Abstract
Endovascular surgery is advantageous in experimentally induced ischemic stroke because it causes fewer cranial traumatic lesions than invasive surgery and can closely mimic the pathophysiology in stroke patients. However, the outcomes are highly variable, which limits the accuracy of evaluations of ischemic stroke studies. In this study, eight healthy adult rhesus monkeys were randomized into two groups with four monkeys in each group: middle cerebral artery occlusion at origin segment (M1) and middle cerebral artery occlusion at M2 segment. The blood flow in the middle cerebral artery was blocked completely for 2 hours using the endovascular microcoil placement technique (1 mm × 10 cm) (undetachable), to establish a model of cerebral ischemia. The microcoil was withdrawn and the middle cerebral artery blood flow was restored. A reversible middle cerebral artery occlusion model was identified by hematoxylin-eosin staining, digital subtraction angiography, magnetic resonance angiography, magnetic resonance imaging, and neurological evaluation. The results showed that the middle cerebral artery occlusion model was successfully established in eight adult healthy rhesus monkeys, and ischemic lesions were apparent in the brain tissue of rhesus monkeys at 24 hours after occlusion. The rhesus monkeys had symptoms of neurological deficits. Compared with the M1 occlusion group, the M2 occlusion group had lower infarction volume and higher neurological scores. These experimental findings indicate that reversible middle cerebral artery occlusion can be produced with the endovascular microcoil technique in rhesus monkeys. The M2 occluded model had less infarction and less neurological impairment, which offers the potential for application in the field of brain injury research.
Keywords: nerve regeneration, brain injury, rhesus monkeys, model middle cerebral artery, microcoil, infarction, stroke, interventional therapy, digital subtraction angiography, magnetic resonance image, neuroimaging, neuroregeneration
Introduction
Although ischemic stroke is one of the leading causes of death, disability, and massive socioeconomic loss worldwide (Roger et al., 2011; Cook et al., 2012), its pathophysiology and biomedicine remain unclear. Because the information obtained from human patients is limited, reproducible animal models of focal ischemic infarction are crucial for studying cerebral ischemia (Liu et al., 2012). In contrast to rodent and other non-primate stroke models that have inherent flaws, nonhuman primate models of focal ischemic stroke provide excellent opportunities for understanding the vascular and cellular pathophysiology of cerebral ischemic injury because they resemble what happens in humans (Fukuda and Zoppo, 2003). In addition to being used for neuroprotective assessment, these models can also facilitate efforts to develop diagnostic tools for identifying and treating stroke symptoms.
Stroke in humans is highly heterogeneous both clinically and radiographically, and to date, no consensus has been reached regarding which nonhuman stroke models most closely mimics human pathology (Kaku et al., 1998; Liu et al., 2012). The use of a gyrencephalic species such as a rhesus monkey (West et al., 2009) is desirable for studies of stroke in nonhuman primates. Indeed, the rhesus monkey is ideal for stroke studies because its brain is structurally and functionally similar to that of humans (D’Arceuil et al., 2006; Hofer et al., 2008; Kumar et al., 2009; Rodriguez-Mercado et al., 2012). Additionally, the immunologic profile of rhesus monkeys is similar to that of humans (Sariol et al., 2007; Valentine and Watkins, 2008). These characteristics make the rhesus monkey the preferred model for ischemic stroke studies.
One difficulty with stroke models is interanimal variability. Until recently, this issue was incompletely addressed by nonhuman primate models of middle cerebral artery occlusion (MCAO), which were plagued by considerable interanimal variability in terms of infarct size. Several experiments were designed to reduce this variability using an open approach surgical technique (Liu et al., 1992; Huang et al., 2000). Endovascular surgery to induce ischemic stroke has been shown to produce fewer traumatic cranial incisions and to more closely model the pathophysiology in humans compared with invasive surgery. However, the variability in stroke volume was high (D’Arceuil et al, 2006). Using the intraluminal microcoil approach, Rink et al. (2008) reported a 15% standard deviation in infarct volume in canines subjected to transient MCAO, which is significantly better than other methods. However, this method has not been developed in nonhuman primates such as rhesus monkeys. Therefore, in an effort to address stroke variability in primate models, we used an intraluminal approach to modify a model of reperfused stroke, and create a unilateral MCAO. By taking advantage of the microcoil approach, we hypothesized that more-consistent infarctions would occur, thereby allowing experiments involving fewer animals to achieve statistically significant results. Among heterogeneous stroke subtypes, the proportions of large-arterial and cortical ischemic strokes were reported as 71% and 22% in humans, respectively (Feigin et al., 2006; Halkes et al., 2006). To simulate these, we established and evaluated two types of ischemic stroke models by obstructing different locations in the middle cerebral artery.
Using an intraluminal technique, we introduced a microcoil into the middle cerebral artery and temporally occluded and then recanalized it. The reliability of infarcts produced by this procedure could be measured by taking magnetic resonance images (MRI) of the monkey brains during the acute phase. The procedure resulted in discrete and limited neurobehavioral deficits. This paper: (1) describes how we created the occluded-stroke model in rhesus monkeys using intraluminal microcoils to generate segmental MCAO; (2) identifies and compares the difference in stroke volume and neurological evaluation scores between ischemic lesions induced at different locations in the middle cerebral artery.
Materials and Methods
Animals
A total of eight adult healthy male rhesus monkeys (Macaca mulatta), with an average age of 8.2 ± 1.2 years and an average body weight of 9.40 ± 0.99 kg, were selected for the study (Academy of Military Medical Sciences, China; animal license No. 2013-26). Animals were housed individually indoors under a 12-hour light/dark cycle (light on from 07:00 to 19:00), and at a constant room temperature of 22–24°C. Laboratory diet was provided twice daily, supplemented with fresh fruit and vegetables and drinking water ad libitum. At 1 month before surgery, animals were screened for general health, epidemic diseases, and neurological disorders. All animal experiments were approved and monitored by the Department of Laboratory Animal Science at the Capital Medical University in China. Every effort was made to ensure that the animals were free from pain and discomfort. The principal investigator and the primate handling staff were present for all procedures.
Anesthesia and monitoring
All animals were deprived of food for 12 hours before the experiment. Anesthesia was induced with intramuscularly administered ketamine (10 mg/kg; Fujian Gutian Pharmaceutical Co., Fujian Province, China) (Woods et al., 2013). Thereafter, monkeys were positioned in a supine position on a plastic stretcher. The trachea was intubated and ventilated. Both the inguinal regions and anterior pectoral region were shaved free of hair and cleaned with betadine. Animals were anaesthetized with propofol 0.5 mg/kg per hour (Astra Zeneca, Caponago, Italy) continuously throughout the operation and MRI scan. Physical parameters including blood pressure by arm cuff, respiratory frequency, O2 saturation, electrocardiogram, and rectal temperature were continuously monitored. Temperature was maintained at 37.5 ± 0.5°C using a heating blanket. Atropine (0.6 mg/kg) was administered intramuscularly before operation. A femoral arterial line was used to monitor blood pressure and blood gases.
MRI scan
We monitored the early changes that followed the experimentally-induced stroke (see below) using MRI. Because infarction volume measured from T2/Flair images was shown to be highly correlated with histological estimates (Huang et al., 2000; Gauvrit et al., 2006; Rink et al., 2008; Neumann et al., 2009; West et al., 2009; Schwartz and Pile-Spellman, 2011), evaluation of the infarct lesion was accomplished based on T2 images. All images were performed on a Magnetom Trio MRI Scanner (3.0T; Siemens AG, Siemens Medical Solutions, Erlangen, Germany). Animals were scanned in the supine position 30 minutes before surgery to collect baseline data in the supine position. During the scans, animals were ventilated with an MRI compatible respirator (MRI2550, Surgivet Corp., USA) and monitored with a multi-parameter monitor device (0500-113, Surgivet Corp) under anesthesia. The MRI scans included T2-weighted (high resolution) and magnetic resonance angiography (MRA) scans. The imaging protocols consisted of an axial turbo spin echo T2-weighted scan (repetition time = 3,000, echo time = 300 ms, matrix = 384 × 384, voxel size = 0.4 mm × 0.4 mm × 1.0 mm, slice thickness = 1 mm), and a 3D time of flight (repetition time = 22, echo time = 4.92 ms, matrix = 256 × 256, voxel size = 0.5 mm × 0.5 mm × 0.5 mm, slice thickness = 0.5 mm). The MRA scans were repeated at the end of the operation to identify the MCAO. The T2 scans were repeated 24 hours after MCAO was induced and the ischemic volume of the stroke region was recorded using the same parameters. Monitoring and ventilation were continued throughout the procedure. USVIEW V1.1 (USCUBE, Beijing, China) was used to calculate infarct volume from axial T2-weighted MR images by two independent observers.
Surgical procedure
To minimize variations, all surgical procedures were conducted by a single surgeon. At the beginning of the operation, the animal was given a 1,000 U heparin intravenous bolus, followed by an intravenous infusion of 500 U/h. An 18 gage needle was used to puncture the femoral artery percutaneously using the Seldinger technique. A sheath was then placed in the femoral artery using a catheter-introducer kit (Radifocus Introducer, Terumo, Tokyo, Japan). Next, a guide wire (Radifocus Guide Wire M, Terumo) was inserted through the sheath and the tip of the guide wire was forced from the femoral artery into the abdominal aorta, until it reached the ascending aorta as viewed under X-ray fluoroscopy (images were digitally acquired at a rate of six frames per second). Then it was further forced from the ascending aorta to the right internal carotid artery. While monitoring with X-ray fluoroscopy a 5F-Guiding Catheter (Cordis, Miami Lakes, FL, USA) was inserted along the guide wire and the tip of the catheter was placed at an inlet of the internal carotid artery. After confirming that the tip of the catheter was placed in the desired position, the guide wire was withdrawn. Subsequently, a Prowler 10 microcatheter (Cordis) was inserted into the guiding catheter and the tip of the catheter was pushed forward through the internal carotid artery to an extent closest to the M1 segment. At this stage, a small amount of a contrast agent was slowly injected with a syringe and the flow of the contrast agent from the tip of the microcatheter into the middle cerebral artery was confirmed. A custom microcoil (1 mm × 10 cm, platinum alloy; Achieva Medical Co., Shanghai, China) was then deployed in the M1 segment of the middle cerebral artery (n = 4), or the distal part of the upper trunk of the M2 segment (n = 4) to occlude the artery for 2 hours. The catheter system was secured in position and the animals were transported to the MR department. Physiological monitoring and support were maintained throughout the operation. After removal of the microcoil, a second angiogram confirmed that blood flow in the middle cerebral artery was restored.
Post-operative management
After the operation, the interventional devices were withdrawn, the puncture points were compressed for 30 minutes, and then pressure dressed with sterile bandages. After the animals were occluded, postoperative veterinary care was provided to the monkeys for 24 hours. After MRA scanning, animals were placed in a supine position on a padded mattress with the head elevated 30°. In case of pain, an injection of buprenorphine (Buprecare, 20 μg/kg, intramuscular injection; Animalcare, Dunnington, York, UK) was given as deemed necessary (Barnes, 2012).
Neurological assessment
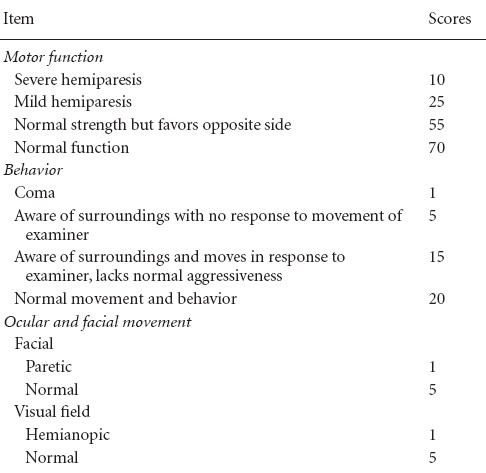
Before the T2-image scanning was performed and 24 hours after MCAO was induced, each monkey was assessed with a commonly used non-human primate neurological evaluation scale (Table 1) by two independent observers who were experienced in evaluating neurological deficits (Spetzler et al., 1980; Frazee et al., 1998; Mack et al., 2003; D’Arceuil et al., 2006; Rodriguez-Mercado et al., 2012). This scale is weighted heavily on motor function, but also takes into account behavior changes (mental status) and ocular and cranial nerve impairment. Lower scores represent worse functional outcomes.
Table 1.
Scoring system for neurological evaluations
Slice preparation and histological detection
After MRI scanning was performed, all animals were euthanized and the brain was harvested for histological study. Animals were deeply anaesthetized with 25 mg/kg pentobarbital and perfused transcardially with physiological saline (pH 6.4) containing heparin (50 U/mL). The brain tissue was harvested immediately post-mortem in all eight animals. Aside from cerebral ischemia, none of the animals had evidence of gross pathology (i.e., intraparenchymal hemorrhage, infection, subdural or epidural hematoma). Continuous 5-mm thick coronal sections were collected from each monkey using a brain matrix for rhesus macaques. These coronal sections were immersed in 10% neutral-buffered formalin/PBS for at least 2 days. Each section was embedded in paraffin, cut into adjacent slices (3–4 μm thickness), and stained with hematoxylin-eosin. Gross pathological study of the brain sections revealed well-defined cerebral infarctions in all animals on the side of vessel occlusion.
Statistical analysis
All data are expressed as the mean ± SD. Statistical analyses were performed using SPSS 22.0 software (IBM Corp, Armonk, NY, USA). Infarction volume and neurological evaluation were analyzed by individual Student's t-test. The correlation among ischemic damage measured by MRI, and the neurological evaluation (stroke scores) was analyzed using a Pearson parametric correlation. A P-value < 0.05 was considered significant.
Results
Changes of life indicators in the rhesus monkey model of ischemic stroke
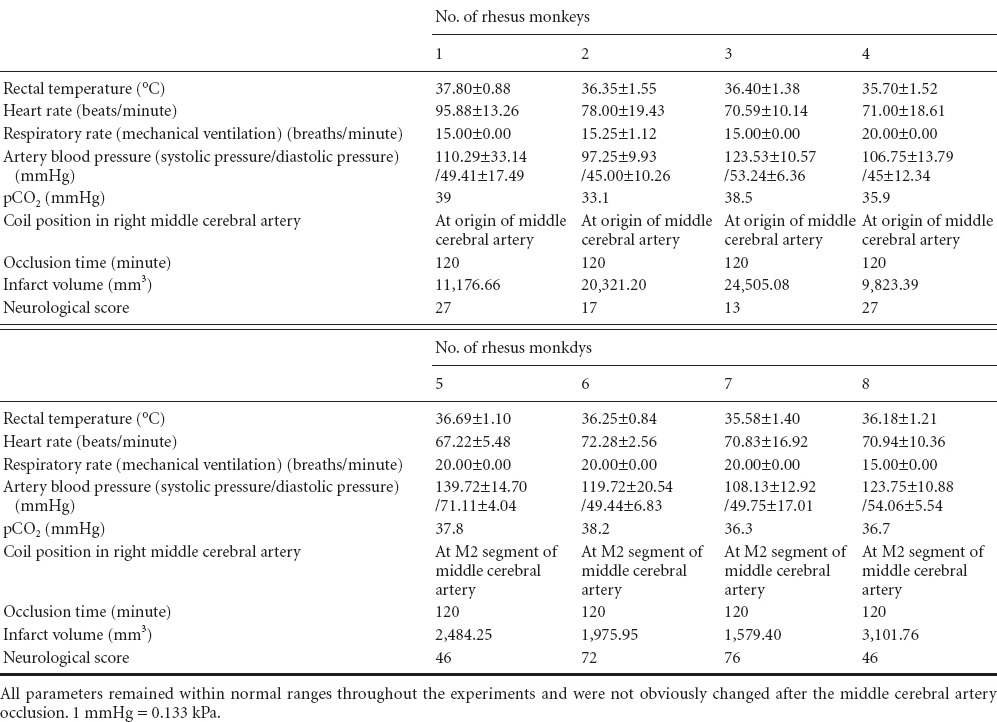
The anesthesia and surgical procedures were well tolerated throughout the procedure by all rhesus monkeys. No parameters were affected by the MCAO in this set of experiments. Physiological parameters remained within the normal range during the experiments (Table 2). All rhesus monkeys recovered from anesthesia and could be evaluated 24 hours after the occlusion was induced.
Table 2.
Life indicators of the rhesus monkeys subjected to middle cerebral artery occlusion
Changes of neurobehavioral function in the rhesus monkey model of ischemic stroke
Ischemia was induced without intraoperative complications in part of the middle cerebral artery in each rhesus monkey using an intraluminal approach for vessel occlusion. Obvious neurological deficits were observed in all rhesus monkeys. MCAO and reperfusion in rhesus monkeys produced a characteristic deficit similar to that in human beings with stroke: Awaking from anesthesia, all rhesus monkeys in the M1 occlusion group exhibited severe neurological impairments, such as depression, decreased activity, lethargy, marked hemiplegia of contralateral limbs, decreased muscle tone, and salivation and drooping of the affected corner of the mouth. Rhesus monkeys in the M2 occlusion group appeared to have contralateral upper limb weakness and apraxia, without severe changes in consciousness or lower limb hemiplegia or weakness. Neurological scores were evaluated by two independent observers, with outcomes in the M1 and M2 occlusion groups being 21.00 ± 7.19 and 60.00 ± 16.25, respectively (Table 2). Lower scores represent worse functional outcomes. There was a statistically significant difference between the two groups (P = 0.0046).
Assessment of a rhesus monkey model of ischemic stroke via histological staining, MRI, and DSA
Anatomical MRI scans of the rhesus monkeys revealed normal brain anatomy before ischemia was induced. MCAO and reperfusion were confirmed by digital subtraction angiography (DSA) and MRA (Figure 1A, B; Figure 2A, B). After releasing the microcoil, DSA and MRA revealed that the distal part of the middle cerebral artery was absent, and all rhesus monkeys showed high signal intensity (ischemia) on T2-weighted images of the corresponding region. In the M1 occlusion group, high-intensity areas indicated that infarcted lesions were localized primarily in the basal ganglia, internal capsule, white matter, and cortex (the middle cerebral artery territory) (Figure 1C). In the M2 occlusion group, infarcts were smaller and localized in the subcortical white matter and in the cortex of the area around the Sylvian fissure (Figure 2C). The total infarct volume in the M1 and M2 occlusion groups were 16,456.58 ± 7,108.45 mm3 (range: 9,823.39–24,505.08) and 2,285.34 ± 658.33 mm3 (range: 1,579.40–3,101.76), respectively (Table 2). The M1 occlusion group showed significantly greater ischemic volume than the M2 group (P = 0.0074).
Figure 1.
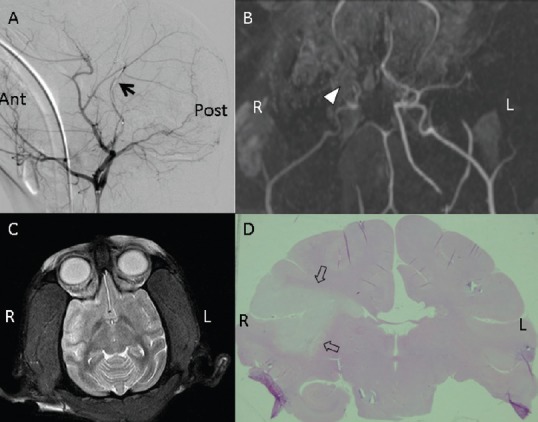
Images of the M1 segment in the rhesus monkey models of middle cerebral artery occlusion.
(A) Digital subtraction of angiographic images. Arrow points to microcoil release in the M1 segment of the middle cerebral artery (normal laterality). (B) Magnetic resonance angiography. Triangle indicates where the middle cerebral artery was occluded in the M1 segment. (C) Representative T2-weighted image of strokes 2 hours after middle cerebral artery occlusion (high signal intensity). (D) Hematoxylin-eosin staining. Arrows point to a large infarction (whitish area) in the right hemisphere. Ant: Anterior; Post: posterior; L: left; R: right.
Figure 2.
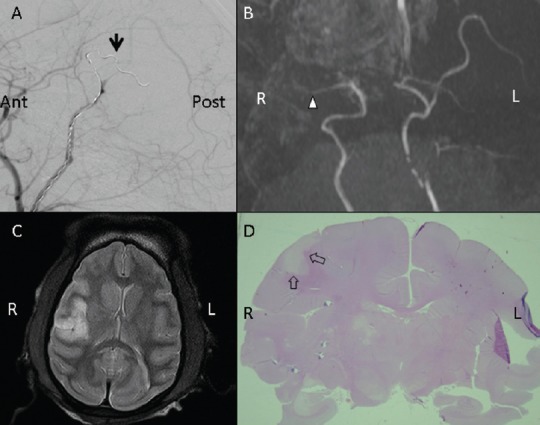
Images of the M2 segment in the rhesus monkey models of middle cerebral artery occlusion.
(A) Digital subtraction of angiographic images. Arrow points to microcoil release in the M2 segment of the middle cerebral artery (normal laterality). (B) Magnetic resonance angiography. Triangle indicates where the middle cerebral artery was occluded in the M2 segment. (C) Representative T2-weighted image of stroke 2 hours after middle cere-bral artery occlusion (high signal intensity). Ischemic area was around the Sylvian fissure. (D) Hematoxylin-eosin staining. Arrows point to a smallish infarction (whitish area) in the right hemisphere that included cortex and sub-cortical white matter. Ant: Anterior; Post: posterior; L: left; R: right.
Microscopic examination of brain pathology in the sections revealed well-defined cerebral infarctions in all rhesus monkeys on the side of vessel occlusion. Hematoxylin-eosin staining revealed clear delineations between infarcted and normal brain areas (Figure 1D; Figure 2D). These findings were confirmed by the MRI results.
Both the MRI images and histological examination confirmed that the M1 occlusion group had a larger infarction volume than did the M2 occlusion group, and was associated with significantly lower neurological evaluation scores. Statistical analysis of infarction volume and neurological evaluation score showed a significant difference between the two groups (Figure 3A, B). The neurological evaluation scores after embolization were significantly negatively correlated with infarct volume (r = −0.8649, P = 0.0056) (Figure 4).
Figure 3.
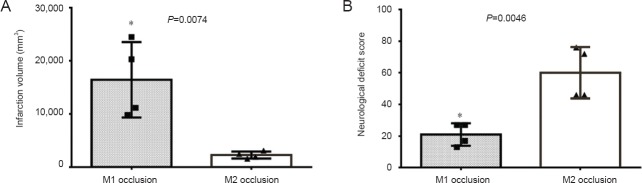
Comparison of infarction volume (A) and neurological deficit scores (B) measured after ischemia in the M1 and M2 occlusion groups.
*P < 0.05, vs. M2 occlusion group. Data are presented as the mean ± SD. Four rhesus monkeys were used in each group. Student's t-tests were performed to determine whether data were significantly different between the two groups (gray bars: M1 occlusion group; white bars: M2 occlusion group).
Figure 4.
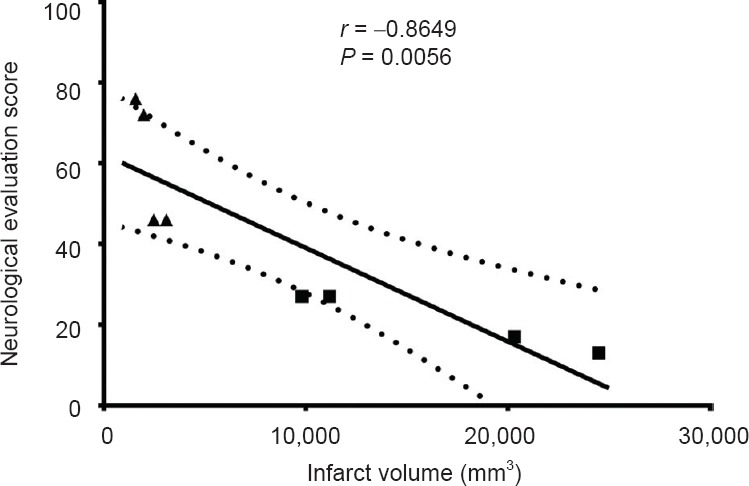
Correlation analysis of infarct volume and neurological evaluation scores in the two groups.
Pearson parametric correlation analysis showed that ischemic damage measured by MRI was significantly negatively correlated with neuro-logical evaluations (stroke scores). “■”: M1 occlusion group; “▲”: M2 occlusion group; “—” : the linear regression line; “::::::::”: the 95% confidence band of the best-fit line.
Discussion
Based on the survival rate and lifespan studies of rhesus monkey colonies, 1 year for a rhesus monkey is equivalent to about 3 to 4 years for a human (Tigges et al., 1988; Bradley et al., 1999; Peters and Kemper, 2012). Adult rhesus monkeys (Macaca mulatta) live 7–14 years (Colman et al., 2009; Mattison et al., 2012). Thus, the rhesus monkeys introduced in our study were all adults. Peters and Kemper (2012) reported that brain structures in these monkeys do not change significantly with age. Consequently, the adult rhesus monkeys here met the standard requirements that have been accepted by other stroke studies, although age was not explicitly mentioned in these studies (West et al., 2009; Cook et al., 2012).
There are three main ways to occlude vessels: surgical methods, endovascular methods, and photothrombotic methods, which are limited in frequency (Cook et al., 2012). With technological advancements, stroke models using non-human primates have been gradually developed from the early-ligated internal carotid-artery model to surgical clipping the middle cerebral artery in transorbital or craniotomy operations, and eventually to interventional access (O’Brien and Waltz, 1973; West et al., 2009; Cook et al., 2012; Gauberti et al., 2012). Throughout the development of these techniques, brain impairments have become smaller and more precise, and closer to the clinical pathophysiological processes of natural stroke. The early efforts that used clipping/ligation had major limitations (traumatic impairment) on pathophysiological and therapeutic manipulations. Because stroke in humans is not necessarily associated with head trauma, the traumatic aspect of invasive surgery might be viewed as a major confounding factor. In contrast, the most important advantage of the endovascular technique is that it is less invasive compared with an invasive surgical approach. Furthermore, the endovascular techniques can maintain cranial cavity integrity and the neurological function defects can be assessed more precisely and comprehensively. Earlier intravascular stroke models in non-human primates have used glue/thrombus injection or inflatable balloon occluders, which often resulted in variations in occlusion location and extent. Furthermore, thicker intraluminal devices such as balloons and microcatheters were unable to reach the upper trunk of the M2 segment. Even thinner intraluminal devices such as microwire or suture have significant limitations that have been extensively documented in the literature and contribute to the high degree of variability in lesion volume and location (Rink et al., 2008). Studies of the intraluminal thread model have reported that the leftover filament in the internal cerebral artery occluded the anterior choroid artery and the hypothalamic artery, thereby producing unintentional subcortical lesions (Gerriets et al., 2004). Consequently, to obtain a relatively consistent stroke model, we have developed an occlusion model using intraluminal techniques that introduce a microcoil into the middle cerebral artery. The microcoil can accurately embolize any segment of the middle cerebral artery trunk. We chose to occlude the middle cerebral artery because strokes are a common and devastating condition, and their treatment remains a major unsolved problem in neuro-critical care (Heinsius et al., 1998; Goldstein et al., 2001; Huttner and Schwab, 2009).
Detailed studies have shown that high clinical and radiographic heterogeneity in stroke patients is primarily determined by the degree of collateral circulation, especially after MCAO (Min et al., 2000; Gerraty et al., 2002; Lee et al., 2009; Shuaib et al., 2011). Because the organization of the vascular systems in the brain is most similar among primates, the variance in collateral circulation between the non-human primates likely explains the differences in lesion severity (considerable variation in both lesion size and functional outcome). One problem with non-human primate stroke models is the occlusion of the collateral artery. Some researchers have made efforts to overcome the effects of bypassed blood supply by blocking the collateral artery (Huang et al., 2000; Cook et al., 2012). This variability is certainly a complicating factor for study design and needs serious consideration (Sasaki et al., 2011). To develop a consistent model of MCAO stroke in the rhesus monkey, we initially performed a pilot study with different positions of reversible middle cerebral artery (single vessel) trunk occlusion. In our study, we hypothesized that infarction can be induced by the proximal MCAO using a microcoil if the orifices of perforating lenticulostriate arteries were involved, which have well-formed distal cerebral collaterals. As shown by Figure 1, occlusion of the M1 segment blocked the blood flow to the whole middle cerebral artery territory including the cortex, subcortical structures, and basal ganglia region. This was attributed to that the occlusion was not a point but a segment of vessel, blocked the opening of collateral arteries branched off to serve these areas. Thus creating a large and consistently sized cerebral infarction may simulate the major hemispheric infarction in humans. Freret et al. (Gao et al., 2006; Liu et al., 2007; Freret et al., 2008) reported a 58% standard deviation around the mean infarct volume from marmosets (n = 4) subjected to transient MCAO, other endovascular models in nonhuman primates have reported larger standard deviations in infarct volume across animals. In our study, when the microcoil approach was used, the standard deviation of the M1 occlusion group was 43% (n = 4), which was less than the aforementioned results. While the standard deviation of the M2 occlusion group was up to 29% (n = 4). The appreciably tighter standard deviation in stroke-induced lesion volumes with the microcoil method suggested that fewer experimental animals would be required to achieve statistical significance in stroke studies. Nevertheless, owing to the presence of leptomeningeal anastomoses and communication between the extracranial and intracranial circulations, the heterogeneous stroke variation could not be eliminated entirely. The results of the present investigation confirmed our hypothesis that the microcoil method could establish a more homogeneous ischemic stroke model in rhesus monkeys.
Another novelty of this study was that introducing the microcoil into the middle cerebral artery at different positions could establish two types of reversible stroke models. Each model would mimic a subclass of thrombotic stroke in humans and offer a relatively homogenous therapeutic target for studies of neuroprotection. While the M1 occlusion group had more severe neurological outcomes than the M2 occlusion group, the M1 occlusion model successfully simulated malignant middle cerebral artery infarction, while the M2 occlusion model successfully simulated cortical infarction. To the best of our knowledge, our study was the first to establish a single forelimb apraxia model using an intraluminal method, which made it particularly suitable for studies of primate motor function. The occlusion at the upper trunk of the middle cerebral artery M2 segment yielded a ischemic infarct area around the Sylvian fissure, including the upper part of Brodmann 4 and 6 areas, which correspond to the distal forelimb in the cortex of primates, thus affecting the hand contralateral to the infarction (Paxinos et al., 2000; Plautz et al., 2003). These results show that the M2 occlusion model may provide a platform for single motor function research. They also confirm the anatomical and species similarity between humans and rhesus monkeys. Although the microcoil has been applied in other non-primate animal stroke models (Rink et al., 2008), they did not take advantage of its superior qualities. Non-human primates have apparent functional differences in fine dexterous ability of the forelimbs, which makes them more suitable for microcoil application. These differences enabled more accurate measurement by neurological assessment of impairments and therapeutic outcome of the Brodmann 4 and 6 in rhesus monkeys. Furthermore, because of the significant correlations between stroke volume and neurological deficiency, the M2 occlusion models had milder neurological deficits and better self-care abilities, which inevitably decreased the expense and effort needed in postoperative care. Because motor function could be assessed precisely while maintaining the aforementioned homogeneity in ischemic volume, the M2-segmental MCAO model provided a relative standard tool for ischemic stroke studies and therapeutic evaluation.
Imaging findings and functional assessment in this study demonstrated that an intraluminally-released microcoil could establish versatile reversible MCAO in rhesus monkey models. These modified intravascular stroke models in rhesus monkeys would mimic two types of ischemic stroke in humans and offer a relatively homogenous therapeutic target for the clinical and fundamental study of ischemic stroke. Comparing M1-occluded and M2-occluded models in rhesus monkeys, brain damage was significantly greater in the M1-occluded models than that in M2-occluded models, and the M2-occluded MCAO model was less variable. The resulting neurological deficits correlated well with ischemic stroke volume.
Footnotes
Funding: This study was financially supported by grants from the National Key Basic Research Program (973 Program) of China, No. 2011CB707804; Beijing Municipal Science and Technology Project, No. 2121100005312016.
Conflicts of interest: None declared.
Copyedited by Norman C, Yang Y, Li CH, Song LP, Wang J, Zhao M
References
- 1.Barnes DC. Subtotal colectomy by rectal pull-through for treatment of idiopathic megacolon in 2 cats. Can Vet J. 2012;53:780–782. [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–229. [PubMed] [Google Scholar]
- 3.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9:371–379. doi: 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 6.D’Arceuil HE, Duggan M, He J, Pryor J, de Crespigny A. Middle cerebral artery occlusion in Macaca fascicularis: acute and chronic stroke evolution. J Med Primatol. 2006;35:78–86. doi: 10.1111/j.1600-0684.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 7.Feigin V, Carter K, Hackett M, Barber PA, McNaughton H, Dyall L, Chen MH, Anderson C. Ethnic disparities in incidence of stroke subtypes: auckland regional community stroke study, 2002-2003. Lancet Neurol. 5:130–139. doi: 10.1016/S1474-4422(05)70325-2. [DOI] [PubMed] [Google Scholar]
- 8.Frazee JG, Luo X, Luan G, Hinton DS, Hovda DA, Shiroishi MS, Barcliff LT, Kontos HA. Retrograde transvenous neuroperfusion: a back door treatment for stroke editorial comment. Stroke. 1998;29:1912–1916. doi: 10.1161/01.str.29.9.1912. [DOI] [PubMed] [Google Scholar]
- 9.Freret T, Bouet V, Toutain J, Saulnier R, Pro-Sistiaga P, Bihel E, Mackenzie ET, Roussel S, Schumann-Bard P, Touzani O. Intraluminal thread model of focal stroke in the non-human primate. J Cereb Blood Flow Metab. 2008;28:786–796. doi: 10.1038/sj.jcbfm.9600575. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda S, Zoppo GJd. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44:96–104. doi: 10.1093/ilar.44.2.96. [DOI] [PubMed] [Google Scholar]
- 11.Gao H, Liu Y, Lu S, Xiang B, Wang C. A reversible middle cerebral artery occlusion model using intraluminal balloon technique in monkeys. J Stroke Cerebrovasc Dis. 2006;15:202–208. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH. Experimental ischemic stroke: a review. Stroke. 1984;15:5–14. doi: 10.1161/01.str.15.1.5. [DOI] [PubMed] [Google Scholar]
- 13.Gauberti M, Obiang P, Guedin P, Balossier A, Gakuba C, Diependaele AS, Chazalviel L, Vivien D, Young AR, Agin V, Orset C. Thrombotic stroke in the anesthetized monkey (Macaca mulatta): characterization by MRI-a pilot study. Cerebrovasc Dis. 2012;33:329–339. doi: 10.1159/000335309. [DOI] [PubMed] [Google Scholar]
- 14.Gauvrit JY, Leclerc X, Girot M, Cordonnier C, Sotoares G, Henon H, Pertuzon B, Michelin E, Devos D, Pruvo JP, Leys D. Fluid-attenuated inversion recovery (FLAIR) sequences for the assessment of acute stroke: inter observer and inter technique reproducibility. J Neurol. 2006;253:631–635. doi: 10.1007/s00415-005-0075-x. [DOI] [PubMed] [Google Scholar]
- 15.Gerraty RP, Parsons MW, Barber PA, Darby DG, Desmond PM, Tress BM, Davis SM. Examining the lacunar hypothesis with diffusion and perfusion magnetic resonance imaging. Stroke. 2002;33:2019–2024. doi: 10.1161/01.str.0000020841.74704.5b. [DOI] [PubMed] [Google Scholar]
- 16.Gerriets T, Stolz E, Walberer M, Muller C, Rottger C, Kluge A, Kaps M, Fisher M, Bachmann G. Complications and pitfalls in rat stroke models for middle cerebral artery occlusion: a comparison between the suture and the macrosphere model using magnetic resonance angiography. Stroke. 2004;35:2372–2377. doi: 10.1161/01.STR.0000142134.37512.a7. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg MD. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 thomas willis lecture. Stroke. 2002;34:214–223. doi: 10.1161/01.str.0000048846.09677.62. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs B, Levine SR, Mosca L, Sacco RL, Sherman DG, Wolf PA, del Zoppo GJ. Primary prevention of ischemic stroke : a statement for healthcare professionals from the stroke council of the american heart association. Stroke. 2001;32:280–299. doi: 10.1161/01.str.32.1.280. [DOI] [PubMed] [Google Scholar]
- 19.Green AR. Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin Exp Pharmacol Physiol. 2002;29:1030–1034. doi: 10.1046/j.1440-1681.2002.03767.x. [DOI] [PubMed] [Google Scholar]
- 20.Halkes PH, Kappelle LJ, van Gijn J, van Wijk I, Koudstaal PJ, Algra A. Large subcortical infarcts: clinical features, risk factors, and long-term prognosis compared with cortical and small deep infarcts. Stroke. 2006;37:1828–1832. doi: 10.1161/01.STR.0000226993.88307.ff. [DOI] [PubMed] [Google Scholar]
- 21.Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory Etiology and outcome patterns. Neurology. 1998;50:341–350. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Hofer S, Merboldt KD, Tammer R, Frahm J. Rhesus monkey and human share a similar topography of the corpus callosum as revealed by diffusion tensor MRI in vivo. Cerebral cortex. 2008;18:1079–1084. doi: 10.1093/cercor/bhm141. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Mocco J, Choudhri TF, Poisik A, Popilskis SJ, Emerson R, DelaPaz RL, Khandji AG, Pinsky DJ, Connolly ES, Zlokovic BV. A modified transorbital baboon model of reperfused stroke editorial comment. Stroke. 2000;31:3054–3063. doi: 10.1161/01.str.31.12.3054. [DOI] [PubMed] [Google Scholar]
- 24.Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol. 2009;8:949–958. doi: 10.1016/S1474-4422(09)70224-8. [DOI] [PubMed] [Google Scholar]
- 25.Kaku S, Umemura K, Mizuno A, Yano S, Suzuki Ki, Kawasaki T, Nakashima M. Evaluation of a GPIIb/IIIa antagonist YM337 in a primate model of middle cerebral artery thrombosis. Eur J Pharmacol. 1998;345:185–192. doi: 10.1016/s0014-2999(98)00005-3. [DOI] [PubMed] [Google Scholar]
- 26.Kumar N, Lee JJ, Perlmutter JS, Derdeyn CP. Cervical carotid and circle of willis arterial anatomy of macaque monkeys: a comparative anatomy study. Anat Rec (Hoboken) 2009;292:976–984. doi: 10.1002/ar.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiecien TD, Sy C, Ding Y. Rodent models of ischemic stroke lack translational relevance. are baboon models the answer? Neurol Res. 2014;36:417–422. doi: 10.1179/1743132814Y.0000000358. [DOI] [PubMed] [Google Scholar]
- 28.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology. 2009;72:1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Hu WX, Zu QQ, Lu SS, Xu XQ, Sun L, Zhou WZ, Shi HB. A novel embolic stroke model resembling lacunar infarction following proximal middle cerebral artery occlusion in beagle dogs. J Neurosci Methods. 2012;209:90–96. doi: 10.1016/j.jneumeth.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Liu XG, Branston NM, Kawauchi M, Symon L. A model of acute focal ischemia in the territory of the anterior cerebral artery in baboons. Stroke. 1992;23:40–44. doi: 10.1161/01.str.23.1.40. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, D’Arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, Pryor J, de Crespigny AJ. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007;38:138–145. doi: 10.1161/01.STR.0000252127.07428.9c. [DOI] [PubMed] [Google Scholar]
- 32.Mack WJ, Komotar RJ, Mocco J, Coon AL, Hoh DJ, King RG, Ducruet AF, Ransom ER, Oppermann M, DeLaPaz R, Connolly ES., Jr Serial magnetic resonance imaging in experimental primate stroke: validation of MRI for pre-clinical cerebroprotective trials. Neurol Res. 2003;25:846–852. doi: 10.1179/016164103771953943. [DOI] [PubMed] [Google Scholar]
- 33.Marshall JW, Duffin KJ, Green AR, Ridley RM, Finklestein SP. NXY-059, a free radical-trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species editorial comment. Stroke. 2001;32:190–198. doi: 10.1161/01.str.32.1.190. [DOI] [PubMed] [Google Scholar]
- 34.Marshall JW, Cummings RM, Bowes LJ, Ridley RM, Green AR. Functional and histological evidence for the protective effect of NXY-059 in a primate model of stroke when given 4 hours after occlusion. Stroke. 2003a;34:2228–2233. doi: 10.1161/01.STR.0000087790.79851.A8. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JW, Green AR, Ridley RM. Comparison of the neuroprotective effect of clomethiazole, AR-R15896AR and NXY-059 in a primate model of stroke using histological and behavioural measures. Brain Res. 2003b;972:119–126. doi: 10.1016/s0006-8993(03)02511-3. [DOI] [PubMed] [Google Scholar]
- 36.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min WK, Park KK, Kim YS, Park HC, Kim JY, Park SP, Suh CK. Atherothrombotic middle cerebral artery territory infarction: topographic diversity with common occurrence of concomitant small cortical and subcortical infarcts. Stroke. 2000;31:2055–2061. doi: 10.1161/01.str.31.9.2055. [DOI] [PubMed] [Google Scholar]
- 38.Neumann AB, Jonsdottir KY, Mouridsen K, Hjort N, Gyldensted C, Bizzi A, Fiehler J, Gasparotti R, Gillard JH, Hermier M, Kucinski T, Larsson EM, Sorensen L, Ostergaard L. Interrater agreement for final infarct MRI lesion delineation. Stroke. 2009;40:3768–3771. doi: 10.1161/STROKEAHA.108.545368. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien MD, Waltz AG. Transorbital approach for occluding the middle cerebral artery without craniectomy. Stroke. 1973;4:201–206. doi: 10.1161/01.str.4.2.201. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. Sat Lake City, Academic Press, USA. 2000 [Google Scholar]
- 41.Peters A, Kemper T. A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging. 2012;33:2357–2372. doi: 10.1016/j.neurobiolaging.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 43.Rink C, Christoforidis G, Abduljalil A, Kontzialis M, Bergdall V, Roy S, Khanna S, Slivka A, Knopp M, Sen CK. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci U S A. 2008;105:14100–14105. doi: 10.1073/pnas.0806678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Mercado R, Ford GD, Xu Z, Kraiselburd EN, Martinez MI, Eterović VA, Colon E, Rodriguez IV, Portilla P, Ferchmin PA, Gierbolini L, Rodriguez-Carrasquillo M, Powell MD, Pulliam JV, McCraw CO, Gates A, Ford BD. Acute neuronal injury and blood genomic profiles in a nonhuman primate model for ischemic stroke. Comp Med. 2012;62:427–438. [PMC free article] [PubMed] [Google Scholar]
- 45.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sariol CA, Munoz-Jordan JL, Abel K, Rosado LC, Pantoja P, Giavedoni L, Rodriguez IV, White LJ, Martinez M, Arana T, Kraiselburd EN. Transcriptional activation of interferon-stimulated genes but not of cytokine genes after primary infection of rhesus macaques with dengue virus type 1. Clin Vaccine Immunol. 2007;14:756–766. doi: 10.1128/CVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki M, Honmou O, Radtke C, Kocsis JD. Development of a middle cerebral artery occlusion model in the nonhuman primate and a safety study of i.v. infusion of human mesenchymal stem cells. PLoS One. 2011;6:e26577. doi: 10.1371/journal.pone.0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M, Consortium SV. stroke therapy academic industry roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawada K, Fukunishi K, Kashima M, Imai N, Saito S, Sakata-Haga H, Aoki I, Fukui Y. Neuroanatomic and magnetic resonance imaging references for normal development of cerebral sulci of laboratory primate, cynomolgus monkeys (Macaca fascicularis) Congenit Anom (Kyoto) 2012;52:16–27. doi: 10.1111/j.1741-4520.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz AE, Pile-Spellman J. New model of reperfused stroke by occlusion of the anterior cerebral artery in baboons. Acta Neurochirurgica. 2011;153:327–331. doi: 10.1007/s00701-010-0816-1. [DOI] [PubMed] [Google Scholar]
- 51.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 52.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U, Investigators SIT. NXY-059 for the treatment of acute ischemic stroke. New Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 53.Spetzler R, Selman W, Weinstein P, Townsend J, Mehdorn M, Telles D, Crumrine R, Macko R. Chronic reversible cerebral ischemia evaluation of a new baboon model. Neurosurgery. 1980;7:5. doi: 10.1227/00006123-198009000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- 55.Valentine LE, Watkins DI. Relevance of studying T cell responses in SIV-infected rhesus macaques. Trends Microbiol. 2008;16:605–611. doi: 10.1016/j.tim.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West GA, Golshani KJ, Doyle KP, Lessov NS, Hobbs TR, Kohama SG, Pike MM, Kroenke CD, Grafe MR, Spector MD, Tobar ET, Simon RP, Stenzel-Poore MP. A new model of cortical stroke in the rhesus macaque. J Cereb Blood Flow Metab. 2009;29:1175–1186. doi: 10.1038/jcbfm.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods SE, Marini RP, Patterson MM. Noninvasive temporal artery thermometry as an alternative to rectal thermometry in research macaques (Macaca spp.) J Am Assoc Lab Anim Sci. 2013;52:295–300. [PMC free article] [PubMed] [Google Scholar]


