images with the Zeiss fluorescent microscope (obtained with the grant 1P30NS069258-01) and Dr. Alan Preston for his editorial revision.
Footnotes
Funding: The project was partially supported by the MBRS-RISE Program (R25 GM061838), MBRS-SCORE (SO6-GM08224), COBRE (5P20-GM103642), SNRP (NS39405) and RCMI (8G12MD007600).
Conflicts of interest: None declared.
Copyedited by Mladinic M, Li CH, Song LP, Zhao M
References
- 1.Akiyama C, Yuguchi T, Nishio M, Tomishima T, Fujinaka T, Taniguchi M, Nakajima Y, Kohmura E, Yoshimine T. Src family kinase inhibitor PP1 reduces secondary damage after spinal cord compression in rats. J Neurotrauma. 2004;21:923–931. doi: 10.1089/0897715041526230. [DOI] [PubMed] [Google Scholar]
- 2.Alluin O, Delivet-Mongrain H, Gauthier MK, Fehlings MG, Rossignol S, Karimi-Abdolrezaee S. Examination of the combined effects of chondroitinase ABC Growth Factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain J, Plater L, Elliot M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23 doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 6.Brandvold KR, Steffey ME, Fox CC, Soellner MB. Development of a highly selective c-Src kinase inhibitor. ACS Chem Biol. 2012;7:1393–1398. doi: 10.1021/cb300172e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Kim H, Chung J, Chun M, Kim SY, Yoon S, Lee M. Activation of Src Tyrosine kinase in microglia in the rat hippocampus following transient forebrain ischemia. Neurosci Lett. 2005;380:1–5. doi: 10.1016/j.neulet.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Orengo L, Figueroa JD, Velazquez I, Torrado A, Ortiz C, Hernandez C, Puig A, Segarra AC, Whittemore SR, Miranda JD. Blocking EphA4 upregulation after spinal cord injury results in enhanced chronic pain. Exp Neurol. 2006;202:421–433. doi: 10.1016/j.expneurol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 10.Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa JD, Benton RL, Velazquez I, Torrado AI, Ortiz CM, Hernandez CM, Diaz JJ, Magnuson DS, Whittemore SR, Miranda JD. Inhibition of EphA7 up-regulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J Neurosci Res. 2006;84:1438–1451. doi: 10.1002/jnr.21048. [DOI] [PubMed] [Google Scholar]
- 13.Fiorgione N, Fehlings MG. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014;82 doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Fitch MT, Silver J. CNS injury glial scars and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor ephexin Cdc42 and CaV2. 1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007a;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, Bikoff JB, Lai KO, Yung WH, Fu AK, Greenberg ME, Ip NY. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007b;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- 18.Gangoso E, Ezan P, Valle-Casuso JC, Herrero-Gonzalez S, Koulakoff A, Medina JM, Giaume C, Tabernero A. Reduced connexin43 expression correlates with c-Src activation, proliferation, and glucose uptake in reactive astrocytes after an excitotoxic insult. Glia. 2012;60:2040–2049. doi: 10.1002/glia.22418. [DOI] [PubMed] [Google Scholar]
- 19.Gilmore ES, Stutts MJ, Milgram SL. Src family kinases mediate epithelial Na+ channel inhibition by endothelin. J Biol Chem. 2001;276:42610–42617. doi: 10.1074/jbc.M106919200. [DOI] [PubMed] [Google Scholar]
- 20.Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Grados-Munro EM, Fournier AE. Myelin-associated inhibitors of axon regeneration. J Neurosci Res. 2003;74:479–485. doi: 10.1002/jnr.10803. [DOI] [PubMed] [Google Scholar]
- 23.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- 24.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel potent and Src family-selective tyrosine kinase inhibitor Study of Lck- and Fyn T-dependent T cell activation. J Biol Chem. 1996;271 doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 25.Hou X, Liu Y, Zhang G. PP2 a potent inhibitor of Src kinases protects against hippocampal CA1 pyramidal cell death after transient global brain ischemia. Neurosci Lett. 2007;420:235–239. doi: 10.1016/j.neulet.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Jadhav V, Matchett G, Hsu PK, Zhang JH. Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. J Neurosurg. 2007;106:680–686. doi: 10.3171/jns.2007.106.4.680. [DOI] [PubMed] [Google Scholar]
- 27.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 28.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 29.Knoll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of non-invasive Pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 2009;27:1–44. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshmi S, Joshi PG. Activation of Src/kinase/phospholipase C/mitogen activated protein kinase and induction of neurite expression byATP independent of nerve growth factor. Neuroscience. 2006;141:179–189. doi: 10.1016/j.neuroscience.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 32.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting inNogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennmyr F, Ericsson A, Gerwins P, Akterin S, Ahlstrom H, Terént A. Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol Scand. 2004;110:175–179. doi: 10.1111/j.1600-0404.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- 34.Liang S, Pong K, Gonzalez C, Chen Y, Ling H, Mark RJ, Boschelli F, Boschelli DH, Ye F, Barrios-Sosa AC, Mansour TS, Frost P, Wood A, Pangalos MN, Zaleska MM. Neuroprotective profile of novel Src kinase inhibitors in rodent models of cerebral ischemia. J Pharmacol Exp Ther. 2009;331:827–835. doi: 10.1124/jpet.109.156562. [DOI] [PubMed] [Google Scholar]
- 35.Lin B, Xu Y, Zhang B, He Y, Yan Y, He MC. Mek inhibition reduces glial scar formation and promotes the recovery of sensorimotor function in rats following spinal cord injury. Exp Ther Med. 2014;7:66–72. doi: 10.3892/etm.2013.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu da Z, Sharp FR, Van KC, Ander BP, Ghiasvand R, Zhan X, Stamova B, Jickling GC, Lyeth BG. Inhibition of Src family kinases protects hippocampal neurons and improves cognitive function after traumatic brain injury. J Neurotrauma. 2014;31:1268–1276. doi: 10.1089/neu.2013.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 38.Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J, Whittemore SR. Induction of Eph B3 after spinal cord injury. Exp Neurol. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- 40.Niclou SP, Ehlert EM, Verhaagen J. Chemorepellent axon guidance molecules in spinal cord injury. J Neurotrauma. 2006;23:409–421. doi: 10.1089/neu.2006.23.409. [DOI] [PubMed] [Google Scholar]
- 41.Ohta M, Anklesaria P, Wheaton M, Ohara A, Pierce JH, Holland CA, Greenberger JS. Retroviral src gene expression in continuous marrow culture increases the self-renewal capacity of multineage hematopoetic stem cells. Leukemia. 1989;3:206–226. [PubMed] [Google Scholar]
- 42.Oudega M, Bradbury EJ, Ramer MS. Combination therapies. Handb Clin Neurol. 2012;109:617–636. doi: 10.1016/B978-0-444-52137-8.00038-3. [DOI] [PubMed] [Google Scholar]
- 43.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 44.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.Robles E, Woo S, Gomez TM. Src-dependent tyrosine phosphorylation at the tips of growth filopodia promotes extension. J Neurosci. 2005;25:7669–7681. doi: 10.1523/JNEUROSCI.2680-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosas OR, Figueroa JD, Torrado AI, Rivera M, Santiago JM, Konig-Toro F, Miranda JD. Expression and activation of ephexin is altered after spinal cord injury. Dev Neurobiol. 2011;71:595–607. doi: 10.1002/dneu.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE, Greenberg ME. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 48.Santiago JM, Rosas O, Torrado AI, Gonzalez MM, Kalyan-Masih PO, Miranda JD. Molecular, anatomical, physiological, and behavioral studies of rats treated with buprenorphine after spinal cord injury. J Neurotrauma. 2009;26:1783–1793. doi: 10.1089/neu.2007.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumann J, Alexandrovich GA, Biegon A, Yaka R. Inhibition of NR2B Phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J Neurotrauma. 2008;25:945–957. doi: 10.1089/neu.2008.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz M, Yoles E. Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J Neurotrauma. 2006;23:360–370. doi: 10.1089/neu.2006.23.360. [DOI] [PubMed] [Google Scholar]
- 51.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 52.Shi L, Butt B, Ip FC, Dai Y, Jiang L, Yung WH, Greenberg ME, Fu AK, Ip NY. Ephexin1 is required for structural maturation and neurotransmission at the neuromuscular junction. Neuron. 2010a;65:204–216. doi: 10.1016/j.neuron.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi L, Fu AK, Ip NY. Multiple roles of the Rho GEF ephexin1 in synapse remodeling. Commun Integr Biol. 2010b;3:622–624. doi: 10.4161/cib.3.6.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 55.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Hu J, She Y, Smith GM, Xu XM. Cortical PKC inhibition promotes axonal regeneration of the corticospinal tract and forelimb functional recovery after cervical dorsal spinal hemisection in adult rats. Cereb Cortex. 2014;24:3069–3079. doi: 10.1093/cercor/bht162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinasec-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama K, Tezuka T, Hoshina N, Nakazawa T, Yamamoto T. Phosphorylation at Tyr-694 of Nogo-A by Src-family kinases. Biochem Biophys Res Comm. 2006;349:1401–1405. doi: 10.1016/j.bbrc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Young W. Spinal cord regeneration. Cell Transplant. 2014;23:573–611. doi: 10.3727/096368914X678427. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Sawada T, Jing X, Yokote H, Yan X, Sakaguchi K. Regulation of ephexin1 a guanine nucleotide exchange factor of Rho family GTPases by fibroblast growth factor receptor-mediated tyrosine phosphorylation. J Biol Chem. 2007;282:31103–31112. doi: 10.1074/jbc.M704430200. [DOI] [PubMed] [Google Scholar]
- 61.Zhao H, Cao X, Wu G, Loh HH, Law P. Neurite outgrowth is dependent on the association of c-Src and lipid rafts. Neurochem Res. 2009;34:2197–2205. doi: 10.1007/s11064-009-0016-7. [DOI] [PubMed] [Google Scholar]
- 62.Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]


