Abstract
Exercise maintenance after supervised cardiac rehabilitation is important in maintaining both physical activity and physiological factors, such as peak 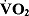 and muscle strength (MS), associated with reduced mortality. However, there is no evidence of the effects of unsupervised exercise training and MS training on physical activity and physiological factors after supervised cardiac rehabilitation of Japanese cardiac patients. We conducted a randomized, controlled trial to evaluate the effect of unsupervised exercise training on physical activity and selected physiological factors after supervised cardiac rehabilitation. Eighteen myocardial infarction (MI) patients (16 men, 2 women; mean age 66.3 years) were recruited following completion of a supervised recovery-phase cardiac rehabilitation program. Patients were randomly assigned to a MS training (n=10) or control group (n=8). Baseline measurements of physical activity, peak
and muscle strength (MS), associated with reduced mortality. However, there is no evidence of the effects of unsupervised exercise training and MS training on physical activity and physiological factors after supervised cardiac rehabilitation of Japanese cardiac patients. We conducted a randomized, controlled trial to evaluate the effect of unsupervised exercise training on physical activity and selected physiological factors after supervised cardiac rehabilitation. Eighteen myocardial infarction (MI) patients (16 men, 2 women; mean age 66.3 years) were recruited following completion of a supervised recovery-phase cardiac rehabilitation program. Patients were randomly assigned to a MS training (n=10) or control group (n=8). Baseline measurements of physical activity, peak 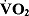 , and MS were performed at the end of supervised recovery-phase cardiac rehabilitation (6 months after the onset of MI: T1). Six months later, after going through an unsupervised exercise program (12 months after the onset of MI: T2) exercise maintenance, peak
, and MS were performed at the end of supervised recovery-phase cardiac rehabilitation (6 months after the onset of MI: T1). Six months later, after going through an unsupervised exercise program (12 months after the onset of MI: T2) exercise maintenance, peak 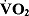 , MS, and physical activity were remeasured. The MS training group performed low-intensity MS training and walking over the second 6-month period; the control group performed walking exercise only. All patients maintained their exercise training. At T2, there were no significant differences in peak
, MS, and physical activity were remeasured. The MS training group performed low-intensity MS training and walking over the second 6-month period; the control group performed walking exercise only. All patients maintained their exercise training. At T2, there were no significant differences in peak 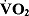 values between the MS training and control groups. There was also no significant difference in physical activity (mean number of steps per week) between the MS training and control groups. However, MS was significantly higher in the MS training group than in the control group. We concluded that unsupervised exercise training and low-level MS training performed after supervised cardiac rehabilitation may effectively maintain not only physical activity and peak
values between the MS training and control groups. There was also no significant difference in physical activity (mean number of steps per week) between the MS training and control groups. However, MS was significantly higher in the MS training group than in the control group. We concluded that unsupervised exercise training and low-level MS training performed after supervised cardiac rehabilitation may effectively maintain not only physical activity and peak 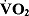 but increase MS.
but increase MS.
Keywords: unsupervised exercise training, physical activity, peak 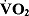 , knee extension muscle strength
, knee extension muscle strength
Cardiac rehabilitation is important to improve health-related quality of life (QOL) and physiological measures such as peak oxygen uptake (peak 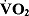 .1)–3) In addition, supervised muscle strength (MS) training is also effective for both apparently healthy people and cardiac patients2)3). Morganti et al.4) reported that great gains in strength were seen after 8–12 weeks of supervised high-intensity strength training (80% of one repetition maximum) and that smaller but significant gains continued for at least another 44 weeks in healthy older women. Izawa et al.2) suggested that middle-intensity MS training (50% of one repetition maximum) performed during a 12-week recovery-phase cardiac rehabilitation program was effective for MS. Although many reports relate the effectiveness of supervised cardiac rehabilitation, long-term maintenance of compliance after supervised cardiac rehabilitation ends has proven to be a problem5)6).
.1)–3) In addition, supervised muscle strength (MS) training is also effective for both apparently healthy people and cardiac patients2)3). Morganti et al.4) reported that great gains in strength were seen after 8–12 weeks of supervised high-intensity strength training (80% of one repetition maximum) and that smaller but significant gains continued for at least another 44 weeks in healthy older women. Izawa et al.2) suggested that middle-intensity MS training (50% of one repetition maximum) performed during a 12-week recovery-phase cardiac rehabilitation program was effective for MS. Although many reports relate the effectiveness of supervised cardiac rehabilitation, long-term maintenance of compliance after supervised cardiac rehabilitation ends has proven to be a problem5)6).
Cardiac rehabilitation in Japan is covered by national health insurance for the first 6 months after acute myocardial infarction (MI) and cardiac surgery; thereafter, patients must continue further exercise at their own volition. Exercise maintenance after supervised cardiac rehabilitation is important in maintaining both exercise capacity and the physical activity associated with reduced mortality1)3). Regular physical activity also produces significant improvements in many risk factors for cardiovascular disease, increases functional capacity and reduces the risk of re-hospitalization among cardiac patients, and improves the prognosis of patients with coronary artery disease7).
When studying the rate of exercise maintenance after supervised cardiac rehabilitation, Oldridge5) found through use of a self-reported questionnaire that a >20% reduction in attendance occurred after 6 months of post-MI cardiac rehabilitation. Some previous reports2)3) discuss improved results of physiological measurements of peak 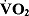 and MS in supervised exercise training for cardiac patients; however, because these outcomes were measured during supervised exercise, it is not clear if these effects also occur with unsupervised exercise training for cardiac patients after completion of supervised recovery-phase cardiac rehabilitation. Thus, we thought that evaluation of the effects of unsupervised exercise and MS training on cardiac patients after supervised cardiac rehabilitation was necessary. The purpose of the present study was to evaluate 1) the effect of unsupervised exercise training on exercise maintenance and physical activity and 2) the effect of MS training on physiological measures over the 6-month period following supervised cardiac rehabilitation.
and MS in supervised exercise training for cardiac patients; however, because these outcomes were measured during supervised exercise, it is not clear if these effects also occur with unsupervised exercise training for cardiac patients after completion of supervised recovery-phase cardiac rehabilitation. Thus, we thought that evaluation of the effects of unsupervised exercise and MS training on cardiac patients after supervised cardiac rehabilitation was necessary. The purpose of the present study was to evaluate 1) the effect of unsupervised exercise training on exercise maintenance and physical activity and 2) the effect of MS training on physiological measures over the 6-month period following supervised cardiac rehabilitation.
Methods
Study design and subjects
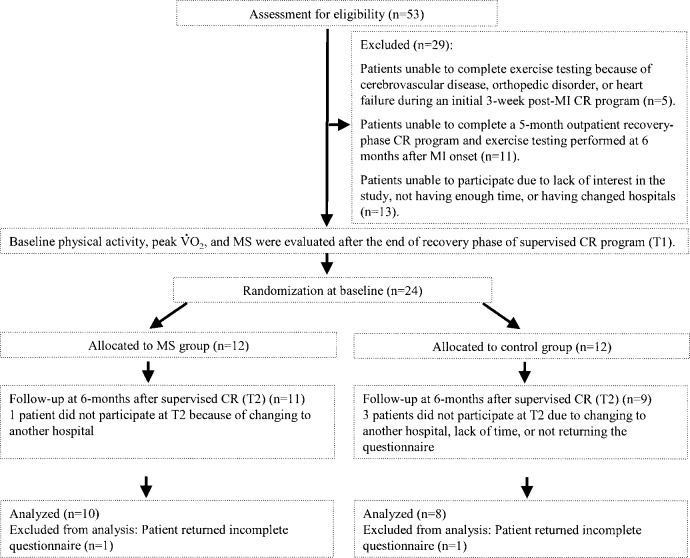
This study was a randomized, controlled trial in which subjects were selected from among 53 patients admitted to St. Marianna University School of Medicine Hospital for evaluation of MI between April 2002 and October 2002. Of these 53 patients, 48 met our criteria and initially were included in the present study. The other 5 patients were excluded due to inability to complete exercise testing because of cerebrovascular disease, orthopedic disorder, or heart failure during an initial 3-week post-MI cardiac rehabilitation program. From these 48 patients, 37 patients were recruited following completion of a 5-month outpatient recovery-phase cardiac rehabilitation program and exercise testing performed at 6 months after MI onset. Twenty-four of these 37 patients were offered participation in this study. The remaining 13 patients had no interest in the study, did not have enough time, or had changed hospitals and thus were not included. On the basis of exercise testing, the patients were randomly assigned to a MS training group (MS group, n=12) or a control group (n=12). The MS training group performed low-intensity MS training and walking during the 6-month study period; the control group performed walking exercise only.
Baseline measurements of physical activity level, peak 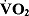 , and MS were performed at the end of the supervised recovery-phase cardiac rehabilitation program (6 months after the onset of MI: T1). Another 6 months later (12 months after the onset of MI: T2), exercise maintenance, physical activity level, peak
, and MS were performed at the end of the supervised recovery-phase cardiac rehabilitation program (6 months after the onset of MI: T1). Another 6 months later (12 months after the onset of MI: T2), exercise maintenance, physical activity level, peak 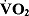 , and MS were reassessed. A flow diagram of patient progress through the phases of the randomized trial is shown in Fig. 1. The ethics committee of St. Marianna University School of Medicine institutional committee on human research approved the study, and written informed consent was obtained from all participants.
, and MS were reassessed. A flow diagram of patient progress through the phases of the randomized trial is shown in Fig. 1. The ethics committee of St. Marianna University School of Medicine institutional committee on human research approved the study, and written informed consent was obtained from all participants.
Fig. 1.
Summary of patient flow through the present study. MI, myocardial infarction; CR, cardiac rehabilitation; MS, muscle strength
Patient clinical characteristics
Peak serum creatine kinase-myocardial band (CK-MB) and left ventricular ejection fraction (LVEF) as an index of cardiac function were assessed by a cardiologist. Age, sex, body mass index (BMI), education (<12 yrs or ≥12 yrs of schooling), marital status, MI location, and medications were also evaluated.
Evaluation of exercise maintenance after the supervised cardiac rehabilitation program
Exercise maintenance after cardiac rehabilitation was evaluated on the basis of the trans-theoretical model of exercise behavior change8)9). This model suggests that the individual progresses through five stages when changing exercise behavior: 1) pre-contemplation: not physically active and does not intend to change; 2) contemplation: not active but intends to change; 3) preparation: doing some activity; 4) action: regularly active but only within the last 6 months; and 5) maintenance: regularly active for longer than 6 months. Evaluation of patients over the 6-month period of unsupervised exercise training was done through use of a self-reported questionnaire completed by the patients and returned to us by mail. On the basis of questionnaire answers, patients who we determined to be in the preparation, action, or maintenance stages were classified as maintaining exercise, and those we determined to be in the pre-contemplation or contemplation stages were classified as not maintaining exercise.
Physical activity at T1 and T2
Physical activity was measured at T1 and T2. We used mean number of steps taken per day over 1 week as the index of objective physical activity. This index was estimated by use of the Kenz Lifecorder electronic pedometer (Suzuken Co., Ltd., Nagoya, Japan), which was chosen for its reliability and validity of the data it outputs10)11). The Lifecorder records number of steps taken on the basis of patient physical characteristics (age, sex, height, and weight) entered by the physical therapist. All patients were taught to attach the Lifecorder themselves and were instructed to use the Lifecorder 24 hours a day for 1 week, except while bathing, from the time they received it. The patients were asked to maintain a log of all physical activity during this period and were instructed to return the Lifecorder to us at the end of each 1-week measurement period. Mean number of steps taken daily over 1 week = total step count over 7 days / 712).
Exercise capacity
Subjects underwent cardiopulmonary exercise testing under a ramp treadmill protocol at T1 and T213). Peak 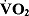 was measured to assess exercise capacity. Measurements made from expired gasses were used as indices of cardiovascular dynamics during exercise. Symptom-limited exercise testing was performed on a MAT-2500 treadmill (Fukuda Denshi Co., Tokyo, Japan). A 12-lead ECG was monitored continuously throughout the test, and heart rate was measured from the R-R interval of the ECG (ML-5000, Fukuda Denshi Co.). Peak
was measured to assess exercise capacity. Measurements made from expired gasses were used as indices of cardiovascular dynamics during exercise. Symptom-limited exercise testing was performed on a MAT-2500 treadmill (Fukuda Denshi Co., Tokyo, Japan). A 12-lead ECG was monitored continuously throughout the test, and heart rate was measured from the R-R interval of the ECG (ML-5000, Fukuda Denshi Co.). Peak 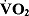 was measured during the exercise period with an AE-300S aero monitor (Minato Ikagaku Co., Tokyo, Japan) and calculated with a personal computer (Pentium 98 SE, EPSON Co., Nagano, Japan). The endpoint of exercise testing was determined according to the criteria of the American College of Sports Medicine14) . Prescribed cardiac medications were continued on the day of the exercise test.
was measured during the exercise period with an AE-300S aero monitor (Minato Ikagaku Co., Tokyo, Japan) and calculated with a personal computer (Pentium 98 SE, EPSON Co., Nagano, Japan). The endpoint of exercise testing was determined according to the criteria of the American College of Sports Medicine14) . Prescribed cardiac medications were continued on the day of the exercise test.
Measurement of knee extension muscular strength
Knee extension muscular strength was measured at T1 and T2 with the Biodex System 2 isokinetic dynamometer (Biodex Medical Systems, Inc., New York, NY, USA) to assess lower limb strength. Testing was performed at a maximum of 5 repetitions for knee extensors at isokinetic speeds of 60°/sec. Isokinetic test results were analyzed with Biodex System 2 software2). We measured the knee extension muscular strength peak torque per body weight value of each knee and used the maximum value obtained as the index of knee extension muscular strength.
Unsupervised exercise training program
After baseline testing, patients in the MS training group performed an unsupervised exercise program at least twice weekly for 1 hour that combined walking as aerobic exercise and resistance training. Exercise sessions were composed of warm-up, aerobic exercise, resistance training, and cool-down periods. Exercise intensity during aerobic exercise was maintained at a rating of perceived exertion of 11 to 13 according to the Borg 6 to 20 scale15). This scale measures the overall feeling of the subjective sensation of effort accompanying exercise. Each session was preceded and followed by series of upper- and lower-extremity and body stretches. Instructions for MS training emphasized smooth, continuous movements to ensure proper breathing to avoid Valsalva maneuvers. Two MS training exercises using body weight only were performed: squats and calf raises. Patients performed four sets of 5 repetitions/session for each exercise at a perceived exertion rating of 11 to 13. Each repetition lasted 5 sec, with a 5-sec rest between repetitions and a 10-sec rest between sets. Subjects started squat and calf exercises in the standing position. For the squat exercise, all subjects were instructed to slowly extend their knees as fully as possible, from 10° to 60° of flexion angle, and then to return to the starting position without trunk movement or use of their hands for support.
After baseline testing, patients in the control group also met at least twice weekly for 1 hour in an unsupervised aerobic exercise program comprised of walking but no MS training. Exercise sessions were composed of warm-up, aerobic exercise, and cool-down periods. Exercise intensity during aerobic exercise was maintained at a rating of perceived exertion of 11 to 13 during walking. Each session was also preceded and followed by series of upper-and lower-extremity and body stretches.
For self-monitoring purposes during the 6-month period of unsupervised exercise training, all patients were asked to record objective physical activity as measured by a pedometer (not the Kenz Lifecorder) owned by each patient to verify results of several earlier studies that reported the self-monitoring approach uses strategies to increase and maintain physical activity behavior12)16)17). Each patient was also asked to continue these recordings for the long term after completion of the 6-month unsupervised exercise period.
Statistical analysis
Results are expressed as mean ± 1 standard deviation. Non-parametric and χ2 tests were used to analyze differences in clinical factors between groups. Physical activity and physiological outcomes were analyzed using repeated-measures analysis of variance (ANOVA). ANOVA was used to compare main or interaction effects over time for period (T1 vs. T2) and group (MS group vs. control group). For each ANOVA model with a significant main or interaction effect, Tukey HSD tests were performed post hoc to localize the effects. Statistical analyses were performed with SPSS 12.0J statistical software (SPSS Japan, Inc., Tokyo, Japan). A p value of < 0.05 was considered significant.
Results
Subjects and response rate to questionnaire
Of the initial 24 patients, one patient in the MS group and two patients in the control group did not participate at T2 for reasons such as change to another hospital or lack of time. With the exception of one control group patient, 20 (95%) of the 21 remaining patients returned the questionnaire. However, one patient in each group returned incomplete questionnaires, so they were also excluded from the present study. Therefore, the study sample consisted of 18 patients, 10 in the MS group and 8 in the control group. Mean length of time from the MI event did not differ significantly between the MS and the control groups (13.8 ± 2.3 vs. 13.7 ± 2.6 months, p=0.67).
Clinical factors
Patient characteristics at baseline were almost identical between the two groups. Oral dosages of drugs did not differ significantly between the two groups (Table 1).
Table 1. Clinical characteristics of the two study groups.
| MS group (n=10) | Control group (n=8) | p value | |
|---|---|---|---|
| Mean age ±SD (yrs) | 65.2 ± 9.7 | 66.8 ± 9.9 | ns |
| Sex (Male/Female) | 9/1 | 7/1 | ns |
| BMI (kg/m2) | 23.5 ± 3.9 | 24.0 ±3.5 | ns |
| Education (yrs) | 15.2 ± 3.1 | 16.5 ±1.0 | ns |
| Married (%) | 84.1 | 85.7 | ns |
| Maximum CK-MB (IU/I) | 191.8 ±107.9 | 199.1 ±142.9 | ns |
| LVEF (%) | 49.2 ±7.8 | 51.8 ±9.1 | ns |
| Location of MI | |||
| Inferior | 5 | 3 | ns |
| Anterior | 5 | 4 | |
| Lateral | 0 | 1 | |
| Medication | |||
| Nitrates | 5 | 4 | ns |
| Calcium antagonists | 2 | 1 | |
| β-blockers | 3 | 2 | |
| ACEI | 7 | 6 | |
| ARB | 4 | 3 |
There were no significant differences between groups. ns, not significant; BMI, body mass index; CK-MB, creatine kinase-myocardial band; LVEF, left ventricular ejection fraction; CR, cardiac rehabilitation; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Exercise maintenance after cardiac rehabilitation
All 10 (100%) of the MS group patients and all 8 (100%) of the control patients continued exercise over the 6-month period of unsupervised exercise training.
Physical activity measurements at T1 and T2
Physical activity over the two time periods is presented in Table 2. Differences in physical activity values in the MS and control groups were evaluated with repeated two-way ANOVA methods. No significant period by group interactions (F [1/16]=3.45, p=0.09) were detected, and there were no significant differences in physical activity values (mean number of steps taken per day for 1 week) between MS and control groups at T1 (10458.7 ± 2210.1 vs. 9622.4 ± 2582.6 steps), and T2 (9945.7 ± 2812.7 vs. 9812.3 ± 2652.3 steps).
Table 2. Effect of unsupervised exercise training on physical activity, peak 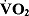 , and knee extension strength.
, and knee extension strength.
| Variable | MS group (n=10) |
Control group (n=8) |
F Value | ||
|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||
| Physical activity (steps) | 10458.7 ±2210.1 | 9945.7 ± 2812.7 | 9622.4 ±2582.6 | 9812.3 ±2652.3 | F=3.45 |
Peak 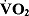 (ml/kg/min) (ml/kg/min) |
30.2 ±7.8 | 30.8 ± 6.6 | 27.4 ±6.6 | 25.9 ±5.9 | F=3.46 |
| Knee extension strength (Nm/kg)*†‡ | 1.8 ±0.4 | 2.1 ±0.4 | 1.9 ±0.3 | 1.6 ±0.3 | F=20.6 |
Main period effect, p< 0.05;
main group effect, p< 0.05;
period by group interaction, p< 0.05.
MS, muscle strength.
Physiological measurements at T1 and T2
The endpoint of exercise testing for both groups was leg fatigue, shortness of breath, or gas exchange ratio (GER) ≥1.20. No patient showed ischemic ST changes, experienced chest pain or serious arrhythmia during exercise testing. Peak 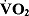 values were not significantly different from initial values within each group, and there were no statistically significant interaction periods by group (T1: 30.2 ± 7.8 vs. 27.4 ± 6.6 ml/min/kg, T2: 30.8 ± 6.6 vs. 25.9 ± 5.9 ml/min/kg; F(1, 16)=3.46, p = 0.08; Table 2). However, MS showed improvement at T2 compared with baseline results in the MS group. The effects of MS training on knee extension strength over the two time periods are presented in Table 2. Although there were no significant differences in knee muscular strength values between the groups at T1, knee muscular strength at T2 in the MS group was significantly higher than that in the control group (T1: 1.8 ± 0.4 vs. 1.9 ± 0.3 Nm/kg, T2: 2.1 ± 0.4 vs. 1.6 ± 0.3 Nm/kg; period by group interactions, F(1, 16)=20.6, p< 0.05).
values were not significantly different from initial values within each group, and there were no statistically significant interaction periods by group (T1: 30.2 ± 7.8 vs. 27.4 ± 6.6 ml/min/kg, T2: 30.8 ± 6.6 vs. 25.9 ± 5.9 ml/min/kg; F(1, 16)=3.46, p = 0.08; Table 2). However, MS showed improvement at T2 compared with baseline results in the MS group. The effects of MS training on knee extension strength over the two time periods are presented in Table 2. Although there were no significant differences in knee muscular strength values between the groups at T1, knee muscular strength at T2 in the MS group was significantly higher than that in the control group (T1: 1.8 ± 0.4 vs. 1.9 ± 0.3 Nm/kg, T2: 2.1 ± 0.4 vs. 1.6 ± 0.3 Nm/kg; period by group interactions, F(1, 16)=20.6, p< 0.05).
Discussion
This study was one of the first randomized controlled trials to investigate the effect of a combined mode of unsupervised exercise training (walking and muscle strengthening using body weight) on physical activity and physiological measures after supervised cardiac rehabilitation. We found that our exercise training program, even though unsupervised, was continued by all patients after completion of formal, supervised cardiac rehabilitation and that physical activity was not significantly different from T1 to T2 all patients.
Several earlier studies reported that the self-monitoring approach uses strategies to increase and maintain physical activity behavior16)17). When compared with standard exercise methods such as aerobic and/or MS exercise only, self-monitoring methods such as continuing to record body weight and/or physical activity in addition to performing exercise significantly improves short-term physical activity levels in sedentary people with diabetes16)17). In the present study, we used self-monitoring methods to promote exercise maintenance after supervised cardiac rehabilitation in our patients. Self-recording of physical activity during exercise maintenance in the unsupervised exercise training period may have contributed to the continuance of exercise in our patients. Thus, the present study suggests that such self-monitoring methods may be effective during unsupervised exercise training to promote short-term exercise compliance.
Adherence to our study protocol was excellent. Prior readiness for exercise has been reported to result in more effective adherence to exercise maintenance in healthy people1)19) . All patients in the present study had participated in a supervised post-MI recovery-phase cardiac rehabilitation program; therefore, it appears that prior readiness for exercise may be essential for exercise maintenance in the 6 months following completion of a cardiac rehabilitation program.
Berlanga et al.20) investigated the impact of focused, individualized advice compared with general advice on physical activity level over a 1-year period in patients with diabetes. Their study reported that in comparison with the patients who received general advice, those who received individualized advice significantly increased their total weekly energy expenditure, as measured by a physical activity questionnaire. However, physical activity was not measured objectively. In the present study, physical activity was evaluated objectively by measuring step count over 1 week. Because evaluation tools differed in these two studies, we could not compare our results directly with those of Berlanga et al.; however, the level of activity performed by our patients compared favorably with that recommended for management of patients with diabetes mellitus and/or hyperlipidemia21)22).
The walking exercise used in the present study is a low-cost, safe therapy with minimal adverse side effects and favorable benefits on a broad spectrum of health parameters, including each component of the metabolic syndrome. Therefore, we consider walking exercise in both groups to be effective from the viewpoint of promoting secondary disease prevention. However, many patients have difficulty maintaining long-term physical activity habits and lifestyle changes after completion of a supervised exercise program; Izawa et al.23) previously reported a reduction of over 19% in exercise maintenance rate 19 months after the onset of acute MI in patients undergoing a post-MI 6-month supervised cardiac rehabilitation program. Therefore, further studies are needed to evaluate the effectiveness of unsupervised exercise training over the long term after the end of supervised cardiac rehabilitation programs.
With regard to exercise adherence, other researchers have shown self-efficacy to be commonly associated with the adoption and maintenance of exercise behavior, physical activity, and health-related QOL12)24). The self-efficacy theory posits that the performance of a specific behavior is strongly influenced by the individual's confidence in his or her ability to perform that behavior25). Carlson et al.26) previously reported that self-efficacy was one of the factors in maintenance of exercise by cardiac patients. Izawa et al.12) also recently reported that the self-monitoring approach during cardiac rehabilitation may effectively increase self-efficacy. In the present study, we could not determine whether self-efficacy was affected differently between groups. Future trials are needed to evaluate the relation between self-efficacy and physiological outcomes of unsupervised exercise training.
There was no significant difference in peak 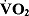 values between the MS and control groups. Several studies have reported greater increases in measures of exercise capacity such as exercise time and peak workload for aerobic exercise in cardiac patients who performed combined resistance and aerobic exercise training compared with patients who performed aerobic exercise training alone27– 29). These studies, however, used exercise time or peak workload to assess changes in aerobic exercise capacity. Another study30) conducted on cardiac patients in supervised exercise sessions used cardiopulmonary gas exchange analysis to measure peak
values between the MS and control groups. Several studies have reported greater increases in measures of exercise capacity such as exercise time and peak workload for aerobic exercise in cardiac patients who performed combined resistance and aerobic exercise training compared with patients who performed aerobic exercise training alone27– 29). These studies, however, used exercise time or peak workload to assess changes in aerobic exercise capacity. Another study30) conducted on cardiac patients in supervised exercise sessions used cardiopulmonary gas exchange analysis to measure peak 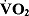 and reported results similar to those of the present study. It is very important to maintain peak
and reported results similar to those of the present study. It is very important to maintain peak 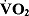 after supervised cardiac rehabilitation because exercise capacity is one of the predictors of mortality in cardiac patients31). Kavanagh et al.31) recently reported that individuals who enter a cardiac rehabilitation program with a low level of aerobic exercise gain 1.8 years of survival for each 1 ml/kg/min increase in measured
after supervised cardiac rehabilitation because exercise capacity is one of the predictors of mortality in cardiac patients31). Kavanagh et al.31) recently reported that individuals who enter a cardiac rehabilitation program with a low level of aerobic exercise gain 1.8 years of survival for each 1 ml/kg/min increase in measured 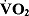 . Cardiorespiratory fitness, the principal determinant of which is physical activity, has been closely associated with reported physical activity habits and metabolic syndrome incidence32)33). Physical activity is also associated with a lower rate of death from all causes and from coronary heart disease among middle-aged and older men33). In the present study, physical activity of all patients was maintained from T1 to T2 during unsupervised exercise training. This finding suggests that even during unsupervised exercise training, continuance of physical activity may maintain exercise capacity. In the general population, promotion of higher levels of exercise capacity though greater physical activity may be the most prudent clinical and public health strategy for the primary and secondary prevention of metabolic syndrome32).
. Cardiorespiratory fitness, the principal determinant of which is physical activity, has been closely associated with reported physical activity habits and metabolic syndrome incidence32)33). Physical activity is also associated with a lower rate of death from all causes and from coronary heart disease among middle-aged and older men33). In the present study, physical activity of all patients was maintained from T1 to T2 during unsupervised exercise training. This finding suggests that even during unsupervised exercise training, continuance of physical activity may maintain exercise capacity. In the general population, promotion of higher levels of exercise capacity though greater physical activity may be the most prudent clinical and public health strategy for the primary and secondary prevention of metabolic syndrome32).
Although only low-intensity MS training was performed in the present study, MS at T2 was significantly higher in the MS group than in the control group. Kubo et al.34) suggested that in apparently healthy young and elderly people, isometric training consisting of 70% of maximal voluntary contraction increases the stiffness and modulus of human tendon structures as well as muscular strength and size. Another study also suggested that low-load MS training (squats using body weight) for 6 months is able to increase the elasticity of tendon aponeurosis structures35). Thus, exercise training can be effective in maintaining muscular strength after supervised cardiac rehabilitation. This finding agrees with those of other studies in which cardiac patients underwent resistance training to increase muscular strength. Importantly, the MS training performed in the present study required no equipment such as weight machines, elastic bands, or weights strapped to the body, and it is possible to easily perform these exercises anywhere. The MS training method used in the present study is convenient and can be performed even by unsupervised patients.
Some studies have suggested that improved strength is associated with increased muscle and bone mass, mobility, and balance, all thought to be important factors in fracture and dependency prevention4)36–38). Another study in older adults showed that resistance exercises are an important adjunct to aerobic training in maintaining MS and endurance and in improving functional capacity, particularly for activities of daily living that contribute to QOL39). Importantly, one recent study reported that MS is an independent predictor of mortality in cardiac patients40). Thus, unsupervised aerobic walking exercise in addition to MS training after supervised cardiac rehabilitation may be effective in reducing mortality and improving QOL.
Study limitations
This study was limited by the small number of patients, no investigation of the patients who had not participated in this study at T2, and unavoidable differences in environment between the two groups. Environment and patient life style may exert effects on exercise capacity and MS. Equalization of the environment between the two groups was not a viable option and could not be controlled in the present study. Also, total exercise training time and frequency of exercise in both groups were not determined. Further studies addressing these limitations should be performed.
Metabolic syndrome is a condition of high-risk phenotypes that include elevated blood pressure, dyslipidemia, impaired glycemic control, and abnormal obesity. A recent study suggested that posture and body movement in daily life influence weight gain41). In the present study, these parameters were not examined long-term after supervised cardiac rehabilitation. Further studies are needed to investigate the relation of coronary risk factors to exercise maintenance after supervised cardiac rehabilitation.
Conclusion
We conclude that the performance of aerobic walking exercise in addition to MS training during an unsupervised period following supervised cardiac rehabilitation may effectively maintain exercise capacity and increase MS. Future trials should be continued for longer periods, and long-term follow-up will be required to evaluate whether these benefits continue over time.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Young Scientists (B) #16700498 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- 1). Oldridge NB, Guyatt GH, et al. : Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA 260: 945-950, 1988. [PubMed] [Google Scholar]
- 2). Izawa K, Hirano Y, et al. : Improvement in physiological outcomes and health-related quality of life following cardiac rehabilitation in patients with acute myocardial infarction. Circ J 68: 315-320, 2004. [DOI] [PubMed] [Google Scholar]
- 3). Goto Y, Itoh H, et al. : Use of exercise cardiac rehabilitation after acute myocardial infarction. Circ J 67: 411-415, 2003. [DOI] [PubMed] [Google Scholar]
- 4). Morganti CM, Nelson ME, et al. : Strength improvements with 1 yr of progressive resistance training in older women. Med Sci Sports Exerc 27: 906-912, 1995. [PubMed] [Google Scholar]
- 5). Oldridge NB: Cardiac rehabilitation services: what are they and are they worth it? Compr Ther 17: 59-66, 1991. [PubMed] [Google Scholar]
- 6). Oldridge NB, Spencer J: Exercise habits and perceptions before and after graduation or dropout from supervised cardiac exercise rehabilitation. J Cardiopulm Rehabil 5: 313-319, 1985. [Google Scholar]
- 7). Niebauer J, Hambrecht R, et al. : Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation 96: 2534-2541, 1997. [DOI] [PubMed] [Google Scholar]
- 8). Prochaska JO: Treating entire populations for disease prevention. Japanese Health Psychology 10: 1-17, 2002. [Google Scholar]
- 9). Marcus BH, Simkin LR: The transtheoretical model: applications to exercise behavior. Med Sci Sports Exerc 26: 1400-1404, 1994. [PubMed] [Google Scholar]
- 10). Schneider PL, Crouter SE, et al. : Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc 35: 1779-1784, 2003. [DOI] [PubMed] [Google Scholar]
- 11). Crouter SE, Schneider PL, et al. : Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc 35: 1455-1460, 2003. [DOI] [PubMed] [Google Scholar]
- 12). Izawa KP, Watanabe S, et al. : Effect of the self-monitoring approach on exercise maintenance during cardiac rehabilitation. Am J Phys Med Rehabil 84: 313-321, 2005. [DOI] [PubMed] [Google Scholar]
- 13). Izawa K, Tanabe K, et al. : Cardiopulmonary response abnormalities during exercise in patients with non-insulindependent diabetes-mellitus complicated acute myocardial infarction. Cardiovasc Rev Rep 22: 734-742, 2001. [Google Scholar]
- 14). Hanson P: Clinical exercise testing. In: Blair SN, Painter P, Pate RR, Smith LK, Taylor CB: (eds) Resource Manual for Guidelines for Exercise Testing and Prescription. Philadelphia, Lea & Febiger, 1988, pp 205-222. [Google Scholar]
- 15). Borg G: Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377-381, 1982. [PubMed] [Google Scholar]
- 16). Loughlan C, Mutrie N: An evaluation of the effectiveness of three interventions in promoting physical activity in a sedentary population. Health Educ J 56: 154-165, 1997. [Google Scholar]
- 17). Kirk AF, Higgins LA, et al. : A randomized controlled trial to study the effect of exercise consultation on the promotion of physical activity in people with Type 2 diabetes: a pilot study. Diabet Med 18: 877-882, 2001. [DOI] [PubMed] [Google Scholar]
- 18). Oka K: Exercise adherence-promote of physical activity and exercise. In: Sakano Y, Maeda M: (eds) Clinical Psychology of Self-efficacy. Kyoto, Kitaouji Shobo, 2002, pp 218-234 (In Japanese). [Google Scholar]
- 19). Bock BC, Marcus BH, et al. : Maintenance of physical activity following an individualized motivationally tailored intervention. Ann Behav Med 23: 79-87, 2001. [DOI] [PubMed] [Google Scholar]
- 20). Berlanga F, Wareham N, et al. : Does a “focused’ advice to increase physical activity work in patients with newly diagnosed Type 2 diabetes? Diabet Med 15: 24-25, 1998. [Google Scholar]
- 21). Stahle A, Mattsson E, et al. : Improved physical fitness and quality of life following training of elderly patients after acute coronary events. A 1 year follow-up randomized controlled study. Eur Heart J 20: 1475-1484, 1999. [DOI] [PubMed] [Google Scholar]
- 22). Guidelines for exercise training in patients with heart disease. Circ J 66 (Suppl IV): 1177-1247, 2002. (In Japanese). [Google Scholar]
- 23). Izawa KP, Yamada S, et al. : Long-term exercise maintenance, physical activity, and health-related quality of life after cardiac rehabilitation. Am J Phys Med Rehabil 83: 884-892, 2004. [DOI] [PubMed] [Google Scholar]
- 24). Fletcher JS, Banasik JL: Exercise self-efficacy. Clin Excell Nurse Pract 5: 134-143, 2001. [DOI] [PubMed] [Google Scholar]
- 25). Bandura A: Self-efficacy mechanism in human agency. Am Psychol 37: 122-147, 1982. [Google Scholar]
- 26). Carlson JJ, Norman GJ, et al. : Self-efficacy, psychosocial factors, and exercise behavior in traditional versus modified cardiac rehabilitation. J Cardiopulm Rehabil 21: 363-373, 2001. [DOI] [PubMed] [Google Scholar]
- 27). Wosornu D, Bedford D, et al. : A comparison of the effects of strength and aerobic exercise training on exercise capacity and lipids after coronary artery bypass surgery. Eur Heart J 17: 854-863, 1996. [DOI] [PubMed] [Google Scholar]
- 28). Beniamini Y, Rubenstein JJ, et al. : High intensity strength training of patients enrolled in an outpatient cardiac rehabilitation program. J Cardiopulm Rehabil 19: 8-17, 1999. [DOI] [PubMed] [Google Scholar]
- 29). McCartney N, McKelvie RS, et al. : Usefulness of weightlifting training in improving strength and maximal power output in coronary artery disease. Am J Cardiol 67: 939-945, 1991. [DOI] [PubMed] [Google Scholar]
- 30). Pierson LM, Herbert WG, et al. : Effects of combined aerobic and resistance training versus aerobic training alone in cardiac rehabilitation. J Cardiopulm Rehabil 21: 94-100, 2001. [DOI] [PubMed] [Google Scholar]
- 31). Kavanagh T, Mertens DJ, et al. : Prediction of long-term prognosis in 12,169 men referred for cardiac rehabilitation. Circulation 106: 666-671, 2002. [DOI] [PubMed] [Google Scholar]
- 32). Paffenbarger RS, Jr, Hyde RT, et al. : The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 328: 538-545, 1993. [DOI] [PubMed] [Google Scholar]
- 33). Lamonte MJ, Baelow CE, et al. : Cardiorespiratory fitness is inversely associated incidence of metabolic syndrome: a prospective study of men and women. Circulation 112: 505-512, 2005. [DOI] [PubMed] [Google Scholar]
- 34). Kubo K, Kanehisa H, et al. : Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 91: 26-32, 2001. [DOI] [PubMed] [Google Scholar]
- 35). Kubo K, Kanehisa H, et al. : Effects of low-load resistance training on the tendon properties in middle-aged and elderly women. Acta Physiol Scand 178: 25-32, 2003. [DOI] [PubMed] [Google Scholar]
- 36). Fiatarone MA, O'Neill EF, et al. : Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330: 1769-1775, 1994. [DOI] [PubMed] [Google Scholar]
- 37). Gutin B, Peterson M, et al. : A screening and counseling program for prevention of osteoporosis. Osteoporos Int 2: 252-256, 1992. [DOI] [PubMed] [Google Scholar]
- 38). Tinetti ME, Liu WL, et al. : Predictors and prognosis of inability to get up after falls among elderly persons. JAMA 269: 65-70, 1993. [PubMed] [Google Scholar]
- 39). Fiatarone MA, Marks EC, et al. : High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029-3034, 1990. [PubMed] [Google Scholar]
- 40). Hulsmann M, Quittan M, et al. : Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail 6: 101-107, 2004. [DOI] [PubMed] [Google Scholar]
- 41). Levine JA, Lanningham-Foster LM, et al. : Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584-586, 2005. [DOI] [PubMed] [Google Scholar]