Abstract
Melatonin has been shown to diminish edema in rats. Melatonin can be used to treat spinal cord injury. This study presumed that melatonin could relieve spinal cord edema and examined how it might act. Our experiments found that melatonin (100 mg/kg, i.p.) could reduce the water content of the spinal cord, and suppress the expression of aquaporin-4 and glial fibrillary acidic protein after spinal cord injury. This suggests that the mechanism by which melatonin alleviates the damage to the spinal cord by edema might be related to the expression of aquaporin-4 and glial fibrillary acidic protein.
Keywords: nerve regeneration, spinal cord injury, melatonin, edema, aquaporin, glial fibrillary acidic protein, immunohistochemistry, western blot assay, neural regeneration
Introduction
Every year between 11.5 and 53.4 million people in the world are reported to suffer from spinal cord injury (SCI), which harms the autonomic, motor and sensory functions (Nakanishi et al., 2014; Wang et al., 2014). A patient's quality of life is strongly associated with the degree of the injury to the spine. Currently, there is no good way to treat SCI, so it is important to investigate possible targets in the field of basic medicine for SCI (Wang et al., 2007; Zhang et al., 2014). However, the complexity of the biological and cytological processes of repairing the spinal cord poses many challenges.
SCI is correlated with spinal cord edema, which if unchecked can lead to severe damage and death (Leypold et al., 2008; Fujiki et al., 2014). Previous studies have proved that the outcomes after SCI are affected greatly by the progress of spinal cord edema, and an ideal strategy includes a reduction of spinal cord edema in vivo (Verkman et al., 2011; Yang et al., 2011). Spinal cord edema is often long lasting and therapy resistant and thus poses a main challenge for neurosurgeons (Pierrefiche et al., 1993; Tan et al., 1993; Reiter et al., 1995; Wang et al., 2009).
So far, there is no particular medicine for treating SCI. Although there has been much research, until now the only clinical pharmacological treatment to improve the neurological dysfunction following SCI was methylprednisolone, which had to be administered within 8 hours of SCI (Bracken et al., 1990, 1992, 1997, 1998). However, the side effects of methylprednisolone make its use to treat SCI controversial.
In many cell types, aquaporins have a significant function in transporting water (Oshio et al., 2004; Saadoun et al., 2010). Previous research has confirmed that the changes in aquaporin-4 are strongly linked with the blood-spinal cord barrier and its permeability in spinal edema. However, if aquaporin-4 expression changes in the injured spinal cord, this will cause changes in water content in the spinal cord (Xu et al., 2008; Wang et al., 2009). Astrocyte swelling plays an important role in cellular edema after acute SCI. Glial fibrillary acidic protein (GFAP) is a specific marker of astrocytes.
Melatonin (N-acetyl-5-methoxytryptamine), a major secretory product from the pineal gland, not only acts as a synchronizer for the circadian rhythm but also has powerful antioxidant activities. The effect of melatonin on spinal cord edema is poorly understood. This study sought to survey the underlying function of melatonin (100 mg/kg) on spinal edema in a rat model of SCI and the mechanisms of that melatonin action.
Materials and Methods
Animals
The Severe Wound and Trauma Laboratory of General Hospital of Shenyang Military Area Command of Chinese PLA, Shenyang, China (license No. SYXK (Liao) 2006-0002) provided 150 clean, healthy, female, Sprague-Dawley rats aged 10–11 weeks and weighing 250 ± 22 g. Animal experiments were approved by the Ethics Committee of General Hospital of Shenyang Military Area Command of Chinese PLA in China. Every effort was made to reduce pain and as few rats as possible were used during experiments. The rats were kept four to a cage. Rats were given water and food libitus under conditions of 12-hour light/dark cycles at 26 ± 2°C.
Model establishment
Rats were anesthetized with chloral hydrate (300 mg/kg, i.p.) and they breathed air naturally and evenly. The rats were prone on the operating table. A medial dorsal incision was made. A laminectomy was conducted. T12 vertebra and the dura were intact. Bone wax and a bipolar coagulator were used to stop the bleeding. The thoracic spinal cord was clipped with an aneurysm clip for 5 minutes at room temperature. After removing the clip carefully, silk stitches were used to sew the paravertebral fascia and the skin respectively. Those rats that had complications were not included in the experiment. The healthy adult rats were carefully observed after surgery, and placed in separate cages. The rats were supplied with food and water. Their urinary bladders were pressed three times every day.
Animal groups
The 150 rats were equally and randomly divided into three groups. The sham surgery group only received laminectomy without dural compression. The SCI group received laminectomy after SCI and the rats were injected with normal saline i.p. instantly after injury for 2 days. Melatonin group received laminectomy after SCI and the rats were administered 100-mg/kg melatonin (Solarbio Biotechnology, Beijing, China) i.p. promptly after SCI for 2 days (Wu et al., 2014). The melatonin was dissolved in anhydrous ethanol, and then diluted with normal saline to a concentration of 12.5 mg/mL. Five rats from each group were used to analyze the water content in the spinal cord. An additional five rats from each group were used for western blot assays, which were performed separately at 12, 24, 48, and 72 hours post SCI (Ma et al., 2010). After the operation, five rats from each group were selected for immunohistochemical staining at 24 and 72 hours.
Measurement of water content in the spinal cord
Spinal edema was evaluated by determining the water content in the spinal cord. At 12, 24, 48, and 72 hours after surgery, spinal cords were removed from five rats in each group. One-cm lengths of crushed spinal cord were weighed for their wet weights. Then the injured spinal cord was dried at 80°C for 48 hours so that water content could be calculated by wet weight – dry weight/wet weight × 100%.
Immunohistochemical staining
Immunohistochemical staining was used to explore the levels of aquaporin-4 and GFAP in the spinal cord 24 and 72 hours after it was injured. Rats were anesthetized, perfused transcardially with saline, and fixed with 4% paraformaldehyde. The spinal cord was exposed from vertebrae T10 to L1. A 1-cm long segment was obtained and placed at 4°C for 24 hours, dehydrated and immersed in paraffin. Tissue at 3 mm above the center of the injured spinal cord was sliced into 10-μm axial sections with a microtome. These sections were treated with 3% H2O2 for 15 minutes to block endogenous peroxidase, incubated with primary antibody rabbit anti-aquaporin-4 polyclonal antibody (1:150; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) or mouse anti-GFAP monoclonal antibody (1:400; Beijing Biosynthesis Biotechnology Co., Ltd.) at 4°C overnight. The sections were washed with PBS, and then incubated with secondary antibody goat anti-rabbit IgG (1:400; Beijing Biosynthesis Biotechnology Co., Ltd.) for 15 minutes at 37°C. Sections were visualized using diaminobenzidine, and observed under a BX43 microscope (× 200; Olympus, Tokyo, Japan). The number of positive cells in a 190 μm × 340 μm rectangular area of the gray matter was counted by two observers independent of the experimenters.
Western blot assay
At 12, 24, 48 and 72 hours after injury, changes in aquaporin-4 and GFAP were observed by western blot assay. One-cm lengths of spinal cord were homogenized in radioimmune precipitation assay buffer, and then centrifuged at 17,000 × g for 15 minutes at 4°C. The Coomassie G250 binding method was used to determine the protein concentration of the soluble material. The protein lysates were transferred to polyvinylidene fluoride membranes (Beijing Biosynthesis Biotechnology Co., Ltd.). The membranes were blocked with 5% skim milk for 2 hours, and then incubated with polyclonal antibody rabbit anti-rat aquaporin-4 (1:400; Solarbio Biotechnology, Beijing, China), rabbit anti-rat GFAP (1:200; Solarbio Biotechnology) or β-actin (1:2,000; Solarbio Biotechnology). Samples were then incubated with goat anti-rabbit lgG (1:2,000; Beijing Biosynthesis Biotechnology Co., Ltd.) for 2 hours at room temperature. Aquaporin-4, GFAP and β-actin antibody bands were tracked by the EC3, an image-forming system (UVP Inc., Upland, CA, USA). The optical density was measured by ImageJ software (NIH, Bethesda, MD, USA). The expression levels of aquaporin-4 and GFAP in each group were determined. The value of the protein was normalized as its ratio to the sham.
Statistical analysis
The data were expressed by the mean ± SD, and analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance and the least significant difference test were used to determine the difference in water content. P < 0.05 was considered statistically significant.
Results
Melatonin effects on water content in rats with spinal cord injury
Compared with the sham surgery group, the water content was significantly higher in the SCI group (P < 0.05). Compared with the SCI group, the water content was significantly lower in the melatonin group at 24, 48 and 72 hours after surgery (P < 0.05). However, the water content in the spinal cord did not change significantly at 12 hours after injury (Figure 1).
Figure 1.
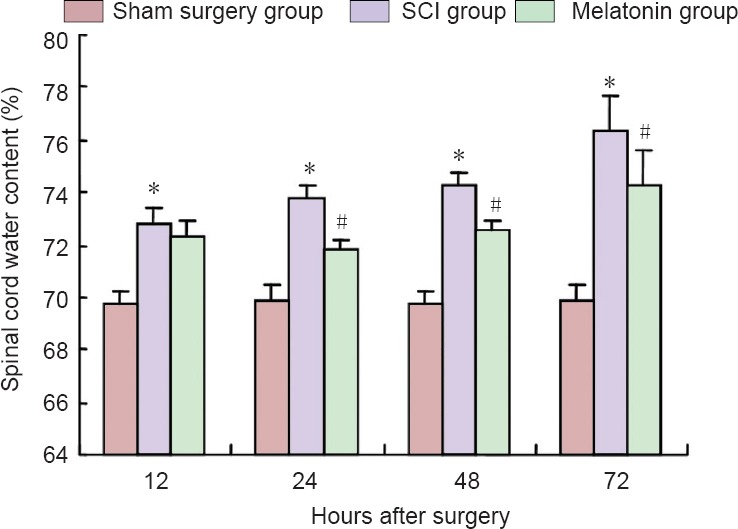
Melatonin effects on water content in rats with spinal cord injury.
*P < 0.05, vs. sham surgery group, #P < 0.05, vs. SCI group. Data are expressed as the mean ± SD (n = 5; one-way analysis of variance and the least significant difference test). SCI: Spinal cord injury.
Melatonin effects on aquaporin-4 expression
Immunohistochemical staining showed strong immunoreactivity in the gray matter. As shown in Figure 2, the number of aquaporin-4-immunoreactive cells was significantly higher compared with the sham surgery group at 24 and 72 hours after surgery (P < 0.05). However, there were fewer aquaporin-4-immunoreactive cells in the melatonin group than in the SCI group (P < 0.05). Figure 3 shows representative western blots for aquaporin-4 (34 kDa) and β-actin (42 kDa). The levels of aquaporin-4 to β-actin were quantified and normalized.
Figure 2.
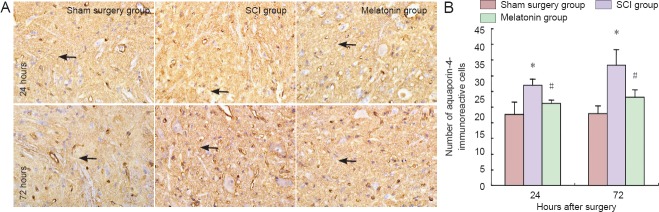
Changes of aquaporin-4-immunoreactive cells at 24 and 72 hours after injury.
(A) Immunohistochemical staining, optical microscope, × 200. (A) Arrows: Aquaporin-4-immunoreactive cells. (B) Changes in the number of aquaporin-4-immunoreactive cells. *P < 0.05, vs. sham surgery group; #P < 0.05, vs. SCI group. Data are expressed as the mean ± SD (n = 5, one-way analysis of variance and the least significant difference test). SCI: Spinal cord injury.
Figure 3.
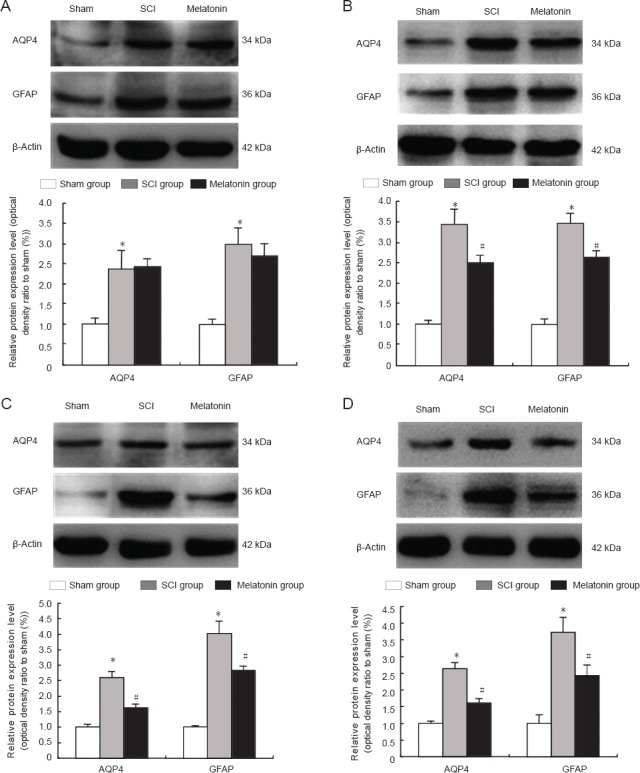
Western blot assay for AQP4 and GFAP protein levels at 12 (A), 24 (B), 48 (C) and 72 hours (D) after injury.
The graph showed significant up-regulation of AQP4 and GFAP expression in the SCI group at 12, 24, 48 and 72 hours after SCI. Treatment of melatonin (100 mg/kg) could markedly decrease AQP4 and GFAP levels in the spinal cord at 24, 48 and 72 hours after injury. *P < 0.05, vs. sham surgery (sham) group; #P < 0.05, vs. SCI group. Data are expressed as the mean ± SD (n = 5, one-way analysis of variance and the least significant difference test). SCI: Spinal cord injury; AQP4: aquaporin-4; GFAP: glial fibrillary acidic protein.
A western blot assay showed a low level of aquaporin-4 expression in the sham surgery group. Aquaporin-4 expression significantly increased at 12, 24, 48 and 72 hours after injury in the model group (P < 0.05; Figure 3A–D). Compared with the SCI group, aquaporin-4 expression was significantly lower in the melatonin group at 24, 48 and 72 hours after injury (P < 0.05, Figure 3B–D). No significant difference in aquaporin-4 expression was detected between the SCI group and melatonin group at 12 hours (P > 0.05; Figure 3A).
Effects of melatonin on GFAP expression
GFAP-positive cells in the SCI group were more numerous than in the sham surgery group at 24 and 72 hours after surgery. Conversely, the quantity of GFAP-immunoreactive cells was significantly lower in the melatonin group compared with the SCI group (P < 0.05; Figure 4).
Figure 4.
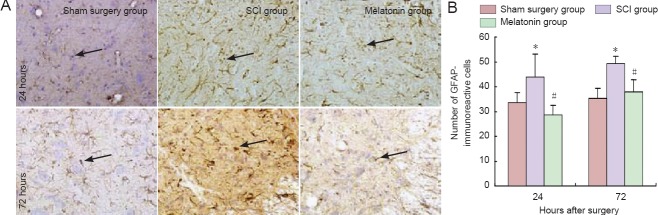
Changes in the expression of GFAP-immunoreactive cells at 24 and 72 hours after injury. (A) Immunohistochemical staining (optical microscope, × 200). Arrows: GFAP-immunoreactive cells. (B) Changes in the number of GFAP-immunoreactive cells. *P < 0.05, vs. sham surgery group; #P < 0.05, vs. SCI group. Data are expressed as the mean ± SD (n = 5; one-way analysis of variance and the least significant difference test). SCI: Spinal cord injury; GFAP: glial fibrillary acidic protein.
A western blot assay showed that aquaporin-4 expression was low in the sham surgery group, but increased significantly at 12, 24, 48 and 72 hours after injury (P < 0.05; Figure 3A–D). Compared with the SCI group, the level of GFAP was significantly lower in the melatonin group at 24, 48 and 72 hours after injury (P < 0.05; Figure 3B–D). However, there was no significant difference between the SCI and melatonin groups for values of GFAP or aquaporin-4 at 12 hours after injury (P > 0.05; Figure 3A).
Discussion
Many previous studies have confirmed the effect of melatonin (50 or 100 mg/kg) on treating SCI (Liu et al., 2004; Esposito et al., 2009; Lee et al., 2011). Gül et al. (2005) studied the effects of different doses of melatonin in rats with SCI. Their study used melatonin at 50 and 100 mg/kg to treat SCI in rats, and found that the 100 mg/kg dose was more effective than the 50 mg/kg dose.
There are two studies that show highly beneficial effects of melatonin on SCI (Fujimoto et al., 2000; Kaptanoglu et al., 2000). Both studies used similar weight drop injury models, but Fujimoto et al. used 50 g-cm, and Kaptanoglu et al. used 25 g per 10 minutes. Additionally, drug dosages were very different in these studies: 100 and 2.5 mg/kg. Fujimoto et al. concluded that melatonin has significant protective effects mainly on myelin sheaths, but also on the nucleus and mitochondria. Similarly, Kaptanoglu et al. suggested that melatonin not only has considerable protective effect against oxidative damage, but also reduces neutrophil-induced toxicity. A variety of SCI models is have been used in experiments; the clip compression model has advantages over the weight drop model. The implementation of anterior and lateral spinal cord compression simulated vertebra corpus fractures in humans (Fujimoto et al., 2000; Kaptanoglu et al., 2000).
This study reveals that melatonin (100 mg/kg) is a effective therapy for treating. The results demonstrated that the use of melatonin for treating SCI decreased water content, aquaporin-4 and GFAP levels in the spine.
SCI leads to disability, sensory disorder and even death (Samantaray et al., 2008). To minimize or prevent these outcomes, we need to understand the pathophysiology of acute SCI to find effective therapeutic interventions. SCI starts with mechanical compression of the spinal cord. The spinal cord will present dropsy, microvascular permeability changes, and altered blood flow (Rowland et al., 2008; Tator et al., 1991). Traumatic injury of the spinal cord will always induce edema, mainly in the gray matter of the spinal cord (Shepard et al., 1999; Oshio et al., 2004). Subsequent pathophysiological injuries cause apoptosis (Ray et al., 2003). The active and progressive damage will soon spread and will continue for some time after the initial injury (Park et al., 2010). The present study in rats examined the protective effects of melatonin (100 mg/kg) on treating edema from 12 to 72 hours after SCI.
The water content of the spinal cord invariably increases after SCI but it could be reduced significantly after 24 and 72 hours after injury by early administration of melatonin (100 mg/kg). However, the effects of melatonin were not seen at 12 hours after injury. Our results demonstrated that long-term use of melatonin could decrease the spinal cord edema. As we described previously, the pathophysiology of SCI could be classified into primary or secondary damage. Primary damage consists of microvascular bleeding, which can increase water content in the spinal cord. The spinal cord water content at 12 hours after SCI may be affected by both primary and secondary damages, but the treatment with melatonin cannot mitigate the primary damage. The action of melatonin needs further investigation to optimize its therapeutic value.
Immunohistochemistry and western blot assay were used to study aquaporin-4 and GFAP protein levels after SCI. Aquaporin-4 is a member of the aquaporin family and is widely expressed in the nervous system. Previous studies showed that aquaporin-4 colocalized with GFAP using double labeling examination with confocal microscopy (Hsu et al., 2011; Tourdias et al., 2011). Other studies showed that aquaporin-4 can be found around capillaries in the human brain. Some researchers found intense aquaporin-4 staining in the spinal cord (Hsu et al., 2011; Tourdias et al., 2011). There was a strong correlation between aquaporin-4 content and the extent of spinal cord edema (Badaut et al., 2011). The studies showed that aquaporin-4 existed in the glial cells of the gray matter and glial foot processes. Recently, some studies applied immunohistochemistry and western blot assay to reveal that the expression of that aquaporin-4 increased significantly from 2 to 3 days after SCI (Kimura et al., 2010; Hemley et al., 2013).
Melatonin (100 mg/kg) can reduce edema. Our results show that melatonin (100 mg/kg) has a beneficial, anti-edema effect from 24 to 72 hours after SCI and that the level of aquaporin-4 decreases, which suggests that a lower level of aquaporin-4 expression may be linked to the decrease in spinal cord edema in rats.
Cellular swelling is significant after the central nervous system is injured and astrocytic swelling was more apparent than neuronal swelling (Marmarou et al., 2014). Our results showed that melatonin (100 mg/kg) reduced GFAP protein levels in the spinal cord 2–3 days after SCI. Melatonin effectively removes astrocytic swelling caused by SCI. In short, in the injured spinal cord segments, the expression of aquaporin-4 and GFAP increases. Melatonin (100 mg/kg) administered after SCI only reduces aquaporin-4 and GFAP levels in spinal cord segments between 24 and 72 hours after SCI. The results of this experiment show that melatonin (100 mg/kg) can ameliorate edema after SCI. The lowered edema may reduce the level of expression of aquaporin-4. The reduction in the expression of GFAP may also help melatonin (100 mg/kg) to reduce astrocytic swelling after SCI. This study of melatonin has shown worthwhile benefits, but the effects of melatonin in SCI patients will require further investigations.
Acknowledgments:
We would like to thank all the staffs from Laboratory of Severe and War-Related Trauma Center, General Hospital of Shenyang Military Area Command of Chinese PLA, Shenyang, China for providing technical support and guidance.
Footnotes
Funding: This study was supported by the China Postdoctoral Science Foundation Grant, No. 2014M552692.
Conflicts of interest: None declared.
Copyedited by Dawes E, Stow A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Badaut J, Ashwal S, Obenaus A. Aquaporins in cerebrovascular disease: a target for treatment of brain edema. Cerebrovasc Dis. 2011;31:521–531. doi: 10.1159/000324328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bracken MB, Shepard MJ, Collins WF. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 3.Bracken MB, Shepard MJ, Collins WF., Jr Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second national acute spinal cord injury study. J Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 4.Bracken MB, Shepard MJ, Holford TR. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 5.Bracken MB, Shepard MJ, Holford TR. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1- year follow up. Results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 6.Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J Pineal Res. 2009;46:79–86. doi: 10.1111/j.1600-079X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujiki M, Furukawa Y, Kobayashi H, Abe T, Ishii K, Uchida Kamida T. Geranylgeranylacetone limits secondary injury, neuronal death, and progressive necrosis and cavitation after spinal cord injury. Brain Res. 2005;1053:175–184. doi: 10.1016/j.brainres.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto T, Nakamura T, Ikeda T, Takagi K. Potent protective effects of melatonin on experimental spinal cord injury. Spine. 2000. 2000;25:769–775. doi: 10.1097/00007632-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gül Ş, Çelik SE, Kalaycı M, Taşyürekli M, Cokar N, Bilge T. Dose-dependent neuroprotective effects of melatonin on experimental spinal cord injury in rats. Surg Neurol. 2005;64:355–361. doi: 10.1016/j.surneu.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Hemley SJ, Bilston LE, Cheng S, Chan JN, Stoodley MA. Aquaporin-4 Expression in Post-Traumatic Syringomyelia. J Neurotrauma. 2013;30:1457–1467. doi: 10.1089/neu.2012.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu MS, Seldin M, Lee DJ, Seifert G, Steinhäuser C, Binder DK. Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus. Neuroscience. 2011;178:21–32. doi: 10.1016/j.neuroscience.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaptanoglu E, Tuncel M, Palaoglu S, Konan A, Demirpençe E, Kilinç K. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J Neurosurg. 2000;93:77–84. doi: 10.3171/spi.2000.93.1.0077. [DOI] [PubMed] [Google Scholar]
- 13.Kimura A, Hsu M, Seldin M, Verkman AS, Scharfman HE, Binder DK. Protective role of aquaporin-4 water channels after contusion spinal cordinjury. Ann Neurol. 2010;67:794–801. doi: 10.1002/ana.22023. [DOI] [PubMed] [Google Scholar]
- 14.Lee JK, Kwak HJ, Piao MS, Jang JW, Kim SH, Kim HS. Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta neurochirurgica. 2011;153:1321–1329. doi: 10.1007/s00701-010-0889-x. [DOI] [PubMed] [Google Scholar]
- 15.Leypold BG, Flanders AE, Burns AS. The early evolution of spinal cord lesions on MR imaging following traumatic spinal cord injury. AJNR Am J Neuroradiol. 2008;29:1012–1016. doi: 10.3174/ajnr.A0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JB, Tang TS, Yang HL, Xiao DS. Antioxidation of melatonin against spinal cord injury in rats. Chin Med J (Engl) 2004;117:571–575. [PubMed] [Google Scholar]
- 17.Ma L, Chen Y, Sang HF, Xiong LZ. The dose-response relationship between ginsenoside Rd injection and spinal cord ischemic injury in rabbit. Zhonghua Shenjing Waike Jibing Yanjiu Zazhi. 2010;9:422–426. [Google Scholar]
- 18.Marmarou CR, Liang X, Abidi NH, Parveen S, Taya K, Henderson SC, Young HF, Filippidis AS, Baumgarten CM. Selective vasopressin-1a receptor antagonist prevents brainedema reduces astrocytic cell swelling and GFAP V1aR and AQP4 expression after focal traumatic brain injury. Brain Res. 2014;1581:89–102. doi: 10.1016/j.brainres.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi K, Tanaka N, Kamei N, Hiramatsu T, Ujigo S, Sumiyoshi N, Rikita T, Takazawa A, Ochi M. Resection of spinous processes can cause spinal cord injury in patient with ossification of the posterior longitudinal ligament in the thoracic spine. Spinal Cord. 2014;52(Suppl 3):S19–21. doi: 10.1038/sc.2014.151. [DOI] [PubMed] [Google Scholar]
- 20.Oshio K, Binder DK, Yang B, Schecter S, Verkman AS, Manley GT. Expression of aquaporin water channels in mouse spinal cord. Neuroscience. 2004;127:685–693. doi: 10.1016/j.neuroscience.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Park K, Lee Y, Park S, Lee S, Hong Y, Kil Lee S, Hong Y. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J Pineal Res. 2010;48:270–281. doi: 10.1111/j.1600-079X.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 22.Pierrefiche G, Topall G, Courboin G, Henriet I, Laborit H. Antioxidant effect of melatonin in mice. Res Commun Chem Pathol Pharmacol. 1993;80:211–223. [PubMed] [Google Scholar]
- 23.Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- 24.Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995;18:1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 25.Rowland JW, Hawryluk GWJ, Kwon B. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on thehorizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 26.Saadoun S, Papadopoulos MC. Aquaporin-4 in brain and spinal cord oedema. Neuroscience. 2010;168:1036–1046. doi: 10.1016/j.neuroscience.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL. Melatonin attenuates calpain upregulation axonal damage and neuronal death in spinal cord injury in rats. J Pineal Res. 2008;44:348–357. doi: 10.1111/j.1600-079X.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepard MJ, Bracken MB. Magnetic resonance imaging and neurological recovery in acute spinal cord injury: observations from the National Acute Spinal Cord Injury Study 3. Spinal Cord. 1999;37:833–837. doi: 10.1038/sj.sc.3100927. [DOI] [PubMed] [Google Scholar]
- 29.Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 30.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 31.Tourdias T, Mori N, Dragonu I, Cassagno N, Boiziau C, Aussudre J, Brochet B, Moonen C, Petry KG, Dousset V. Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J Neuroinflammation. 2011;8:143. doi: 10.1186/1742-2094-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verkman AS, Ratelade J, Rossi A, Zhang H, Tradtrantip L. Aquaporin-4: orthogonal array assembly, CNS functions, and role in neuromyelitis optica. Acta Pharmacol. 2011;32:702–710. doi: 10.1038/aps.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Zhu Q, Man X, Guo L, Hao L. Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury. Neural Regen Res. 2014;15:1678–1687. doi: 10.4103/1673-5374.141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Cao X, Nagel DJ, Yin G. Activation of ASK1 during reperfusion of ischemic spinal cord. Neurosci lett. 415:248–252. doi: 10.1016/j.neulet.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 35.Wang YF, Gu YT, Xu WB, Lv G. Temporary loss of perivascular aquaporin-4 in white matter after the spinal cord ischemic injury of rats. Mol Neurosci. 2009;20:145–149. doi: 10.1097/WNR.0b013e32831c6c44. [DOI] [PubMed] [Google Scholar]
- 36.Wu Q, Jing Y, Yuan X, Zhang X, Li B, Liu M, Wang B, Li H, Liu S, Xiu R. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J Mol Neurosci. 2014;54:714–722. doi: 10.1007/s12031-014-0430-4. [DOI] [PubMed] [Google Scholar]
- 37.Xu WB, Gu YT, Wang YF, Lu XH, Jia LS, Lv G. Bradykinin preconditioning modulates aquaporin-4 expression after spinal cord ischemic injury in rats. Brain Res. 2008;1246:11–18. doi: 10.1016/j.brainres.2008.09.087. [DOI] [PubMed] [Google Scholar]
- 38.Yang YB, Piao YJ. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol. 2011;24:703–710. [PubMed] [Google Scholar]
- 39.Zhang N, Yin Yi, Xu SJ, Wu YP, Chen WS. (2012) Inflammation and apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]


