Abstract
The present study was undertaken to evaluate the effects of intermittent weight-bearing (IWB) combined with β2-agonist clenbuterol (Cb) medication for suppressing muscle atrophy during progressive disuse atrophy. Male Wistar rats (age: 8weeks, body weight: 232 ± 14 g) were divided into a control group (CON) and an experimental group. The experimental group was further subdivided into a Cb medication group under normal conditions and a hindlimb unweighting (HU) treatment group. The HU treatment group was composed of four groups: HU treatment-only, HU treatment + IWB, HU treatment + Cb medication and HU treatment + IWB + Cb medication. IWB was performed by temporarily removing the suspension device for one hour daily. On Day 14, bilateral soleus muscle (SOL) and extensor digitorum longus muscle (EDL) were extracted. Muscles from the right side were used for the measurement of contractile properties (physiological functional evaluations). Muscles from the left side were used for histochemical and biochemical analysis. During HU, IWB combined with Cb medication worked to preserve the wet weight and relative weight of SOL as compared to CON. Its contractile properties were affected by weight-bearing, while the cross-sectional area of type I fiber and protein concentration were affected by Cb. This combined therapy had marked effects on the morphology of EDL, particularly on the cross-sectional area of type II fiber. The protein concentration and contractile properties of EDL were unaffected by this combined therapy. The effect of a combination of IWB and Cb medication was specific to fiber-type and region. The data suggested that 1) IWB was effective on functional aspects such as contractile properties and useful for physical therapy, 2) Cb medication exerted the atrophy-suppressive effect in morphological parameters and manifested less effect on functional aspects. The results in this study indicated the possibility of elevating the efficacy of IWB by Cb medication in SOL.
Keywords: intermittent weight-bearing, clenbuterol, disuse muscle atrophy, rat, atrophy prevention
Physical therapy is being extensively practiced nowadays, as advances have been made in our understanding of the possibilities of rehabilitation. Often, patients with various diseases or injuries experience atrophy of certain muscles. It is desired to establish valid methods for the prevention and treatment of muscle atrophy. The etiology of skeletal muscle atrophy is myogenic, neurogenic or associated with disuse of the muscles. Disuse atrophy is viewed as a kind of adaptation to the disuse of the muscles1). It occurs in postoperative or bed-ridden patients and astronauts exposed to microgravitational environments2). The adverse effect of muscle atrophy on daily life is greater among elderly people whose absolute muscle strength has been reduced by aging3)4). However, unlike myogenic or neurogenic muscle atrophy, disuse muscle atrophy is reversible, if the cause of atrophy is eliminated. Physical therapists are therefore eager to establish appropriate methods for alleviating this condition.
To create an experimental model of disuse atrophy, researchers have developed a hindlimb suspension method5). In this method, the hindlimbs of experimental animals are suspended to deprive the opportunity of weight-bearing. Unlike fixation with plaster or internal fixation, hindlimb suspension allows active movement of the leg joints. The condition created by this method thus resembles a bed-ridden state. This model has been used by investigators at the United States National Aeronautics and Space Agency (NASA) and other facilities to devise countermeasures to prevent muscle atrophy among astronauts. A number of reports on the features, time course and other data concerning this condition have been published6)7). On the basis of these data, astronauts perform training in space for several hours a day, using various specific exercise machines (treadmills, etc.)8). However, little data has been collected concerning the effectiveness of weight-bearing actually, since it is not possible in space. For the physical therapy of people who live on the ground, weight-bearing does seem to provide a valid means of stimulation. But, it is difficult for physical therapists to prescribe for bed-ridden patients to perform treadmill or other such exercises. Weight-bearing exercise would seem to provide the safest means of stimulation in clinical practice. This means is frequently used. However, in the past, weight-bearing types of physical therapy relied too much on the experience and skill of the individual physical therapist and varied with the condition of each individual patient. The authors recognized the need to establish an efficient and valid means of providing weight-bearing exercise, and have evaluated the effects under various weight-bearing conditions to prevent muscle atrophy9–16). Although they take note of the importance of treating and alleviating atrophy once it has occurred, the authors have emphasized the suppression of progressive atrophy and shortening the time required for recovery from atrophy. In fact, weight-bearing becomes more effective as it is applied over longer periods of time. In this connection, Someya et al.17) have reported that weight-bearing needs to be practiced for 18 hours/day in order to prevent a decrease in muscle cross-sectional area. In practice, however, it is difficult to achieve such prolonged weight-bearing periods, and the authors have adopted intermittent weight-bearing (IWB) for 1.2 hours per day. Brown et al.18) also have reported that daily one-hour weight-bearing is useful for preventing the progression of disuse muscle atrophy. Our previous study revealed that the duration9), timing of the start14), frequency10–12), interval13) of weight-bearing and the duration of cessation periods15) are important factors for determining the efficacy of weight-bearing. Our previous results also suggested that applying IWB when treating progressive disuse atrophy affects myonucleus kinetics, stimulating proliferation of myonuclei and suppressing apoptosis16). However, it was not possible to achieve complete prevention of atrophy by IWB alone.
Roy et al.19) examined the effects of growth hormone (GH), insulin-like growth factor (IGF-I), and exercise (climb up the ladder) on unweighting rat soleus muscle. The cross-sectional area of the muscle remained unaffected by GH/IGF-I medication or exercise alone. However, when medication and exercise were used simultaneously, interactions were observed. This result indicates that the anabolic effect of GH/IGF-I differed from the effect of exercise on the soleus muscle. Allen et al.20) have also reported that muscle atrophy was suppressed by GH/IGF-I medication combined with resistive exercise. They suggested that a mechanism of this effect may be that loads on muscles stimulate the secretion of other growth factors or transcription factors, thus potentiating the effects of extrinsic growth factors. Their results also suggest the possibility that loads on muscles elevate the sensitivity of the muscles through enhanced binding capabilities to GH/IGF-I receptors. In other words, it seems likely that the administration of hormones such as GH during the progression of disuse muscle atrophy elevates the effect of exercise in suppressing muscle atrophy. Recently, a report was published on a study evaluating the effects of β2-adrenergic agonist, designed to devise means of preventing skeletal muscle atrophy, delaying the atrophy of denervated muscles and healing burns21). β2-adrenergic agonist is attracting close attention as a possible means of preventing atrophy in microgravitational environments, through its protein-anabolic action on skeletal muscles22–25). Pharmacological analyses26) have been conducted to examine changes in the distribution of this agonist during hindlimb suspension. Clenbuterol (Cb) is a β2-adrenergic agonist27). Clinically, Cb has been prescribed to patients with bronchial asthma or urinary incontinence with abdominal pressure. The use of Cb by body builders and athletes with the object of protein-anabolic and fat-removing effects has become a problem, and the International Olympic Committee has added this drug to its doping list28). However, the usefulness of this drug when administered appropriately has also been reported28). Zeman et al.29) conducted an experiment on mdx mice and found a decrease in muscle degeneration following Cb medication, suggesting its utility for dealing with human diseases. Also, attempts to use Cb on patients with muscular dystrophy have been made in Japan. In these attempts, Cb did not suppress the progression of the disease but it was useful for retaining muscle mass and strength30). Herrera et al.21) administered Cb to rats with progressive disuse atrophy and determined the drug's effectiveness at suppressing atrophy. Their results suggest that early Cb medication can result in a beneficial delay of atrophy among treated patients. Hayes et al.31) reported that Cb medication in combination with low intensity exercise (swimming) favorably affected the muscular contractile properties of mdx mice. On the basis of these results, author hypothesize that treatment with protein-anabolic agents such as Cb will elevate the effect of IWB against the progression of disuse muscle atrophy. If a method for safe and appropriate use of this drug is established, Cb is clinically beneficial. To date, however, no report has been published concerning the effects of IWB applied in combination with Cb medication.
The present study was undertaken to evaluate the effects of IWB combined with Cb medication for suppressing muscle atrophy during progressive disuse atrophy. The extent of muscle atrophy is often evaluated using morphological indicators such as muscle weight and cross-sectional area. Author believes, however, that evaluation of the muscle strength, i.e., functional evaluation, is also important for assessing the extent of disuse atrophy in clinical cases. In the present study, therefore, author conducted morphological evaluations (using histochemical analysis) and physiological functional evaluations (by measuring muscle tension). Furthermore, the myofibrillar protein level was measured biochemically as an indicator of anabolic effect. Thus, the effects of IWB and Cb were evaluated in a comprehensive manner. On the basis of the data thus collected, the effects of intervention (IWB and/or Cb medication), its clinical possibility and problems as a physical therapy are discussed. In practice, medication is beyond the scope of physical therapy. However, if medication can elevate the efficacy of IWB administered as physical therapy and can be beneficial to the patient, it will be significant to evaluate the usefulness of such a drug within the framework of physical therapy. If this combined therapy is proven effective, it may be possible to use it in cooperation with physicians and under informed consent of individual patients as a means of minimizing disuse atrophy of muscles in future.
Mehtods
Materials
Eighty-two male Wistar rats (age; 7 weeks) were prepared. After one week, the rats were used in this study (body weight; 232 ± 14 g). As experimental materials, the soleus muscle (SOL) was selected as the slow muscle, and the extensor digitorum longus muscle (EDL) was selected as the fast muscle in the leg. The rats were housed in individual cages under a 12-hour light-dark cycle, and maintained on standard rat feed and water ad libitum.
Protocols
This experimental protocol was approved by the committee on animal experimentation of Kanazawa University (No. 031668). The rats were divided into a control group (CON, n=12) and an experimental group. The experimental group was further subdivided into a Cb medication group under normal conditions (CON+Cb, n=10) and a hindlimb unweighting (HU) treatment group. The HU treatment group was composed of four groups: HU treatment-only group (HU, n=15), HU treatment + IWB group (HU+IWB, n=15), HU treatment + Cb medication group (HU+Cb, n=15) and HU treatment + IWB + Cb medication group (HU+IWB+Cb, n=15). The three groups without Cb medication (CON, HU and HU+IWB) were injected with physiological saline under the same conditions. The period of this experiment was set up for 2 weeks. Disuse atrophy was induced by the HU treatment (hindlimb suspension) device described in our previous study15). The hindlimb was suspended so that it did not touch the floor. During suspension, the rats could move using their forelimbs to consume food and water. IWB was performed by temporarily removing the suspension device so that the rats supported their body weight on all four limbs for one hour daily. The rats were subcutaneously injected with Cb (1.0 mg/kg, clenbuterol hydrochloride, Sigma Chemical Co., St. Louis, MO, USA) or saline (1.0 ml/kg) following a 2-day on/2-day off dosing regimen26) for 2 weeks (days 0, 1, 4, 5, 8, 9, 12 and 13).
Muscle preparation
On Day 14, body weight was measured, and bilateral SOL and EDL were extracted under anesthesia (pentobarbital sodium, 50 mg/kg, ip). Muscles from the right side were used for the measurement of contractile properties and water content. Muscles from the left side were rapidly frozen and stored at −80°C for histochemical and biochemical analysis.
Measurement of contractile properties and water content
After measurement of muscle length and girth at rest, isometric contractile properties were measured in vitro. The muscle length was measured the interval between proximal and distal myotendonous junction using slide caliper. The muscle girth was measured the length of the suture thread after ligation at muscle-belly. The muscle was mounted onto a force-recording device (LTS-500GA, Kyowa, Japan) and bathed in Ringer's solution (25°C), which had been aerated with oxygen gas. The muscle was stretched to 110% of its rest length, stimulated with a supramaximal square wave (0.2 ms duration) delivered via two parallel platinum electrodes using an electric stimulator (SC6, Medelec, UK). The following measurement of peak twitch tension (Pt), tetanus was elicited at 0.2 ms duration, 100 Hz, 640 ms trains. Contractile responses were recorded and analyzed on an analog-to-digital converter (MacLab/4S, Castle Hill, Australia) coupled to a computer (Power Macintosh G4, Apple Computer Inc., USA). The analysis parameters (Fig. 1) were Pt, latent time (LT), contraction time (CT), one-half relaxation time (1/2RT) at twitching and peak tetanic tension (Po) at tetanus. The crosssectional area of muscle was estimated by dividing muscle wet weight (mg) by muscle length (mm) using a modification of the technique described by Fitts et al.32). The muscle wet weight was measured after blotting the extra solution three times using absorbent paper. After freeze-drying (FD-1000, Tokyo Rika, Japan) for 48 hours, the muscle dry weight was measured. The water content was calculated from the difference between the muscle wet and dry weight.
Fig. 1.
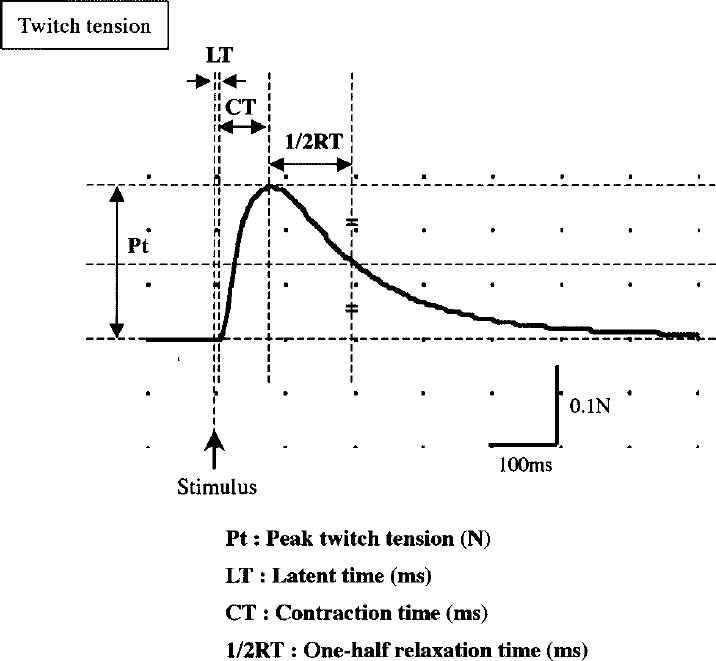
Parameters of contractile properties.
Histochemical analysis
Transverse sections (10 µm in thickness) were cut in a cryostat microtome at −25°C and classified into muscle fiber type (I and II) with routine ATPase staining (pH 10.6). The proportion of each type in number of muscle fibers was calculated as a qualitative index of atrophy according to the method described in a previous study16). As quantitative index of atrophy, the cross-sectional area of more than 200 muscle fibers in each muscle was measured16). Furthermore, the atrophy factor (AF), which is the modified method of Hachisuka et al.33), was calculated from the histograms in the cross-sectional area. To obtain AF, the number of muscle fibers in the histograms with a crosssectional area of less than 500 µm2, between 500 and 1000 µm2, between 1000 and 1500 µm2, and between 1500 and 2000 µm2, were multiplied by 4, 3, 2 and 1, respectively, and these products were added and then divided by the total number of muscle fibers. The larger the AF compared with CON, the more remarkable the atrophy.
Biochemical analysis
The muscle protein concentration was obtained using a modification of the technique described by Wineski et al.22) and Caiozzo et al.34). All tissue preparation was performed on ice with buffer at 4°C. The muscle was homogenized in 10 vol of a solution (containing 250 mM sucrose, 100 mM KCl, 20 mM imidazole and 5 mM EDTA, adjusted to pH 6.8) using a homogenizer (ULTRA-TURRAX T8, IKA-WERKE, Germany). A total of 100 µl aliquots of the homogenate was used to determine the total protein (TP) concentration. The remaining aliquots of the above were centrifuged at 1,000 × g for 10 min. using a centrifugal separator (5415R, EPPNDORF, Japan). The pellet was resuspended in 10 vol of a solution (containing 175 mM KCl and 20 mM imidazole, adjusted to pH 7.0 : Solution A). This homogenate was centrifuged as described above. The final pellet was suspended in solution A of 300 µl and was used to determine the myofibrillar protein (MP) concentration. The protein concentration was determined using a bicinchoninic acid (BCA) protein assay35) (Regent Kit 23227, Pierce Chemical Co., USA). Bovin serum albumin (BSA) was used as the standard. The TP and MP concentrations were expressed as milligrams per gram muscle wet weight.
Statistical analysis
The data are expressed as mean ± SD. The differences among all groups were statistically evaluated using a one-way analysis of variance (ANOVA). Additionally, a two-way ANOVA among the HU treatment groups was performed to test whether IWB, Cb medication, and/or interactions between these, significantly affected the parameters measured in this study. When a significant difference was recognized (p<0.05), paired comparisons were performed using Scheffe's post hoc test. To investigate the correlation among muscle weight, tension and protein concentration, Pearson's correlation coefficient was calculated, and correlation analysis was performed.
Results
In the HU treatment group, three rats (two in HU and one in HU+Cb) were removed from the device, which resulted in the hindlimb temporarily bearing the weight. The final number of rats used in the analysis was 79 because the data of these rats were excluded.
Muscle weight (Table 1)
Table 1. Muscle mass and water content.
| Group | n | Muscle wet wt (mg) | Wet wt/body wt (mg/g) | Muscle length (mm) | Muscle girth (mm) | Water content (mg) |
|---|---|---|---|---|---|---|
| SOL | ||||||
| CON | 12 | 133.0 ± 8.4† | 0.47 ± 0.04† | 26.2 ± 1.6† | 8.4 ± 0.4 | 98.4 ± 11.1† |
| CON+Cb | 10 | 140.4 ± 7.6† | 0.51 ± 0.04† | 25.3 ± 0.6† | 9.7 ± 0.3*† | 107.9 ± 7.5† |
| HU | 13 | 74.4 ±10.0* | 0.35 ± 0.04* | 21.4 ± 1.5* | 7.5 ± 0.4 | 62.4 ± 11.5* |
| HU+IWB | 15 | 93.9 ± 9.8*† | 0.44 ± 0.04† | 23.6 ± 0.5*† | 8.5 ± 0.5† | 89.0 ± 11.0† |
| HU+Cb | 14 | 113.6 ± 9.8*† | 0.49 ± 0.05† | 20.6 ± 1.3* | 10.1 ± 0.8*† | 92.2 ± 13.1† |
| HU+IWB+Cb | 15 | 122.4 ±10.4† | 0.51 ± 0.02† | 22.3 ± 1.4* | 10.7 ± 0.6*† | 100.8 ± 14.5† |
| EDL | ||||||
| CON | 12 | 151.4 ±12.4† | 0.54 ± 0.03 | 23.9 ± 0.6† | 10.2 ± 0.8† | 114.5 ± 8.5† |
| CON+Cb | 10 | 165.8 ±17.3† | 0.60 ± 0.04*† | 24.9 ± 0.6† | 10.7 ± 0.5† | 136.4 ± 17.9*† |
| HU | 13 | 113.9 ± 7.5* | 0.54 ± 0.04 | 21.9 ± 0.3* | 8.5 ± 0.6* | 87.9 ± 8.2* |
| HU+IWB | 15 | 115.0 ± 9.1* | 0.54 ± 0.04 | 23.7 ± 0.7† | 9.3 ± 0.4 | 98.3 ± 8.8 |
| HU+Cb | 14 | 139.5 ± 6.6† | 0.60 ± 0.04*† | 23.3 ± 1.0† | 10.4 ± 0.5† | 109.7 ± 7.0† |
| HU+IWB+Cb | 15 | 132.9 ±10.8*† | 0.56 ± 0.03 | 24.1 ± 0.8† | 0.5 ± 0.4† | 106.1 ± 8.7† |
n : the number of rats. wt : weight.
*: p<0.05 (compared with CON).
† : p<0.05 (compared with HU).
The wet weight of SOL in CON+Cb tended to increase compared with CON, but the difference was not significant. Compared with CON, the parameter significantly decreased to 55.9% in HU, 70.6% in HU+IWB, 85.4% in HU+Cb. The wet weight in HU+IWB+Cb was 92.0% of the values for CON, and no significant difference between either group was recognized. In the HU treatment groups, a significant difference was recognized between any of the two groups, except between HU+Cb and HU+IWB+Cb. The muscle-to-body weight ratio (relative weight) of SOL in CON was not significantly different in any of the experimental groups, except for HU. The ratio of intervention groups (HU+IWB, HU+Cb and HU+IWB+Cb) was significantly greater than in HU. The wet weight of EDL in CON+Cb and HU+Cb was not significantly different from CON. Compared with CON, the parameter significantly decreased to 75.2% in HU, 76.0% in HU+IWB, 87.8% in HU+IWB+Cb. Among the HU treatment groups, a significant difference was recognized between any of the two groups, except between HU and HU+IWB and between HU+Cb and HU+IWB+Cb. The relative weight of EDL in CON+Cb and HU+Cb was significantly greater than CON.
Muscle length and girth (Table 1)
The muscle length of SOL in the HU treatment groups significantly decreased compared with CON. Among the HU treatment groups, the muscle in HU+IWB was significantly longer than in HU. The muscle girth of SOL in the three groups medicated with Cb (CON+Cb, HU+Cb and HU+IWB+Cb) was significantly greater than CON. Among the HU treatment groups, the muscle girth in the intervention groups was significantly greater than HU. The muscle length of EDL in HU significantly decreased compared with CON. Among the HU treatment groups, the muscle of the intervention groups was significantly longer than HU. The muscle girth of EDL in HU was significantly smaller than CON. Among the HU treatment groups, the muscle girth of intervention groups was significantly greater than HU.
Water content (Table 1)
The water content of SOL in HU significantly decreased compared with CON. Among the HU treatment groups, the water content in the intervention groups was significantly greater than HU. Compared with CON, the water content of EDL in CON+Cb increased, and that in HU decreased. Among the HU treatment groups, the water content of EDL in HU+Cb and HU+IWB+Cb was significantly greater than HU. There was no change in the proportion of water in either SOL or EDL.
Contractile properties (Table 2, Figs. 2, 3)
Table 2. Contractile properties of peak twitch tension in SOL and EDL.
| Group | CT (ms) | 1/2RT (ms) | Pt (N) | Pt/cm2 (N/cm2) |
|---|---|---|---|---|
| SOL | ||||
| CON | 80.0 ± 19.3† | 116.5 ± 51.9 | 0.19 ± 0.03† | 3.66 ± 0.47† |
| CON+Cb | 74.5 ± 10.7 | 99.0 ± 29.9 | 0.24 ± 0.02*† | 4.22 ± 0.46† |
| HU | 59.6 ± 8.8* | 97.7 ± 29.0 | 0.05 ± 0.02* | 1.56 ± 0.42* |
| HU+IWB | 79.3 ± 12.1† | 111.3 ± 29.1 | 0.11 ± 0.04*† | 2.78 ± 1.01† |
| HU+Cb | 72.9 ± 19.1 | 114.6 ± 31.8 | 0.07 ± 0.03* | 1.21 ± 0.54* |
| HU+IWB+Cb | 79.3 ± 8.0† | 113.7 ± 25.5 | 0.14 ± 0.03*† | 2.51 ± 0.61*† |
| EDL | ||||
| CON | 34.5 ± 6.0 | 29.5 ± 12.1 | 0.43 ± 0.10† | 7.08 ± 1.65 |
| CON+Cb | 33.0 ± 4.8 | 34.0 ± 14.3 | 0.49 ± 0.09† | 7.37 ± 1.79 |
| HU | 31.9 ± 3.8 | 26.9 ± 6.3 | 0.32 ± 0.07* | 6.38 ± 1.30 |
| HU+IWB | 30.3 ± 2.3 | 29.7 ± 3.5 | 0.27 ± 0.05* | 5.49 ± 1.10 |
| HU+Cb | 33.2 ± 3.2 | 34.3 ± 8.3 | 0.43 ± 0.09† | 7.32 ± 1.53 |
| HU+IWB+Cb | 33.7 ± 4.4 | 36.0 ± 10.2 | 0.39 ± 0.06 | 7.06 ± 0.89 |
CT : contraction time. 1/2RT : one-half relaxation time. Pt : peak twitch tension.
*: p<0.05 (compared with CON).
†: p<0.05 (compared with HU).
Fig. 2.
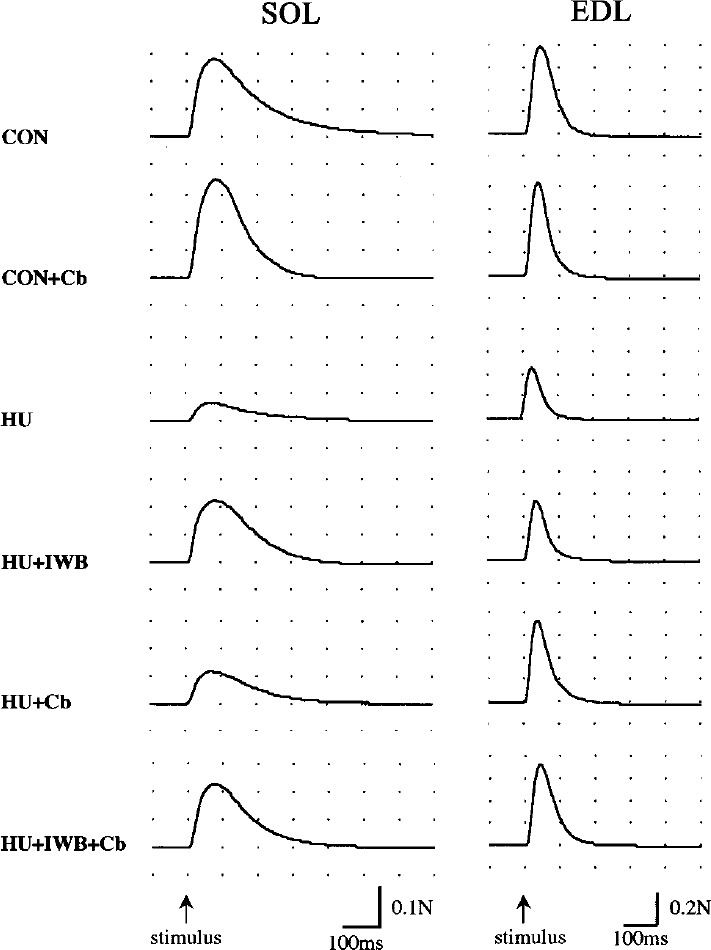
The typical wave of peak twitch tension in SOL and EDL.
Fig. 3.
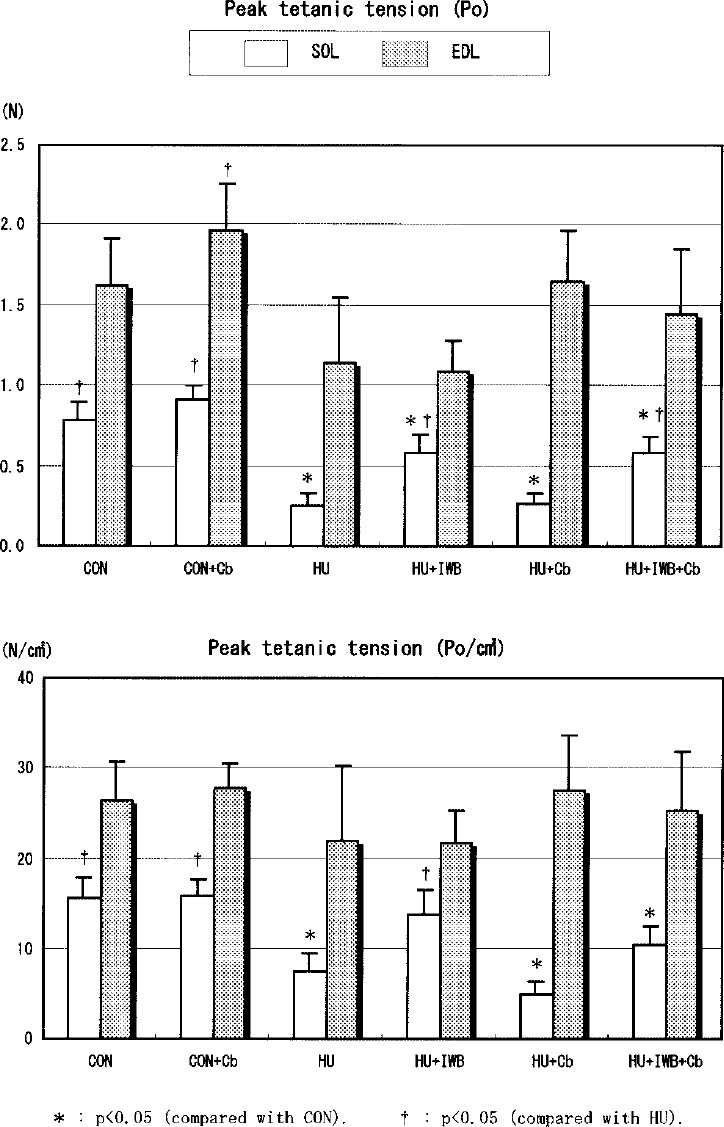
Peak tetanic tension in SOL and EDL.
LT and 1/2RT of SOL did not differ among each group. 1/2RT of EDL in HU+IWB+Cb was longer than HU. CT of SOL in HU was much shorter compared with CON. The parameters of SOL in HU+IWB and HU+IWB+Cb were significantly longer than HU. There was no change in CT of EDL in this study. Pt and Po of SOL in the HU treatment group significantly decreased compared with CON. Among the HU treatment groups, the parameters in HU+IWB and HU+IWB+Cb were significantly greater than HU. No statistically significant difference in Pt/cm2 of SOL was recognized between HU+IWB and CON. There was no difference in the parameters between HU and HU+Cb. Po/cm2 of SOL in HU+IWB was significantly greater than HU+Cb. Pt of EDL in HU and HU+IWB significantly decreased compared with CON. The parameter in HU+Cb was significantly greater than HU. Pt/cm2 of EDL in HU+Cb was significantly greater than HU+IWB. No statistically significant difference in Po and Po/cm2 of EDL in CON was recognized among the other groups.
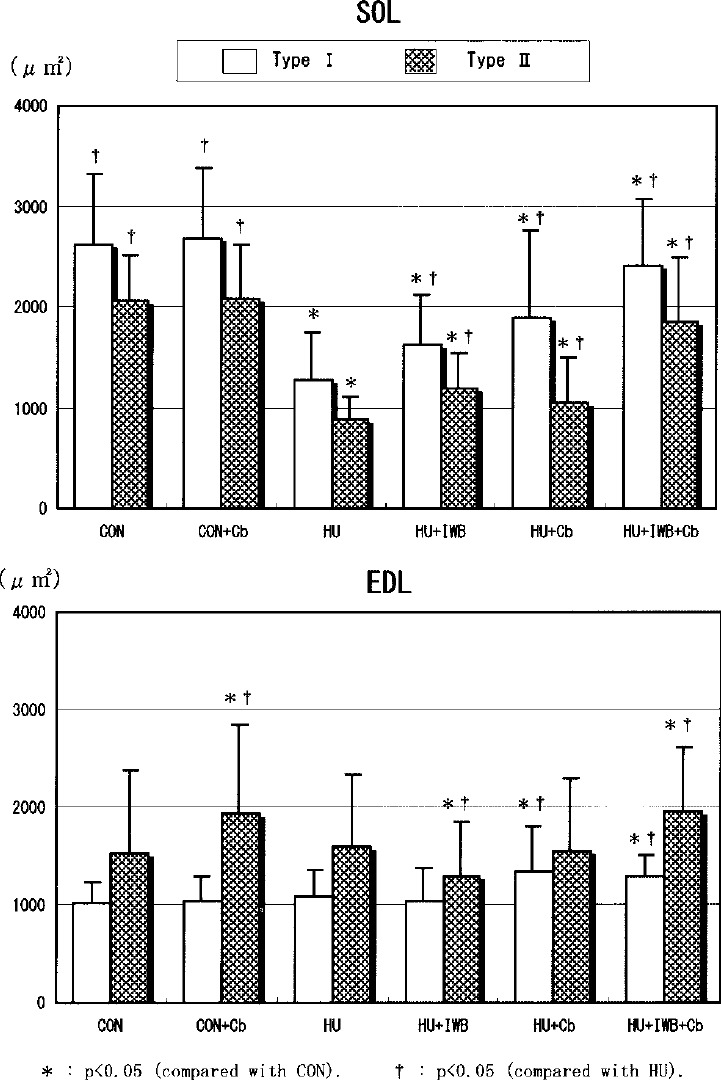
Type proportion and cross-sectional area of muscle fibers (Figs. 4, 5, 6)
Fig. 4.
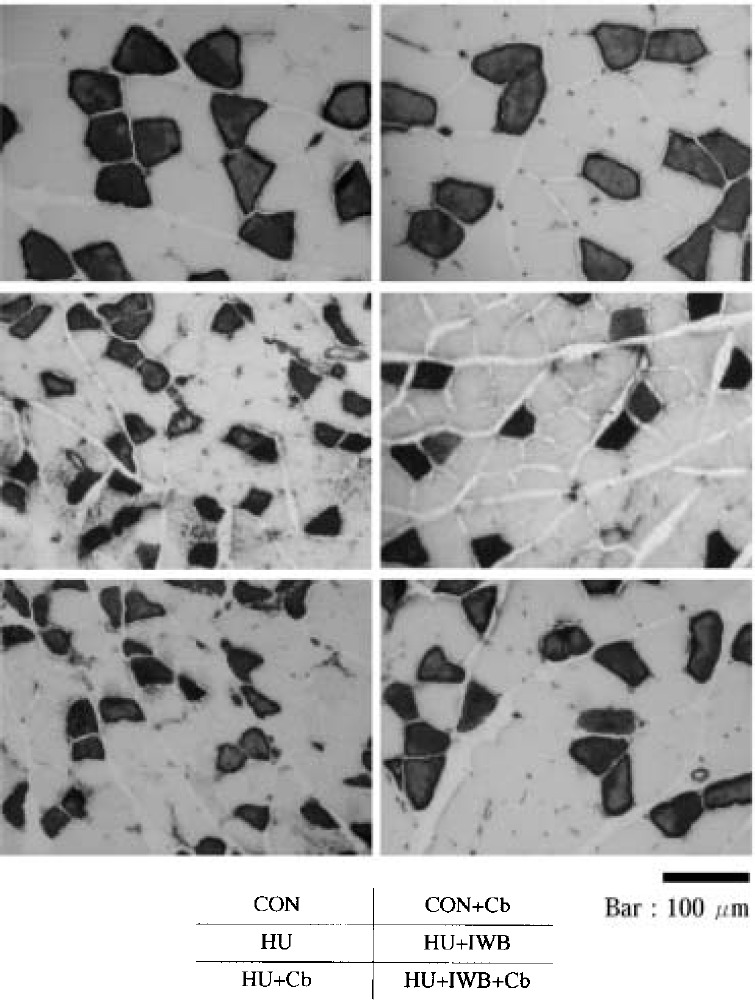
Photomicrograph of SOL cross-section (ATPase stain, pH 10.6).
Fig. 5.
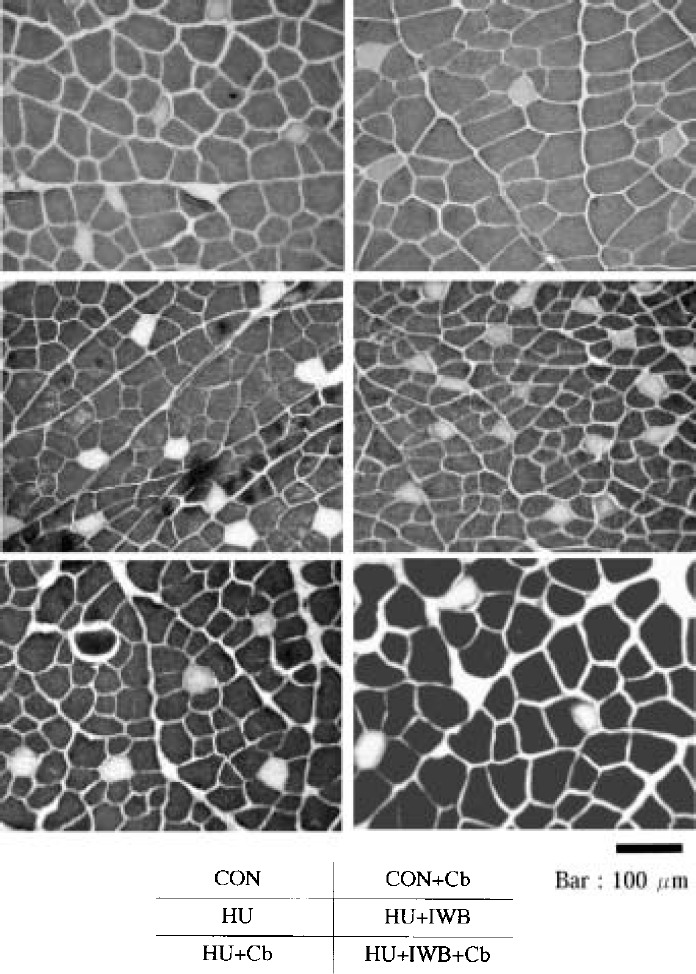
Photomicrograph of EDL cross-section (ATPase stain, pH 10.6).
Fig. 6.
Cross sectional area of SOL and EDL fibers.
The proportion of SOL type I fibers in the HU treatment groups (66.5–70.9%) tended to decrease compared with CON (71.3%). However, there was no statistical difference between each group. The proportion of EDL type II fibers in the HU treatment groups (93.8–95.8%) significantly increased compared with CON (90.7%). Compared with CON (AF=0.25), the mean crosssectional area of SOL type I fibers did not differ statistically from CON+Cb (AF=0.18). The parameter significantly decreased in HU (AF=1.96), HU+IWB (AF=1.30), HU+Cb (AF=1.17) and HU+IWB+Cb (AF=0.36). Compared with CON (AF=1.55), the mean cross-sectional area of EDL type II fibers did not statistically differ from HU (AF=1.49) and HU+Cb (AF=1.57). The parameter significantly increased in CON+Cb (AF=1.13) and HU+IWB+Cb (AF=1.09). Conversely, it significantly decreased in HU+IWB (AF=1.69).
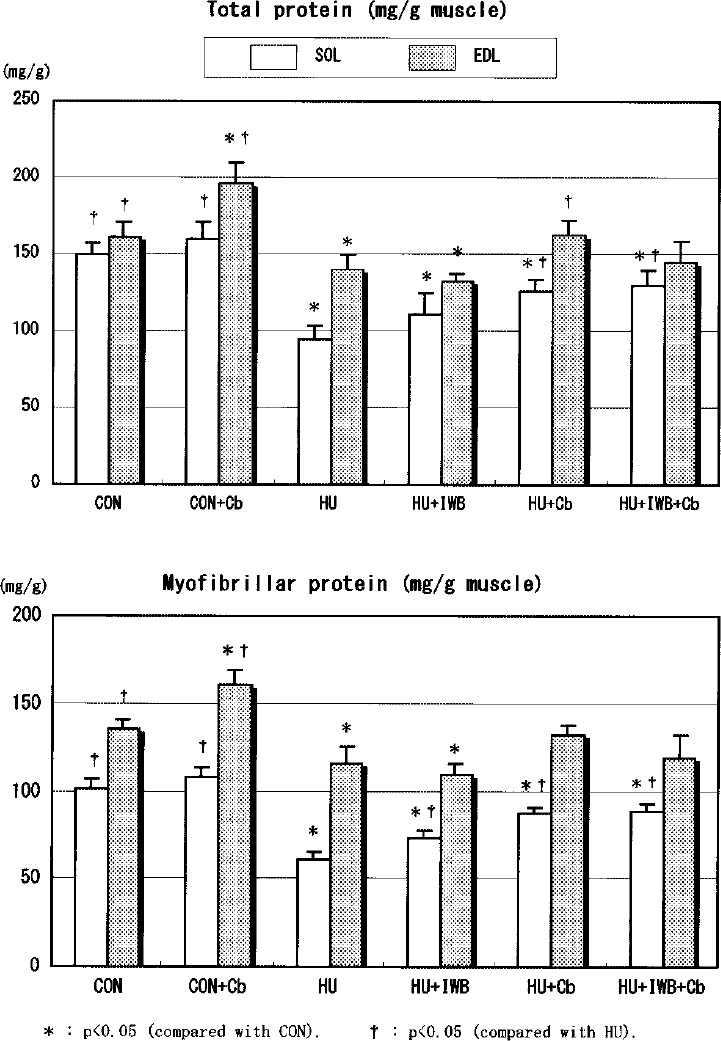
Protein concentrations (Fig. 7)
Fig. 7.
Total and myofibrillar protein content in SOL and EDL.
The TP and MP concentration of SOL in the HU treatment group significantly decreased compared with CON. Among the HU treatment groups, the TP and MP concentration in intervention groups were significantly greater than HU. The parameters of SOL in HU+Cb were greater than HU+IWB. The TP and MP concentrations of EDL in HU+Cb and HU+IWB+Cb did not statistically differ from CON. The parameters increased in CON+Cb, and decreased in HU and HU+IWB, compared with CON. The TP and MP concentrations of EDL in HU+Cb were greater than HU+IWB.
Interaction
The results of a two-way ANOVA showed that no statistically significant interaction in all parameters was recognized between IWB and Cb medication. As to IWB, a significant influence was recognized in all parameters, except for 1/2RT, of SOL. As to Cb medication, a significant influence was recognized in all parameters of EDL.
Correlation
The results of the correlation analysis showed significant positive correlation among muscle wet weight, Po and MP concentration. The correlation between muscle wet weight and MP concentration (SOL: r=0.944, EDL: r=0.936) was higher than between wet weight and Po (r=0.671 r=0.690) and between MP concentration and Po (r=0.672, r=0.689).
Discussion
The present study was undertaken to examine the effects of IWB and Cb medication in suppressing atrophy of rat skeletal muscles during progression of disuse muscle atrophy. First, the validity of inducing disuse muscle atrophy in this study is discussed. The wet weight of SOL in HU decreased to 55.9% of that in CON, and the water content of SOL in HU also decreased to 63.4% of CON. The relative weight also decreased significantly in HU. Analysis of the contractile properties revealed that CT decreased significantly in HU, suggesting that the muscle in this group had a tendency to fast muscle. Muscle tension, cross-sectional area and protein content also decreased significantly in HU. These findings indicate that marked disuse atrophy was induced in SOL of HU. The wet weight of EDL in HU was 75.2% of that in CON. The water content of EDL also decreased in HU to 76.8% of the control level. The relative weight of EDL did not differ between HU and CON. Of the indicators of contractility, Pt decreased in HU, while Pt/cm2 and other parameters remained unchanged. The muscle protein concentration decreased significantly in HU. These changes seem to represent temporary growth failure36) due to HU treatment and to support the previous reports32)37) that EDL is less prone to the influence of unweighting state than is SOL.
Next, the effects of Cb are discussed by comparing the data from CON with those from the CON+Cb. Of the indicators of contractility, Pt of SOL increased significantly, while Pt/cm2 of the same muscle did not change. The muscle girth increased significantly. The other parameters showed no significant inter-group difference. These results indicate that SOL did not hypertophy adequately during two weeks of treatment. The contractile properties and muscle girth of EDL showed no significant inter-group difference. However, the relative weight, cross-sectional area of type II fiber and protein content of EDL increased significantly, suggesting that the anabolic effect of Cb induced muscle hypertrophy. These results support the results of previous studies22)26) that Cb is more effective on the EDL than on SOL. When the type proportion of muscle fiber was analyzed, HU treatment resulted in a tendency to fast muscles, although this change was not statistically significant in SOL. This change seems to serve as an important qualitative indicator of atrophy when long-term effects of treatment are evaluated. This point needs additional study from now on. The correlation between muscle wet weight and MP protein concentration was higher than the correlation between wet weight and Po, suggesting that the myofibrillar protein concentration reflected a morphological indicator of atrophy.
IWB during hindlimb suspension exerted effects on the following parameters of SOL: wet weight, water content, muscle length, girth, contractile properties (CT, Pt, Pt/cm2, Po and Po/cm2), cross-sectional area and protein concentration. However, IWB worked unfavorably on the EDL, causing a decrease in the cross-sectional area of type II fibers of this muscle. When interpreting these results, we should consider the ankle joint condition. During suspension, the ankle joint gradually assumes the position of plantar-flexion7), and SOL is shortened approximately 10 mm and EDL is lengthened38). It has been reported that atrophy of plantar flexors during suspension was prevented by fixation of the ankle joint in the dorsi-flexion position and passive stretch39). This means that weight-bearing during suspension induces passive dorsi-flexion of the ankle joint, leading to lengthening of SOL. It seems likely that the increase in muscular work, which was needed to support the body weight, effectively worked to suppress progression of atrophy. The EDL, which is stretched during suspension, is less likely to undergo atrophy. So if weight is loaded during suspension, EDL will shorten and adversely affect the goal of suppressing atrophy.
Cb medication during hindlimb suspension favorably affected wet weight, water content, muscle girth, cross-sectional area and protein concentration of SOL. It favorably affected wet weight, water content, Pt, muscle length, girth, cross-sectional area of type I fiber and protein concentration of EDL. The wet weight, water content, muscle length, girth, cross-sectional area of type II fiber and protein concentration of EDL were kept at the levels comparable to those of CON. In both muscles, Cb exerted the atrophy-suppressive effect primarily in morphological parameters and manifested less effect on functional aspects such as contractile properties. Firstly, this result seems to be associated with the fact that during suspension, the development of muscle tension is limited even when the muscle is active6). In fact, the activity of SOL, as measured by EMG, decreased for a while immediately after the start of suspension but it normalized gradually thereafter38). However, since significant atrophy was observed, it has been pointed out that the suspension-induced shortening of SOL limits the production of muscle tension. Secondly, it seems possible that muscle hypertrophy due to Cb did not lead to development of significant tension. The MP concentration primarily reflects the contractile proteins (actin and myosin) and is usually thought to correlate with contractility. However, muscle hypertrophy induced by Cb medication during hindlimb suspension had no effect on muscle tension, suggesting that there was discrepancy between morphology and function. When identifying the cause of this discrepancy, it is noteworthy that as compared to CON, HU+Cb showed a decrease in the length of SOL but an increase in the girth of this muscle. It seems likely that the change caused by hypertrophy in the muscular shape40)41) is one possible factor for this discrepancy. In the case of pennate muscles like SOL, hypertrophy can elevate the pennation angle, leading to a discrepancy between anatomical cross-sectional area and physiological crosssectional area. This means that an increase in pennation angle due to hypertrophy enlarges the loss during the transmission of muscle tension to the tendons and serves as a factor disadvantageous for production of tension42).
During hindlimb suspension, IWB combined with Cb medication worked to preserve the wet weight and relative weight of SOL as compared to CON. Indicators of contractile properties (Pt, Pt/cm2 and Po) were significantly greater in HU+IWB+Cb than in HU+Cb but did not differ between HU+IWB+Cb and HU+IWB. The cross-sectional area of muscle fibers was greater in HU+IWB+Cb than in any other intervention group. Muscle protein concentration in this group was significantly higher than that in HU+IWB but did not differ from that in HU+Cb. These results suggest that when SOL is exposed to IWB and Cb medication during suspension, its contractile properties are affected by weight-bearing, while the cross-sectional area and protein concentration are affected by Cb. When either IWB or Cb medication was applied separately, the wet weight of the muscle was not preserved although its decrease was smaller. In any event, the success in preserving the wet weight of SOL as compared to CON by the combined application of IWB and Cb medication indicates that this therapy is significant from the viewpoint of preventing muscle atrophy. The cross-sectional area of EDL increased significantly following this combined therapy, as compared to CON. Pt in HU+IWB+Cb was larger than that in HU+IWB, but did not differ from that in HU+Cb. The MP concentration of EDL remained unaffected by this combined therapy. These results suggest that this combined therapy has marked effects on the morphology of EDL, particularly on cross-sectional area of type II fiber. The EDL is less prone to the influence of unweighting on hindlimbs than SOL22)26). However, considering that type II fibers are predominant in EDL (accounting for more than 90%) and that this type is more likely to undergo atrophy with aging3)4), the observed effects of the combined therapy suggest its usefulness.
Direct and indirect actions21) have been suggested to explain the mechanism of the effects of Cb, β2-adrenergic agonist. Its direct activity involves the binding of Cb to β2-receptor on membrane of skeletal muscle fiber, which stimulates adenylate cyclase activity and causes an increase in skeletal muscle cyclic adenosine monophosphate (AMP) and cyclic AMP-dependent protein kinase, leading to an increase in protein synthesis and a decrease in protein degradation21). Hinkle et al.27) reported that the effect of Cb in inducing muscle hypertrophy or inhibiting muscle atrophy is mediated by β2-adrenergic receptor. The indirect activity of Cb pertains to the activity of increasing the production of other factors or hormones mediated by β-receptors on non-muscular cells21). Awede et al.43) suggested that Cb is associated with increased local expression of muscular IGF-I and that Cb activates skeletal muscle IGF-I expression both at the mRNA and peptide levels. It was recently shown that IGF-I induces muscle hypertrophy mediated by the calcium calcincurin pathway44). It has also been suggested that Cb activates satellite cells, leading to stimulation of the production of growth factors45). Although the exact mechanism for interactions between specific cells has not yet been clarified, these results suggest that Cb is associated with increased protein synthesis and thus elevates cell size and muscle strength21).
Pharmacokinetics of Cb in rats during hihndlimb suspension have been reported26), demonstrating that the Cb level in skeletal muscles was higher in unweighting rats than in normal rats. Von Doutsch et al.26) reported that the Cb concentration in SOL and EDL of unweightimg rats was 1.8 times and 1.2 times as high as that of normal rats, respectively. These differences seem to be attributable to influence of suspension-induced shift of body fluid on drug clearance and difference in blood flow to each muscle on Cb concentration. It has also been reported that the response to Cb during suspension was larger in the EDL (in which type II fibers are predominant) and smaller in SOL (in which type I fibers are predominant)22)46). These reports and the results in this study suggest that responses to Cb during suspension vary depending on the type of muscle fibers (fiber-type specificity). In addition, the possibility that a suspension-induced change in muscle length modifies the responses to Cb has been suggested, indicating the necessity of taking into account region-specific responses to Cb (region specificity)22)26). IWB during suspension seems to have more complex influences. IWB during suspension caused changes in the muscle length (stretching of SOL and shortening of EDL). It is known that stretch of skeletal muscles stimulates local production of IGF-I in autocrine and pracrine manners, leading to muscle hypertrophy43). The authors examined the effects on myonuclei and reported that IWB during progressive disuse atrophy stimulates the proliferation of myonuclei and suppresses apoptosis16). In rat skeletal muscles, the metabolic characteristic (oxidative potential) correlated with the density of β-receptors, and type I fibers have more β-receptors and higher adenylate cyclase activity47). If these findings and knowledge are combined with the fact that physical training elevates the number of β-receptors and adenylate cyclase activity48), it seems likely that a combination of IWB and Cb medication is useful in suppressing atrophy of the SOL (in which type I fibers are predominant) caused by the absence of weight-bearing. It has been reported that the effect of Cb in suppressing atrophy is higher on denervated muscles than in normally innervated muscles49)50). Some factors of the nervous system may affect this effect of Cb. In the absence of weight-bearing, normal muscular activity is impossible, and afferent information from muscles to the center decreases, making it impossible for muscles to remain normal function. Even when Cb stimulates hypertrophy of muscles under such circumstances, this may not be accompanied by improvement in functional aspects (e.g., muscle strength). It is therefore expected that a combination of IWB and Cb medication will exert effects in a more comprehensive manner. It is significant that the present study involving IWB and Cb medication to SOL during suspension succeeded in supporting the effectiveness of a combination of exercise (resistive exercise and swimming) and protein-anabolic agent medication previously reported by Roy et al.19), Allen et al.20) and Hayes et al.31). In the EDL, effects of the combined therapy were seen in the cross-sectional area of type II fibers which had shown aging-related atrophy, but no marked effects were seen in contractility. Thus, the efficacy of the combined therapy was found to vary depending on muscle fiber-type. For efficient utilization of the effect of Cb on morphological aspects of type II fibers in the EDL, it seems necessary to determine the optimal frequency and timing of IWB so that this therapy can exert efficacy on muscle function. To make a general evaluation of the effects of a combination of IWB and Cb medication, it is necessary to consider not only the effects on each individual muscle but also the effects on the whole body. Weight-bearing is a physiologically normal stimulus. The movement of the individual associated with weight-bearing is expected to have large impacts on systemic condition. Under the conditions of the present study, the combined therapy was effective on SOL but had little effects on the function of EDL. This result needs to be taken into account when considering the clinical applicability of this therapy. Thus, the present study demonstrated that the effect of a combination of IWB and Cb medication in suppressing the progression of disuse muscle atrophy is specific to fiber-type and region. Taken the results together, this combined therapy is promising.
Concerning the methods of Cb medication, subcutaneous injection21)26), oral administration (with drinking water)51), subcutaneous implantation (in the form of pellet)52), minipump transplantation27) and gavage (with a stomach tube)53) have been reported. Oral administration is simple but its results can vary among individuals. The present study used subcutaneous injection because this method was expected to administer the specified amount of the drug in a reliably manner and its validity had been shown in previous studies22)26). To minimize downregulation of β-receptor, while providing sufficient drug to achieve quantifiable levels and induce skeletal muscle anabolism in vivo, this study adopted the 2-day on/2-day off regimen22)26). Maltin et al.50) suggested the necessity of determining the valid dose level of Cb on the basis of metabolism in vivo. Chen et al.52) reported that Cb administered at a physiological dose level cannot prevent atrophy of the skeletal muscles of aged rats. In past studies of a single dose of Cb, the possibility of this drug to exert myotoxic effects (e.g., specific necosis) was suggested54). It is desirable to evaluate the efficacy of Cb in relation to the method of dosing and the dose level (concentration) after this. Various reports have been published concerning the clinical efficacy of Cb. Maltin et al.55) medicated Cb to patients after meniscectomy of the knee joint and reported its effectiveness. The study conducted by Oya et al.30) demonstrated the effect of Cb in keeping or increasing the muscle mass and strength of retained muscle in patients with muscular dystrophy. Because of its effect in improving athletic performance, Cb has been viewed as a substitute for anabolic steroids28).
In conclusion, the effect of a combination of IWB and Cb medication was specific to fiber-type and region. The data suggested that 1) IWB was effective on functional aspects such as contractile properties and useful for physical therapy, 2) Cb medication exerted the atrophy-suppressive effect in morphological parameters and manifested less effect on functional aspects. The results in this study indicated the possibility of elevating the efficacy of IWB by Cb medication in SOL.
Acknowledgement
The author would like to thank Prof. Katsuhiko Tachino and Shimpachiro Ogiwara, School of Health Sciences, Faculty of Medicine, Kanazawa University, for their advice. The author would also like to thank Miyako Mabuchi, Tomomi Shindo, Kazuaki Kawasumi and Megumi Maruoka, RPT for their valuable contributions to this study.
References
- 1). Allen DL, Roy RR, et al. : Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22: 1350-1360, 1999. [DOI] [PubMed] [Google Scholar]
- 2). Baldwin KM: Effect of spaceflight on the functional, biochemical, and metabolic properties of skeletal muscle. Med Sci Sports Exerc 28: 983-987, 1996. [DOI] [PubMed] [Google Scholar]
- 3). Thompson LV: Effects of age and training on skeletal muscle physiology and performance. Phys Ther 74: 71-81, 1994. [DOI] [PubMed] [Google Scholar]
- 4). Williams GN, Higgins MJ, et al. : Aging skeletal muscle: physiologic changes and the effects of training. Phys Ther 82: 62-68, 2002. [DOI] [PubMed] [Google Scholar]
- 5). Morey-Holton ER, Globus RK: Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92: 1367-1377, 2002. [DOI] [PubMed] [Google Scholar]
- 6). Thomason DB, Booth FW: Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68: 1-12, 1990. [DOI] [PubMed] [Google Scholar]
- 7). Riley DA, Slocum GR, et al. : Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol 69: 58-66, 1990. [DOI] [PubMed] [Google Scholar]
- 8). Convertino VA: Exercise as a countermeasure for physiological adaptation to prolonged spaceflight. Med Sci Sports Exerc 28: 999-1014, 1996. [DOI] [PubMed] [Google Scholar]
- 9). Yamazaki T, Tachino K, et al. : Effect of weight-bearing on disuse muscle atrophy in rats: study of weight-bearing time in a day. Memoirs Al Med Prof Kanazawa Univ 17: 63-67, 1993. (In Japanese). [Google Scholar]
- 10). Yamazaki T, Haida N, et al. : Effect of weight-bearing in prevention of disuse atrophy in rat hindlimb muscles: study of weight-bearing frequency in a week. Rigaku ryohogaku 22: 108-113, 1995. (In Japanese). [Google Scholar]
- 11). Yamazaki T, Haida N, et al. : Effect of weight-bearing frequency per day in retarding disuse atrophy in rat soleus muscle. Rigaku ryoho janaru 30: 53-57, 1996. (In Japanese). [Google Scholar]
- 12). Yamazaki T: Effect of weight-bearing on disuse muscle atrophy in rats. Rigaku ryohogaku 23: 417-420, 1996. (In Japanese). [Google Scholar]
- 13). Yamazaki T, Haida N, et al. : Effects of weight bearing intervals on disuse atrophy of rat soleus muscle. J Jpn Phys Ther Assoc 1: 19-24, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Yamazaki T, Haida N, et al. : Influence of the time when weight bearing is started on disuse atrophy in rat soleus muscle. J Jpn Phys Ther Assoc 4: 13-18, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Yamazaki T, Haida N, et al. : Influence of weight bearing intervals on the prevention of disuse atrophy in rat soleus muscle. J Tsuruma Health Sci Soc 26: 45-50, 2002. (In Japanese). [Google Scholar]
- 16). Yamazaki T: Influence of hindlimb unweighting and intermittent weight bearing on dynamics of nuclei in rat soleus muscle. J Jpn Phys Ther Assoc 6: 1-8, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Someya F, Tachino K: Effects of various daily weightbearing periods on rat soleus muscle during hindlimb suspension: histochemical and mechanical properties. Jpn J Rehabil Med 34: 410-417, 1997. [Google Scholar]
- 18). Brown M, Hasser EM: Weight-bearing effects on skeletal muscle during and after simulated bed rest. Arch Phys Med Rehabil 76: 541-546, 1995. [DOI] [PubMed] [Google Scholar]
- 19). Roy RR, Tri C, et al. : IGF-I, growth hormone, and/or exercise effects on non-weight-bearing soleus of hypophysectomized rats. J Appl Physiol 81: 302-311, 1996. [DOI] [PubMed] [Google Scholar]
- 20). Allen DL, Linderman JK, et al. : Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol 83: 1857-1861, 1997. [DOI] [PubMed] [Google Scholar]
- 21). Herrera NM, Zimmerman AN, et al. : Clenbuterol in the prevention of muscle atrophy: a study of hindlimbunweighted rats. Arch Phys Med Rehabil 82: 930-934, 2001. [DOI] [PubMed] [Google Scholar]
- 22). Wineski LE, von Deutsch DA, et al. : Muscle-specific effects of hindlimb suspension and clenbuterol in mature male rats. Cells Tissues Organs 171: 188-198, 2002. [DOI] [PubMed] [Google Scholar]
- 23). Canu M-H, Stevens L, et al. : Effect of the β2-agonist clenbuterol on the locomotor activity of rat submitted to a 14-day period of hypodynamia-hypokinesia. Behav Brain Res 122: 103-112, 2001. [DOI] [PubMed] [Google Scholar]
- 24). Ricart-Firinga C, Stevens L, et al. : Effects of β2-agonist clenbuterol on biochemical and contractile properties of unloaded soleus fibers of rat. Am J Physiol Cell Physiol 278: C582-C588, 2000. [DOI] [PubMed] [Google Scholar]
- 25). Dodd SL, Koesterer TJ: Clenbuterol attenuates muscle atrophy and dysfunction in hindlimb-suspended rats. Aviat Space Environ Med 73: 635-639, 2002. [PubMed] [Google Scholar]
- 26). Von Deutsch DA, Abukhalaf I, et al. : Distribution and muscle-sparing effects of clenbuterol in hindlimb-suspended rats. Pharmacology 65: 38-48, 2002. [DOI] [PubMed] [Google Scholar]
- 27). Hinkle RT, Hodge KMB, et al. : Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the β2-adrenergic receptor. Muscle Nerve 25: 729-734, 2002. [DOI] [PubMed] [Google Scholar]
- 28). Prather ID, Brown DE, et al. : Clenbuterol: a substitute for anabolic steroids? Med Sci Sports Exerc 27: 1118-1121, 1995. [PubMed] [Google Scholar]
- 29). Zeman RJ, Peng H, et al. : Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve 23: 524-528, 2000. [DOI] [PubMed] [Google Scholar]
- 30). Oya Y, Ogawa M, et al. : Therapeutic trial of β2-adrenergic agonist clenbuterol in muscular dystrophies. Clin Neurol 41: 698-700, 2001. (In Japanese). [PubMed] [Google Scholar]
- 31). Hayes A, Williams DA: Contractile properties of clenbuteroltreated mdx muscle are enhanced by low-intensity swimming. J Appl Physiol 82: 435-439, 1997. [DOI] [PubMed] [Google Scholar]
- 32). Fitts RH, Metzger JM, et al. : Model of disuse: a comparison of hindlimb suspension and immobilization. J Appl Physiol 60: 1946-1953, 1986. [DOI] [PubMed] [Google Scholar]
- 33). Hachisuka K, Umezu Y, et al. : Disuse muscle atrophy of lower limbs in hemiplegic patients. Arch Phys Med Rehabil 78: 13-18, 1997. [DOI] [PubMed] [Google Scholar]
- 34). Caiozzo VJ, Herrick RE, et al. : Response of slow and fast muscle to hypothyroidism: maximal shortening velocity and myosin isoforms. Am J Physiol 263: C86-C94, 1992. [DOI] [PubMed] [Google Scholar]
- 35). Smith PK, Krohn RI, et al. : Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76-85, 1985. [DOI] [PubMed] [Google Scholar]
- 36). Jaspers SR, Tischler ME: Atrophy and growth failure of rat hindlimb muscle in tail-cast suspension. J Appl Physiol 57: 1472-1479, 1984. [DOI] [PubMed] [Google Scholar]
- 37). Loughna PT, Goldspink DF, et al. : Effects of hypokinesia and hypodynamia upon protein turnover in hindlimb muscles of the rat. Aviat Space Environ Med 58: 133-138, 1987. [PubMed] [Google Scholar]
- 38). Ohira M, Hanada H: Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn J Physiol 52: 235-245, 2002. [DOI] [PubMed] [Google Scholar]
- 39). Ohira Y, Yoshinaga T, et al. : Effects of hindlimb suspension with stretched or shortened muscle length on contractile properties of rat soleus. J Appl Biomech 16: 80-87, 2000. [Google Scholar]
- 40). Taylor JA, Kandarian SC: Advantage of normalizing force production to myofibrillar protein in skeletal muscle crosssectional area. J Appl Physiol 76: 974-978, 1994. [DOI] [PubMed] [Google Scholar]
- 41). Segal SS, White TP, et al. : Architecture, composition, and contractile properties of rat soleus muscle grafts. Am J Physiol 250: C474-C479, 1986. [DOI] [PubMed] [Google Scholar]
- 42). Kawakami Y, Abe T, et al. : Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol 74: 2740-2744, 1993. [DOI] [PubMed] [Google Scholar]
- 43). Awede BL, Thissen J-P, et al. : Role of IGF-I and IGFBPs in the change of mass and phenotype induced in rat soleus muscle by clenbuterol. Am J Physiol Endocrinol Metab 282: E31-E37, 2002. [DOI] [PubMed] [Google Scholar]
- 44). Semsarian C, Wu MJ, et al. : Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signaling pathway. Nature 400: 576-581, 1999. [DOI] [PubMed] [Google Scholar]
- 45). Maltin CA, Delday MI: Satellite cells in innervated and denervated muscle treated with clenbuterol. Muscle Nerve 15: 919-925 1992. [DOI] [PubMed] [Google Scholar]
- 46). Stevens L, Firinga C, et al. : Effects of unweighting and clenbuterol on myosin light and heavy chains in fast and slow muscles of rat. Am J Physiol Cell Physiol 279: C1558-C1563, 2000. [DOI] [PubMed] [Google Scholar]
- 47). Williams RS, Caron MG, et al. : Skeletal muscle β-adrenergic receptors: variations due to fiber type and training. Am J Physiol 246: E160-E167, 1984. [DOI] [PubMed] [Google Scholar]
- 48). Buckenmeyer PJ, Goldfarb AH, et al. : Endurance training, not acute exercise, differentially alters β-receptors and cyclase in skeletal fiber types. Am J Physiol 258: E71-E77, 1990. [DOI] [PubMed] [Google Scholar]
- 49). Zeman RJ, Ludemann R, et al. : Clenbuterol, a β2-agonist, retards atrophy in denervated muscles. Am J Physiol 252: E152-E155, 1987. [DOI] [PubMed] [Google Scholar]
- 50). Maltin CA, Delday MI, et al. : Denervation increases clenbuterol sensitivity in muscle from young rats. Muscle Nerve 14: 188-192, 1992. [DOI] [PubMed] [Google Scholar]
- 51). Frerichs O, Fansa H, et al. : Regeneration of peripheral nerves after clenbuterol treatment in a rat model. Muscle Nerve 24: 1687-1691, 2001. [DOI] [PubMed] [Google Scholar]
- 52). Chen KD, Alway SE: A physiological level of clenbuterol does not prevent atrophy or loss of force in skeletal muscle of old rats. J Appl Physiol 89: 606-612, 2000. [DOI] [PubMed] [Google Scholar]
- 53). Hunt DG, Ding Z, et al. : Clenbuterol prevents epinephrine from antagonizing insulin-stimulated muscle glucose uptake. J Appl Physiol 92: 1285-1292, 2002. [DOI] [PubMed] [Google Scholar]
- 54). Burniston JG, Ng Y, et al. : Myotoxic effects of clenbuterol in the rat heart and soleus muscle. J Appl Physiol 93: 1824-1832, 2002. [DOI] [PubMed] [Google Scholar]
- 55). Maltin CA, Delday MI, et al. : Clenbuterol, a β-adrenoreceptor agonist, increases relative muscle strength in orthopedic patients. Clin Sci 84: 651-654, 1993. [DOI] [PubMed] [Google Scholar]


