Studies on the selective retinal degeneration induced by sodium iodate (NaIO3) date back to 1941; Sorsby (1941) described the effect of intravenously injected NaIO3 solution on the rabbit retina. Since then, NaIO3-induced retinal degeneration has been described in different mammalian species including sheep, rabbit, rat and mouse with varying doses and routes of administration. At the present time, the murine NaIO3 model is the most widely used because it results in reproducible, patchy retinal degeneration (Figure 1) and can be studied in a wide variety of wild type and genetic knockout strains. Many studies reporting the pathologic changes in retinal structure at specific time points and electrophysiological changes in retinal function over time have followed (Franco et al., 2009; Redfern et al., 2011). More recently, the model has gained a resurgence of interest because of the ability to utilize high resolution in vivo imaging to study structural changes in the retina in live animals. Techniques such as spectral domain optical coherence tomography (OCT) and confocal scanning laser ophthalmoscopy (cSLO) allow longitudinal evaluation of retinal structure (Machalinska et al., 2014; Yang et al., 2014); thus making this model amenable to evaluation of novel therapeutic approaches to retinal degeneration such as cellular therapies.
Figure 1.
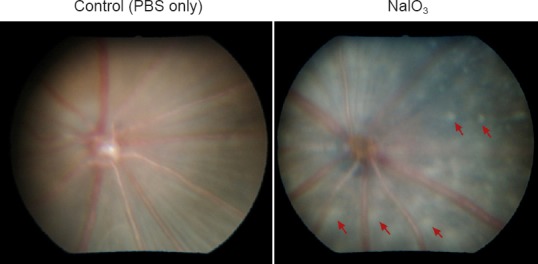
Fundus photograph of murine retina 3 weeks after intravenous injection of NaIO3 (129S6/SvTac strain).
Normal control fundus is shown on the left; fundus of NaIO3 injected animal shows extensive, patchy retinal degeneration (representative lesions labeled with arrows).
The sequence of events leading to retinal damage after NaIO3 administration has been extensively evaluated; however, comparisons of different studies is at times difficult because of differences in the source of NaIO3, methods of administration, animals (type, strain, and age) and time points analyzed after injection. A classic study using high dose NaIO3 (100 mg/kg) reported rapid and selective necrotic cell death of the retinal pigment epithelium (RPE; the layer of pigmented epithelial cells that support the function and survival of the photoreceptors) beginning 6 hours after injection (Kiuchi et al., 2002). Others have evaluated lower doses of NaIO3 treatment and found progressively increasing RPE damage from 15–35 mg/kg (Franco et al., 2009; Machalinska et al., 2014) but no discernible change at 10 mg/kg (Wang et al., 2014). The death of RPE is followed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cell death of photoreceptors, presumably by apoptosis (Kiuchi et al., 2002). Recently, the mechanism of photoreceptor dysfunction and death has been questioned. Carido et al. (2014) demonstrated increased calpain activity, without any increase in caspase 3 activation, 3 days after NaIO3 injection, and suggested that NaIO3 may be triggering a TUNEL-positive, non-apoptotic cell death mechanism in photoreceptors. As well, others suggest a direct effect of NaIO3 on photoreceptors, with visual dysfunction and rapid suppression of photoreceptor genes noted with 20–30 mg/kg intravenous dose in adult C57BL/6J mice (Wang et al., 2014).
The molecular mechanism of RPE damage that occurs after NaIO3 administration is thought to be mediated through induction of oxidative stress. We have shown that murine RPE cultured in the presence of NaIO3 show accumulation of reactive oxygen species (ROS) that co-localizes with mitochondria (Zhou et al., 2014). These results suggest that future studies focused on therapy with antioxidants such as superoxide dismutase could be of considerable interest. With respect to signaling mechanisms, it is known that a rapid activation of AKT and reduction of RPE65 (a RPE-specific protein) occurs in mice subjected to oxidative stress. NaIO3-induced oxidative stress caused inactivation of PTEN and loss of its interactions with junctional proteins suggesting an important role for PTEN in normal RPE cell function as well in response to oxidative stimuli. Another target that has been recently suggested is Nrf2. Sachdeva et al. (2014) showed that Nrf2 signaling is impaired in the aging RPE when an oxidative insult with NaIO3 was imposed. Not only did the RPE of older mice (15 months) show impaired induction of Nrf2 as compared to young (2 months old) mice, they exhibited higher levels of superoxide anion and malondialdehyde. When Keap1, the negative regulator of Nrf2 was conditionally knocked down, the Nrf2 signaling and protection of RPE was restored.
Our laboratory has presented evidence that the small heat shock protein αB crystallin protects the retina from NaIO3 induced retinal degeneration (Zhou et al., 2014). Low dose NaIO3 (20 mg/kg) injection in αB crystallin knockout mice augmented RPE cell death and subsequent retinal degeneration when compared to wild type control mice. Further, using cultured human RPE cells we showed that NaIO3 induced increased AKT phosphorylation and PPARγ expression in RPE cells which could be partially blocked by αB crystallin siRNA knockdown. Our findings suggest that αB crystallin plays a significant role in protection of NaIO3-induced oxidative stress and retinal degeneration in part through upregulation of AKT phosphorylation and PPARγ expression. We are currently evaluating the potential of αB crystallin or αB crystallin peptide nanoparticles as a therapeutic approach for treatment of retinal degeneration.
The NaIO3 model has emerged as a valuable model to study RPE regeneration and cellular therapies in the retina. Machalinska et al. (2014) have shown that regeneration of the RPE occurs 3 months after induction of low dose sodium iodate induced retinal degeneration. Carido et al. (2014) transplanted human embryonic stem cell (hESC)-derived RPE cells 1 week after a single intravenous injection of NaIO3 (70 mg/kg) to mice. While NaIO3 injection caused severe RPE cell loss, photoreceptor degeneration and altered gene and protein expression in the inner nuclear layer (INL) and outer nucler layer (ONL) of the retina, transplantation of donor hESC-derived RPE cells formed extensive monolayers showing mature RPE cell morphology, organization and function. Furthermore, the transplanted RPE within these monolayers showed RPE-specific functions such as phagocytosis of host photoreceptor outer segments. In addition, hESC-derived RPE produced considerable collagen IV which is a component of the underlying Bruch membrane. Although most studies deal with hESC transplantation, the beneficial role of stem-cell derived neural progenitor cells to restore retinal degeneration in the NaIO3 model has also been reported recently (Amirpour et al., 2012). As well, after transplantation of rat mesenchymal stem cells (with and without erythropoietin gene modification) into the subretinal space of NaIO3 treated rats, the transplanted cells adopted RPE morphology and function 8 weeks after transplantation (Guan et al., 2013).
Many investigators see considerable potential of the NaIO3 model for the study of novel therapies for geographic atrophy, a blinding complication of age-related macular degeneration (AMD) for which there is currently no effective treatment. While no animal model fully recapitulates each of the pathologic features found in the disorder, further refinement of the NaIO3 model to better mimic the defined geographic degeneration seen in AMD is underway in our lab and should represent a valuable advance. Finally, geographic atrophy is a slowly progressive disease occurring in an elderly population. The NaIO3 model is typically performed in young mice; it will be important to evaluate whether aging alters NaIO3 toxicity and sensitivity using older mice in various strains. The advent of high resolution imaging will allow longitudinal studies over extended periods of time and provides impetus for use of imaging endpoints for evaluation of preclinical efficacy of novel therapeutics for geographic atrophy.
This work is supported in part by grants EY01545 and by core grant EY03040, the Arnold and Mabel Beckman Foundation, and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness Inc., New York, NY. We thank Dr. Parameswaran G. Sreekumar for the fundus images in Figure 1.
References
- 1.Amirpour N, Karamali F, Rabiee F, Rezaei L, Esfandiari E, Razavi S, Dehghani A, Razmju H, Nasr-Esfahani MH, Baharvand H. Differentiation of human embryonic stem cell-derived retinal progenitors into retina cells by Sonic hedgehog and/or retinal pigmented epithelium and transplantation into the subretinal space of sodium iodate-injected rabbits. Stem Cells Dev. 2012;21:42–53. doi: 10.1089/scd.2011.0073. [DOI] [PubMed] [Google Scholar]
- 2.Carido M, Zhu Y, Postel K, Benkner B, Cimalla P, Karl MO, Kurth T, Paquet-Durand F, Koch E, Münch TA, Tanaka EM, Ader M. Characterization of a mouse model with complete RPE loss and its use in RPE cell transplantation. Invest Ophthalmol Vis Sci. 2014;55:5431–5444. doi: 10.1167/iovs.14-14325. [DOI] [PubMed] [Google Scholar]
- 3.Franco LM, Zulliger R, Wolf-Schnurrbusch UE, Katagiri Y, Kaplan HJ, Wolf S, Enzmann V. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Invest Ophthalmol Vis Sci. 2009;50:4004–4010. doi: 10.1167/iovs.08-2898. [DOI] [PubMed] [Google Scholar]
- 4.Guan Y, Cui L, Qu Z, Lu L, Wang F, Wu Y, Zhang J, Gao F, Tian H, Xu L, Xu G, Li W, Jin Y, Xu GT. Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr Mol Med. 2013;13:1419–1431. doi: 10.2174/15665240113139990071. [DOI] [PubMed] [Google Scholar]
- 5.Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr Eye Res. 2002;25:373–379. doi: 10.1076/ceyr.25.6.373.14227. [DOI] [PubMed] [Google Scholar]
- 6.Machalińska A, Lejkowska R, Duchnik M, Kawa M, Rogińska D, Wiszniewska B, Machaliński B. Dose-dependent retinal changes following sodium iodate administration: application of spectral-domain optical coherence tomography for monitoring of retinal injury and endogenous regeneration. Curr Eye Res. 2014;39:1033–1041. doi: 10.3109/02713683.2014.892996. [DOI] [PubMed] [Google Scholar]
- 7.Redfern WS, Storey S, Tse K, Hussain Q, Maung KP, Valentin JP, Ahmed G, Bigley A, Heathcote D, McKay JS. Evalauation of a convenient method of assessing rodent visual function in safety pharmacology studies: effects of sodium iodate on visual acuity and retinal morphology in albino and pigmented rats and mice. J Pharmacol Toxicol Methods. 2011;63:102–114. doi: 10.1016/j.vascn.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorsby A. Experimental pigmentary degeneration of the retina by sodium iodate. Br J Ophthalmol. 1941;25:58–62. doi: 10.1136/bjo.25.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Iacovelli J, Spencer C, Saint-Geniez M. Direct effect of sodium iodate on neurosensory retina. Invest Ophthalmol Vis Sci. 2014;55:1941–1952. doi: 10.1167/iovs.13-13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Ng TK, Ye C, Yip YW, Law K, Chan SO, Pang CP. Assessing sodium iodate induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:1696–1705. doi: 10.1167/iovs.13-12477. [DOI] [PubMed] [Google Scholar]
- 12.Zhou P, Kannan R, Spee C, Sreekumar PG, Dou G, Hinton DR. Protection of retina by αB crystallin in sodium iodate induced retinal degeneration. PLoS One. 2014;9:e98275. doi: 10.1371/journal.pone.0098275. [DOI] [PMC free article] [PubMed] [Google Scholar]


