Abstract
Previous experimental studies have shown that cerebral infarction can be effectively reduced following treatment with scutellaria baicalensis stem-leaf total flavonoid (SSTF). However, the mechanism of action of SSTF as a preventive drug to treat cerebral infarction remains unclear. In this study, Sprague-Dawley rats were pretreated with 50, 100, 200 mg/kg SSTF via intragastric administration for 1 week prior to the establishment of focal cerebral ischemia/reperfusion injury. The results showed that pretreatment with SSTF effectively improved neurological function, reduced brain water content and the permeability of blood vessels, ameliorated ischemia-induced morphology changes in hippocampal microvessels, down-regulated Fas and FasL protein expression, elevated the activity of superoxide dismutase and glutathione peroxidase, and decreased malondialdehyde content. In contrast to low-dose SSTF pretreatment, the above changes were most obvious after pretreatment with moderate- and high-doses of SSTF. Experimental findings indicate that SSTF pretreatment can exert protective effects on the brain against cerebral ischemia/reperfusion injury. The underlying mechanisms may involve reducing brain water content, increasing microvascular recanalization, inhibiting the apoptosis of hippocampal neurons, and attenuating free radical damage.
Keywords: nerve regeneration, scutellaria baicalensis stem-leaf total flavonoid, pretreatment, cerebral ischemia/reperfusion, hippocampus, apoptosis, vascular permeability, free radicals, neural regeneration
Introduction
Cerebral ischemia/reperfusion injury is caused by several complex pathogenic mechanisms (Fan et al., 2010; Huang et al., 2011) of which there is no effective treatment. Therefore, studies focusing on identifying potential therapeutic drugs for the treatment of cerebral ischemia/reperfusion injury are of great importance (Wang and Yang, 2012).
Growing evidence from recent studies have revealed good efficacy of Ginkgo biloba leaf (Liu et al., 2013b), Pueraria (Wang, 2013), and other single herbs or Chinese herbal prescriptions (Wu et al., 2011; Liu et al., 2012; Li et al., 2013) for the treatment of neurological impairments caused by cerebral infarction. These Chinese herbs have become the focus of studies addressing treatment of cerebral ischemic injury. Pretreatment with Chinese herbal extracts can block the apoptotic process (Liu et al., 2013a; Mou et al., 2013), accelerate neurologic functional recovery (Wang et al., 2013), reduce infarct volume (Ge et al., 2013), produce an ischemic preconditioning effect, and attenuate ischemia/reperfusion injury (Zhao et al., 2013) in experimental animals. Scutellaria baicalensis stem-leaf total flavonoid (SSTF) is the active ingredient of the aerial part of Radix Scutellariae, which exerts obvious preventive effects on cerebrovascular infarction (Liu et al., 2002; Chen et al., 2010, 2012; Wang et al., 2011; Zheng et al., 2011, 2012; Zhao et al., 2013). However, few efforts have been made to understand the preventive effect of SSTF in the treatment of cerebral ischemia/reperfusion injury. In this study, SSTF was administered to rats prior to focal cerebral ischemia/reperfusion, and microvascular and permeability in the hippocampus, brain water content, Fas and FasL protein expression in hippocampal neurons, and antioxidant changes were monitored in a broader attempt to investigate the mechanisms associated with the protective effect of SSTF.
Materials and Methods
Animals
A total of 144 healthy adult Sprague-Dawley rats, half males and half females, weighing 200 ± 20 g, were provided by the Experimental Animal Center of Hebei Medical University of China (license No. SCXK (Ji) 2008-1-003). The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee from Chengde Medical College in China. Rats were fed for 1 week in ventilated cages at 18–22°C and 30–50% humidity, and had free access to food and water to adapt to the feeding environment. Experimental rats were randomly divided into six groups (n = 24): control group, sham group, model group, and SSTF pretreated groups (low-, moderate-, and high-dose groups).
Administration of SSTF
SSTF was provided by the Provincial Key Laboratory of Chengde Medical College, Institute of Chinese Medicine, China (purity 61.8%). The stem and leaves of the aerial part of Radix Scutellariae were collected from Weichang County, Chengde City, Hebei Province, China, and identified by Professor Huang ZS from Hebei Medical University, China.
The stem and leaves of Radix Scutellariae were crushed, the enzymes were inactivated and the remaining components were dissolved in water. The supernatant was concentrated and dried to prepare the SSTF extract. The total flavonoid content was 56–75% and scutellarin content was 6–18%. Using high-performance liquid chromatography (Shimadzu, Tokyo, Japan), we identified the main chemical components of SSTF, including scutellarin, baicalin, chrysin-7-O-β-D-glucuronide, and isoflavones. The extracts were dissolved in PBS to prepare a 5% (v/v) suspension for further use.
Each rat in the SSTF groups was administrated 50, 100, or 200 mg/kg SSTF per day intragastrically for 1 week before operation. Rats from the sham group and control group were only administrated 10 mL/kg (4 mL) normal saline for 7 days. The drug dose was based on previous findings (Liu et al., 2002; Chen et al., 2010, 2012; Wang et al., 2011; Zheng et al., 2011, 2012; Zhao et al., 2013).
Establishment of cerebral ischemia/reperfusion injury model
Twenty-four hours after the last administration, rats from the model group and the three SSTF groups were subjected to focal cerebral ischemia/reperfusion injury via occlusion of the middle cerebral artery using the modified Longa's suture method (Longa et al., 1989). In brief, the right common carotid artery was cut, and the embolus was inserted, occluding the middle cerebral artery for 2 hours; then the embolus was removed, and blood flow to the middle cerebral artery was returned, followed by another 24 hours of reperfusion. After rats regained consciousness, neurological deficit scores were evaluated based on the Longa 5-point scale (Longa et al., 1989). 0: No neurological deficit symptoms, normal activities; 1: the left forelimb cannot fully extend; 2: rats circling to the left side when walking; 3: rats slumped to the left side upon independent movement; 4: rats cannot spontaneously walk, consciousness level is reduced. Rats were excluded if they had excessive intraoperative bleeding or subarachnoid hemorrhage, but animal numbers were supplemented. In the sham and control groups, the right common carotid artery was only isolated, without suture insertion.
Measurement of vascular permeability and brain water content
After 24-hour reperfusion, six rats in each group were injected with 2% (w/v) Evans Blue (2 mL/kg; Shanghai Yue Tang Biotechnology Co., Ltd., Shanghai, China) via the tail vein, for 2 hours, and then anesthetized with 3% (v/v) pentobarbital sodium (30 mg/kg) and perfused with 200 mL saline. Rats were decapitated and the right brain was harvested to measure the wet weight. Subsequently, the brain was incubated with formamide (1 mL/100 g brain tissue) at 60°C for 24 hours, and centrifuged at 1,000 r/min for 5 minutes. The absorbance value of the supernatant was measured using a spectrophotometer (Shanghai Yuan Analysis Instruments Inc., Shanghai, China) at 632 nm. An Evans Blue standard curve was plotted as previously described (Yao et al., 2009) and Evans Blue content (μg/g) was calculated. The left hemisphere was harvested to measure the wet weight using an electronic analytical balance (Shanghai Precision & Scientific Instrument Factory, Shanghai, China), then the brain was oven dried and weighed again. The water content in the brain was calculated according to the formula: water content (%) = (wet weight – dry weight)/wet weight × 100%.
Quantitative analysis of hippocampal microvessels using tannic acid-ferric chloride staining
After 24 hours of reperfusion, six rats from each group were killed and perfused with tannic acid-ferric chloride as previously described (Zhao and Kong, 2001). Brain tissue was sectioned into 30 μm thick slices, exposing the hippocampal microvessels (Paxinos and Watson, 2005). The microvascular density and microvascular area ratio were quantitatively analyzed utilizing the Mivnt microscopical image analysis system (Optimas, Seattle, WA, USA). Five random sections of each rat were examined at five different visual fields under 100× magnification (Nikon, Tokyo, Japan) to calculate the microvascular density and microvascular area ratio, which were then averaged.
Fas and FasL expression in the hippocampus detected by immunohistochemical staining
After 24 hours of reperfusion, rats were anesthetized with 10% (v/v) chloral hydrate and then fixed in 4% (w/v) paraformaldehyde through cardiac perfusion. The right hemisphere was harvested and sliced into paraffin-embedded sections at a thickness of 5 μm. Slices were blocked with normal goat serum (1:10, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China), and incubated with rabbit anti-rat Fas and FasL polyclonal antibodies (1:100, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at 4°C overnight; and then incubated with biotinylated goat anti-rabbit IgG (1:300, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 12 minutes at room temperature. Horseradish peroxidase-conjugated streptavidin working solution (1:300) was added and incubated for a further 12 minutes. Negative controls were incubated with PBS instead of antibodies. Three random sections from each rat were examined at five different visual fields under 200× magnification. The MiVnt image analysis system was used to count the number of Fas- and FasL-positive cells in the right hippocampus. The results were averaged.
Detection of biochemical indices
After 2 hours of ischemia followed by 24 hours of reperfusion, six rats from each group were sacrificed by decapitation. The ischemic hemisphere was harvested and weighed, then homogenated with cold saline at a ratio of 1:9 (10%), and centrifuged at 4°C, 3,500 r/min, for 10 minutes. The supernatant was collected to detect the following biochemical indices. Serum malondialdehyde content was detected using the thiobarbituric acid assay, serum superoxide dismutase activity was assayed using the xanthine oxidase method, and serum glutathione peroxidase activity was detected using the modified DTNB colorimetric method. All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China.
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA) and expressed as the mean ± SD. Differences among groups were compared using one-way analysis of variance, and paired comparisons between the two groups were performed using the least significant difference test. A P < 0.05 was considered statistically significant.
Results
Effects of different concentrations of SSTF pretreatment on neurological function in rats with cerebral ischemia/reperfusion injury
In the control and sham groups, no rats had neurological deficits. Neurological deficit scores significantly increased after cerebral ischemia/reperfusion injury (model group) (P < 0.01). SSTF pretreatment at different doses significantly decreased neurological deficit scores of rats compared with the model group (P < 0.01 or P < 0.05). In addition, the moderate-, and high-dose SSTF groups had lower neurological deficit scores than the low-dose SSTF group (P < 0.05), and there was no significant difference between the moderate-dose and high-dose SSTF groups (P > 0.05; Figure 1).
Figure 1.
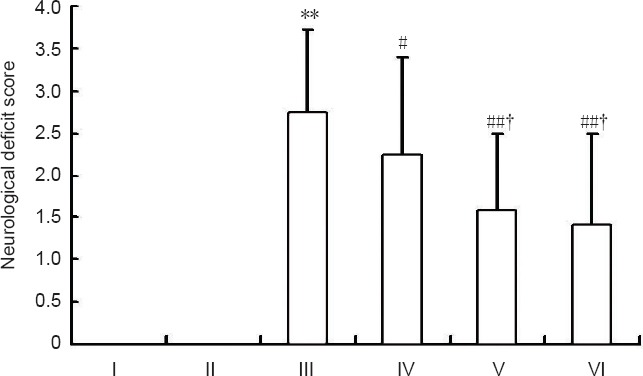
Effects of different concentrations of scutellaria baicalensis stem-leaf total flavonoid (SSTF) pretreatment on neurological deficit scores in rats with cerebral ischemia/reperfusion injury.
The higher scores in the Longa 5-point scale indicates more severe neurological deficits. Data are expressed as the mean ± SD of six rats in each group. Differences among groups were compared using one-way analysis of variance, and paired comparisons between the two groups were performed using the least significant difference test. **P < 0.01, vs. control group/sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, vs. low-dose SSTF group. I–VI: Control, sham, model, low-, moderate-, and high-dose SSTF groups, respectively.
Effect of different concentrations of SSTF pretreatment on brain water content and vascular permeability in rats with cerebral ischemia/reperfusion injury
Brain water content and Evans Blue content of rats from the model group were increased significantly compared with the control group and sham group (P < 0.01), whereas SSTF pretreatment significantly reduced brain water content and Evans Blue content compared with the model group (P < 0.01 or P < 0.05). Furthermore, the moderate- and high-dose SSTF groups had a lower water content than the low-dose SSTF group (P < 0.05). There was no significant difference between the moderate-dose and high-dose SSTF groups (P > 0.05; Figure 2).
Figure 2.
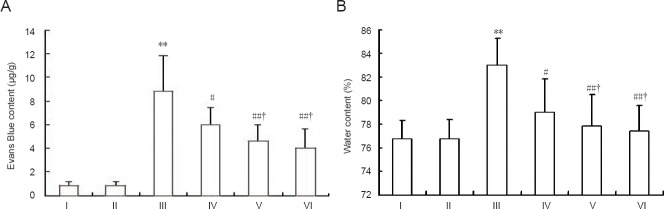
Effect of different concentrations of scutellaria baicalensis stem-leaf total flavonoid (SSTF) pretreatment on Evans Blue content (A) and brain water content (B) in rats with cerebral ischemia-reperfusion injury.
Data are expressed as the mean ± SD of six rats in each group. Differences among groups were compared using one-way analysis of variance, and paired comparisons between the two groups were performed using the least significant difference test. **P < 0.01, vs. control group/sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, vs. low-dose SSTF group. I–VI: Control, sham, model, low-, moderate-, and high-dose SSTF groups, respectively.
Effect of different concentrations of SSTF pretreatment on hippocampal microvessels in rats with cerebral ischemia/reperfusion injury
Tannic acid-ferric chloride staining showed that there were a large number of dense, hollow-shaped microvessels in the hippocampus of rats in the control group and sham group, and large blood vessels elicited branches with abundant capillary networks. In the model group, the number of microvessels was obviously reduced in the hippocampus, only some large vessels with few branches were found. Moreover, microvessels were distorted, rigid or closed, and microvascular density and the microvascular area ratio were significantly decreased (P < 0.05). After pretreatment with different concentrations of SSTF, the microvessels were tubiform and the numbers of microvessels were increased. Compared with the model group, microvascular density and microvascular area ratio were significantly increased in three SSTF groups (P < 0.01 or P < 0.05) in a dose dependent manner (P < 0.05), However, there was no significant difference between the moderate-dose and high-dose SSTF groups (P > 0.05; Figure 3).
Figure 3.
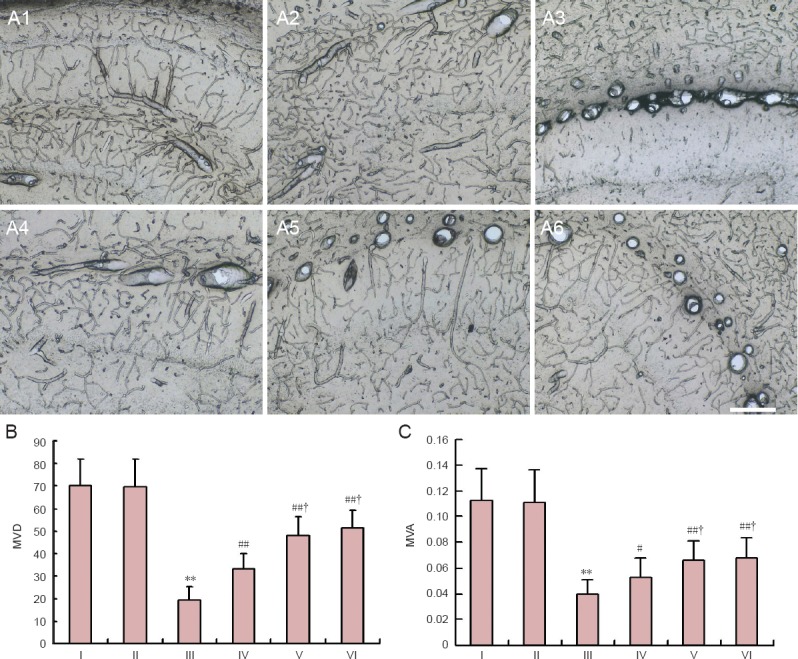
Effect of different concentrations of scutellaria baicalensis stem-leaf total flavonoid (SSTF) pretreatment on microvessels in rats with cerebral ischemia/reperfusion injury.
(A) Effects of different concentrations of SSTF pretreatment on the morphology of microvessels in rats with cerebral ischemia/reperfusion injury (tannic acid-ferric chloride staining, × 100). (B) Effects of different concentrations of SSTF pretreatment on microvascular density (MVD) and microvascular area ratio (MVA) in rats with cerebral ischemia/reperfusion injury. Data are expressed as mean ± SD of six rats in each group. Differences among groups were compared using one-way analysis of variance, and paired comparisons between the two groups were performed using the least significant difference test. **P < 0.01, vs. control group/sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, vs. low-dose SSTF group. I–VI: Control, sham, model, low-, moderate-, and high-dose SSTF groups, respectively.
Effect of different concentrations of SSTF pretreatment on Fas- and FasL-positive expression in the hippocampus of rats with cerebral ischemia/reperfusion injury
Immunohistochemical staining showed that there were no cells in the control and sham groups that stained positive for Fas and FasL expression. In the model group, Fas and FasL were highly and extensively expressed in the hippocampus, mainly in neuronal and glial cells of the cerebral cortex and the hippocampus, and particularly in hippocampal pyramidal neurons that are sensitive to ischemic injury. Compared with the model group, SSTF pretreatment at different concentrations markedly down-regulated Fas and FasL expression in rat hippocampal neurons (P < 0.01 or P < 0.05). The moderate- and high-dose SSTF groups had lower expression levels than the low-dose SSTF group (P < 0.05), and there was no significant difference between the moderate- and high-dose SSTF groups (P > 0.05; Figure 4).
Figure 4.
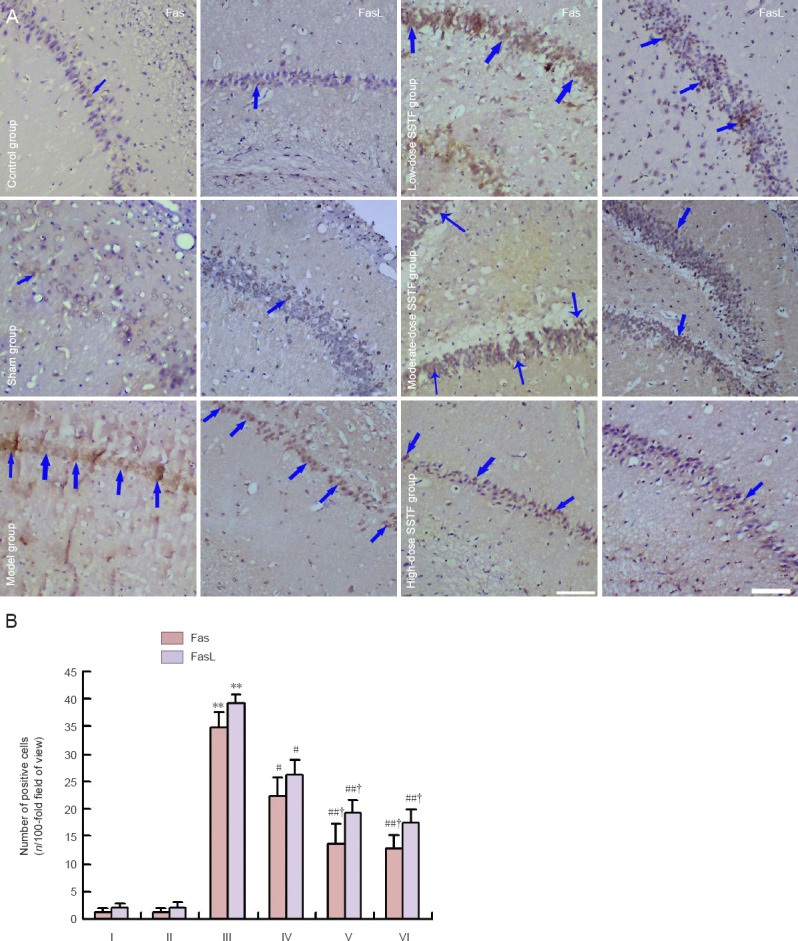
Effects of different concentrations of scutellaria baicalensis stem-leaf total flavonoid (SSTF) pretreatment on Fas and FasL expression in hippocampal neurons following cerebral ischemia-reperfusion injury.
(A) Effects of different concentrations of SSTF pretreatment on Fas and FasL expression in rats with cerebral ischemia-reperfusion injury (immunohistochemical staining, × 200). Fas was expressed in the cytoplasm and cell membrane, and FasL expression was located in the cell membrane. Arrows indicate positive expression. (B) Effects of different concentrations of SSTF pretreatment on the numbers of Fas- and FasL-positive cells in rats with cerebral ischemia-reperfusion injury. Data are expressed as the mean ± SD of six rats in each group. Differences among groups were compared using one-way analysis of variance, and paired comparisons between the two groups were performed using the least significant difference test. **P < 0.01, vs. control group/sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, vs. low-dose SSTF group. I–VI: Control, sham, model, low-, moderate-, and high-dose SSTF groups, respectively.
Effects of different concentrations of SSTF pretreatment on oxidation indices in the ischemic brain of cerebral ischemia/reperfusion injured rats
Superoxide dismutase and glutathione peroxidase activities were normal in brain tissue of rats from the control and sham groups, but were significantly decreased in the model group (P < 0.01, P < 0.05). While malondialdehyde content was significantly increased in the model group (P < 0.01), SSTF pretreatment at different concentrations significantly increased superoxide dismutase and glutathione peroxidase activity when compared with the model group (P < 0.01 or P < 0.05), and decreased malondialdehyde levels (P < 0.01 or P < 0.05). The moderate- and high-dose SSTF groups had higher activities of superoxide dismutase and glutathione peroxidase than the low-dose SSTF group (P < 0.05), while malondialdehyde levels were lower (P < 0.05). There was no significant difference between the moderate-dose and high-dose SSTF groups (P > 0.05; Figure 5).
Figure 5.
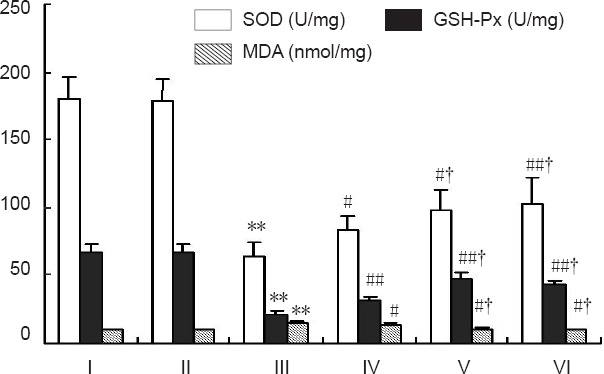
Effect of different concentrations of scutellaria baicalensis stem-leaf total flavonoid (SSTF) pretreatment on oxidative indices in the ischemic brain of cerebral ischemia/reperfusion injured rats.
Data are expressed as the mean ± SD of six rats in each group. The difference among the groups was compared using one-way analysis of variance, and paired comparisons between the two groups were performed using the least significant difference test. **P < 0.01, vs. control group/sham group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, vs. low-dose SSTF group. I–VI: Control, sham, model, low-, moderate-, and high-dose SSTF groups, respectively.
Discussion
SSTF pretreatment protects hippocampal microvessels against ischemia/reperfusion injury
Following cerebral infarction, brain tissue develops edema, and neuronal loss and neurological deficit symptoms are observed due to insufficient blood supply caused by the occlusion of blood vessels (Lee et al., 2000). If blood supply is not promptly restored, irreversible damage occurs. Recanalization of functional microvessels may inhibit the development of brain edema and promote its regression, improve neurological deficit symptoms, and prevent neuronal death (Zhu et al., 2012; Cheng et al., 2013). Microvessels are responsible for tissue-blood exchange, and the microvascular density and microvascular area ratio can reflect the microcirculation material exchange state and local blood flow (Liu et al., 2008), which are critically involved in maintaining the number and functional activity of nerve cells in the brain (Porzionato et al., 2005; Wu et al., 2011). Our findings indicate that SSTF pretreatment contributes to increasing the number of recanalized microvessels, improves microcirculation blood supply, reduces neuronal loss at ischemic area, and promotes neurological recovery after cerebral ischemia and reperfusion.
SSTF pretreatment inhibits pro-apoptotic gene expression and protects hippocampal neurons
After cerebral ischemia and reperfusion were performed, Fas-positive cells were widely distributed in the cerebral cortex and hippocampus, with many neurons and glial cells staining positive, even hippocampal pyramidal cells, which are comparatively sensitive to ischemia (Wang et al., 2005; Chen et al., 2013). Martin-Villalba et al. (1999) found that Fas and FasL were highly expressed in ischemic neurons after middle cerebral artery occlusion in rats, and in vitro application of recombinant FasL triggered apoptosis of primary neurons and neuron-like cells. Clinical and experimental studies have shown that Chinese herbs and compound preparations down-regulate the expression of Fas and FasL, ultimately inhibiting apoptosis of hippocampal neurons following ischemia/reperfusion (Li and Wang caused, 2003; He et al., 2009; Ma et al., 2012; Feng et al., 2013; Chang et al., 2014). The results of this study showed that Fas and FasL were highly expressed in the hippocampus of rats with focal cerebral ischemia/reperfusion injury, and were mainly found in hippocampal neurons and glial cells. This evidence indicates that Fas mediates apoptosis after cerebral ischemia and may induce the activation of early signals responsible for delayed neuronal death, and also confirms the contribution of apoptosis factors for delayed neuronal death. Different doses of SSTF pretreatment markedly down-regulated hippocampal Fas and FasL expression, and reduced the number of positive cells in a dose-dependent manner. We conclude that SSTF pretreatment inhibits the Fas- and FasL-mediated apoptosis signaling pathway, inhibits neuronal apoptosis, and protects the brain against cerebral ischemia/reperfusion injury.
SSTF pretreatment enhances the anti-peroxidation effect and protects hippocampal nerve tissue
Cerebral ischemia/reperfusion injury causes a series of biochemical changes in the brain, among which excessive accumulation of free radicals is the main mediator for brain injury (Sweeney, 1997). Determination of malondialdehyde content can indirectly reflect the content of oxygen free radicals and lipid peroxidation, while superoxide dismutase and glutathione peroxidase activities are indicators of anti-lipid peroxidation capability (Zhao et al., 2006). Free radicals act with unsaturated fatty acids on membrane structures to form lipid peroxides such as malondialdehyde, which then cross-link with macromolecules on cell membranes, which degenerate into polymers that are cytotoxic. The brain is the most vulnerable to damage by free radicals. Free radicals also attack capillary endothelial cells, destroy the blood-brain barrier, increase vascular permeability, and induce brain edema. Furthermore, free radicals contribute to mitochondrial damage (Han, 2013), and prompt the depletion of ATP and other high-energy phosphate substances (Hu et al., 2005). Under physiological conditions, there is a radical scavenging system in vivo, such as superoxide dismutase and glutathione peroxidase. The production and elimination of free radicals are often homeostatic, and no excessive free radicals accumulate. When cerebral ischemia/reperfusion injury occurs, free radical scavenging systems are weakened and homeostasis is broken. Thus, the accumulation of free radicals attacks structures that are rich in unsaturated fatty acids at the ischemic area, causing a “waterfall-like” lipid peroxidation reaction, and destruction of membrane structures (Stanyer et al., 2008), ultimately aggravating brain tissue damage (Fernandez-Lopez et al., 2006).
The flavonoids are the main components of SSTF. Because of the multi-hydroxy structure, flavonoids can be self-oxidized to protect cell membrane systems, indirectly attenuate the impairment caused by free radicals and toxic aldehydes, and regulate superoxide dismutase and glutathione peroxidase activities and malondialdehyde content, thereby protecting the cell (Li et al., 2013). Our findings are supported by previous studies (Gong et al., 2013). SSTF pretreatment can improve the antioxidant capacity of brain tissue, reduce damage to the cell membrane, and exert a protective effect on brain tissue against ischemia/reperfusion.
In summary, SSTF has a protective effect against cerebral ischemia/reperfusion injury, and may be a potential therapeutic treatment for high-risk individuals.
Footnotes
Funding: This study was supported by the grants from Hebei Provincial Science and Technology Department, No. 07276101D-46.
Conflicts of interest: None declared.
Copyedited by Diwakarla S, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Chang MZ, Tian Y, Qiao LN, Di ZL, Hu BL, Zhang R, Wang HQ, Qu HQ, Liu Y. Protective effects of puerarin preconditioning on focal cerebral ischemia/reperfusion injury in rats and its therapeutic time window. Jilin Daxue Xuebao: Yixue Ban. 2014;40:23–27. [Google Scholar]
- 2.Chen M, Zhao SM, Li H, Kong XY. Effect of pretreatment with scutellaria baicalensis stem-leaf total flavonoid on cerebral infarction volume and lipid peroxidation induced by focal cerebral ischemia reperfusion in rats. Jiepou Xue Zazhi. 2010;33:495–497. [Google Scholar]
- 3.Chen M, Kong W, Zhao SM, Zhang SH, Kong XY, Li H, Zheng XY. Effect of pretreatment with scutellaria baicalensis stem-leaf total flavonoid on morphologic changes of hippocampus neurons and blood brain barrier followed by focal cerebral ischemia reperfusion in rats. Jiepou Xue Zazhi. 2012;35:341–344. [Google Scholar]
- 4.Chen M, Zhao SM, Li H, Kong XY, Zheng XY. Effect of Scutellaria baicalensis stem-leave total flavonoid on Fas and FasL expression and anti-oxidation in hippocampal neurons of rats induced by focal ischemia reperfusion. Zhongguo Shiyan Fangji Xue Zazhi. 2013;19:228–232. [Google Scholar]
- 5.Cheng X, Huang Y, Sun JB. Effect of neuroprotective of microvessels in treatment stroke. Jiepou Xue Yanjiu. 2013;35:139–141. [Google Scholar]
- 6.Fan QL, Jiao Y, Liu GJ, Zhou ZH, Zhang B, Chen L, Gan LX, Chen KN. Relationship between HAX-1 expression and neuron apoptosis after cerebral ischemia reperfusion injury in rats. Disan Junyi Daxue Xuebao. 2010;32:83–86. [Google Scholar]
- 7.Feng F, Hong MM, Gao Y. Effects of Puerarin on matrix metalloproteinase-9 expression in brain tissue and brain edema following cerebral ischemia-reperfusion in rats. Zhejiang Zhongxiyi Jiehe Zazhi. 2013;23:440–442. [Google Scholar]
- 8.Fernandez-Lopez D, Martinez-Orgado J, Nunez E, Romero J, Lorenzo P, Moro MA, Lizasoain I. Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr Res. 2006;60:169–173. doi: 10.1203/01.pdr.0000228839.00122.6c. [DOI] [PubMed] [Google Scholar]
- 9.Ge YS, Liu XS, Xue JY, Xin SM. Neuroprotective mechanism of Butylphthalide injection pretreatment on protect cerebral ischemica reperfusion injury viathrough the PI3K/Akt pathway in rats. Zhongfeng yu Shenjing Jibing Zazhi. 2013;30:32–36. [Google Scholar]
- 10.Gong JW, Ye L, Zhang XL, Fan QL. Effects of Dihuang Yinzi on the SOD, CAT, GSH-Px activities and MDA contents in the serum and brain of cerebral ischemia-reperfusion model rats. Zhongguo Shiyan Fangji Xue Zazhi. 2013;19:247–250. [Google Scholar]
- 11.Han J. Clinical curative effects observation of recombinant tissue plasminogen activator (rt-PA) intravenous thrombolytic therapy for super early cerebral infarction. Zuzhong yu Shenjing Jibing Zazhi. 2013;30:833–835. [Google Scholar]
- 12.He Q, Li H, Zhou XQ, Liu WH, Liu JX, Zhang GM. Effect of Danlong Xingnao tablet on expression of AI, Fas/FasL and TNF-á after cerebral ischemia-reperfusion in rats. Hunan Zhongyiyao Daxue Xuebao. 2009;29:23–25. [Google Scholar]
- 13.Hu J, Shi SG, Li LS. Nervous system diseases and superoxide dismutase. Zhongguo Linchuang Kangfu. 2005;9:130–132. [Google Scholar]
- 14.Huang J, Li LR, Zhao RB. Effect of mild hypothermia at the focal side on expressions of survivin and caspase-3 in rats after focal cerebral ischemia and reperfusion. Shandong Daxue Xuebao: Yixue Ban. 2011;49:24–27. [Google Scholar]
- 15.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Wang J. Effect of Naoluotong on apoptosis and relating gene in ischemic reperfusive injury model rats. Zhongguo Zhongyi Jichu Yixue Zazhi. 2003;9:33–35. [Google Scholar]
- 17.Li LR, Huang CF, Ma YH, Wang SQ. Effects of Monkshood and Pinellia preconditioning on SOD, MDA and apoptosis in rats with myocardial ischemia-reperfusion injury. Zhongyao Yaoli yu Linchuang. 2013;29:97–100. [Google Scholar]
- 18.Liu H, Kitazato KT, Uno M, Yagi K, Kanematsu Y, Tamura T, Tada Y, Kinouchi T, Nagahiro S. Protective mechanisms of the angiotensin II type 1 receptor blocker candesartan against cerebral ischemia: in-vivo and in-vitro studies. J Hypertens. 2008;26:1435–1445. doi: 10.1097/HJH.0b013e3283013b6e. [DOI] [PubMed] [Google Scholar]
- 19.Liu HX, Wang X, Yu S, Yang C, Cui Y. The effect of huomai tongluo decoction on Bcl-2/Bax expression in cerebral ischemia and reperfusion rats. Jiepou Kexue Jinzhan. 2013a;19:229–232. [Google Scholar]
- 20.Liu JX, Deng SH, Yang HS, Shi YH, Gao S, Zhao TH. A study on anti-inflammatory effects mechanism of total flavone of Scutellaria stem and leaf. Zhongguo Yaoli Xue Tongbao. 2002;18:713–714. [Google Scholar]
- 21.Liu JX, Li JS, Yu W, Hei CC, Liu HX, Ren FF. Effects of Xinglou Chengqi Decoction and Buyang Huanwu Decoction on Fas/Fasl and Caspase-3 pathway of apoptosis in rats with cerebral ischemia. Zhongguo Shiyan Fangji Xue Zazhi. 2012;18:187–191. [Google Scholar]
- 22.Liu XY, Zheng YQ, Liu JX. The mechanism of the constituents of Ginkgo Biloba against cerebral ischemia reperfusion-injury---a literature review. Shijie Zhongyiyao. 2013b;8:1142–1146. [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Ma Q, Geng Q, Xue Q, Zou YA. Effect of Kangnao Liquid on expression of Fas and FasL protein of ischemic cerebral reperfusion injury in rat. Zhongguo Linchuang Yaolixue Zazhi. 2012;28:370–372. [Google Scholar]
- 25.Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, Schenkel J, Herdegen T, Debatin KM. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci. 1999;19:3809–3817. doi: 10.1523/JNEUROSCI.19-10-03809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mou FF, Yu YJ, Shao SJ, Tan MH, Lu PP, Zhu J, Zhang ZA. Effect of Folium Ginkgo Extract (EGb761) on apoptosis after ischemia- reperfusion injury and expression of Bcl-2/Bax proteins in rats. Shanghai Zhongyiyao Daxue Xuebao. 2013;26:78–81. [Google Scholar]
- 27.Paxinos G, Watson C. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 28.Porzionato A, Macchi V, Morsut L, Parenti A, De Caro R. Microvascular patterns in human medullary tegmentum at the level of the area postrema. J Anat. 2005;206:405–410. doi: 10.1111/j.1469-7580.2005.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanyer L, Jorgensen W, Hori O, Clark JB, Heales SJ. Inactivation of brain mitochondrial Lon protease by peroxynitrite precedes electron transport chain dysfunction. Neurochem Int. 2008;53:95–101. doi: 10.1016/j.neuint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney MI. Neuroprotective effects of adenosine in cerebral ischemia: window of opportunity. Neurosci Biobehav Rev. 1997;21:207–217. doi: 10.1016/s0149-7634(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang CY, Huang P, Zhang ZH. Effects of 3β-double salicyloyl diosgenin on infarct volume and PI3K/Akt signal path in rats with focal cerebral ischemia/reperfusion injury. Zhongguo Yao Li Xue Bao. 2013;29:1672–1675. [Google Scholar]
- 32.Wang J, Hu JP, Li J, Hao L. Effects of naoluoxintong on protein expression of Fas and FasL, cerebral edema and neural signs in rat with cerebral IR. Zhonghua Zhongyiyao Zazhi. 2005;20:87–89. [Google Scholar]
- 33.Wang LY, Yang SJ. Advance research on mechanism and drug treatment method of cerebral ischemia-reperfusion injury. Jinlin Daxue Xuebao: Yixue Ban. 2012;38:1229–1233. [Google Scholar]
- 34.Wang WW. Puerarin protection against cerebral ischemia via inhibiting nNOS in rats. Jiangsu Daxue Xuebao: Yixue Ban. 2013;23:373–376. [Google Scholar]
- 35.Wang YM, Liu YP, Cao K, Shang YZ. Effects of flavonoids from Scutellaria stems and leaves on memory impairment and nerve inflammation in chronic cerebral ischemia rats. Zhongguo Yaoli Xue yu Duli Xue Zazhi. 2011;25:135–140. [Google Scholar]
- 36.Wu XG, Li YX, Liu HX, Yin YH, Zhao SM, Guo YY. Microvessel changes in the gerbil hippocampus after cerebral ischemia and reperfusion by Buyang Huanwu decoction pretreatment. Neural Regen Res. 2011;6:656–660. [Google Scholar]
- 37.Yao ST, Liu XH, Tang XM, Sun S, Wang JF. Ischemic postconditioning ameliorates pia mater microcirculation in rats subjected to cerebral ischemia reperfusion. Zhongguo Bingli Shengli Zazhi. 2009;25:451–455. [Google Scholar]
- 38.Zhao SM, Kong XY. The new method of mordanting vascular-tannin-iron chloride method. Jiepou Xue Zazhi. 2001;24:91–92. [Google Scholar]
- 39.Zhao SM, Liu S, Yang HG, Kong XY, Song CJ, Liu YP. Protective effect of scutellaria baicalensis stem-leaf total flavonoid on lipid peroxidation induced by myocardial ischemia reperfusion in rats. Jiepou Xue Zazhi. 2006;29:450–452. [Google Scholar]
- 40.Zhao SM, Kong W, Zhang SF, Chen M, Zheng XY, Kong XY. Pretreatment with scutellaria baicalensis stem-leaf total flavonoid prevents cerebral ischemia-reperfusion injury. Neural Regen Res. 2013;8:3183–3192. doi: 10.3969/j.issn.1673-5374.2013.34.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng XY, Kong XY, Zhao SM. Protective effect of scutellaria baicalensis stem-leaf total flavonoid on expression of heat shock protein 70 and ultrastructure in cortex neurons following cerebral ischemia reperfusion. Jiepou Xue Zazhi. 2011;34:52–54. [Google Scholar]
- 42.Zheng XY, Kong W, Kong XY, Zhang SF, Zhao SM, Li H, Chen M. Protective effect of scutellaria baicalensis stem-leaf total flavonoid on the injury of microvascular architecture and lipid peroxidation induced by cerebral ischemia reperfusion in rats. Jiepou Xue Zazhi. 2012;35:198–200. [Google Scholar]
- 43.Zhu DK, Han DF, Zhang XN. Effect of edaravone on functional recovery and pathologic changes of hippocampus after focal cerebral ischemic injury in rats. Zhonghua Naoxueguan Bing Zazhi: Dianzi Ban. 2012;6:121–129. [Google Scholar]


