Abstract
Falling due to unstable standing balance is considered to be the main cause of bone fractures, which lead elderly persons to becoming bedridden. Thus, the standing balance of elderly persons is being given increasingly greater attention. On the other hand, postural deformation caused by deformation in the spine and lower leg joints is considered to have an effect on standing balance. The objective of this study is to clarify the effect of postural deformation on the following three categories of standing balance; 1) the ability to immobilize Center of Gravity (COG) in standing statically, 2) the ability to control COG during movement and 3) the postural response induced by postural sway. Fifty elderly persons (age:77.7 ± 6.4 years old, fifty females) participated in this study. Postural deformation was measured using a Spinal Mouse, a device for non-invasive measurement of spinal curvature and photographic image in sagittal plane. In line with to Nakata's classification of postural deformation, subjects were classified by extension type, S-character deformation type, flexion type, hands on the knee type and normal group. In order to assess the ability to immobilize COG in static standing, Center of Pressure (COP) in static standing was measured for 30 sec. In order to assess the ability to control COG during movement, functional reach, maximal length of stride and the period of 10 m gait were measured. Postural response was induced by fore-aft perturbation of the platform on which the subjects stood. Postural responses were assessed by measuring both COP, and electromyography (EMG) of muscles in the lower legs. There was little significant difference among the five groups concerning postural deformation in every measured item, neither in the ability to immobilize COP in static standing, nor in the postural response induced by postural sway. However, the results of measured items concerning the ability to control COG during movement were significantly worse in flexion type and hands on the knee type compared with the normal group. It was suggested that postural deformation in elderly persons effects exclusively on the ability to control COG during movement in standing balance.
Keywords: posture, elderly person, standing balance
In recent years, among elderly persons, the number of injuries caused by falls has been markedly increasing. Bone fractures (e.g., hip bone fracture) are the most common injuries suffered in these falls, and can easily cause elderly persons to become bedridden, with important consequences for today's health care system1). Thus, identifying risk factors of falls and preventing falls are important issues in health care of elderly persons.
Risk factors of falls are classified as either internal factors (related to physical and mental condition) or external factors (related to the environment)2)3). Standing balance is considered the most important inner factor of falls, and is classified into several hierarchical phases4)5). The most elementary phase is postural stability when standing statically. In order to assess this stability, center of pressure (COP) is measured while standing statically, usually using a force plate. The next phase is the ability to control posture during active motion; i.e., keeping center of gravity (COG) of the body mass within the allowable area of the base of support. For example, when walking, we have to control COG during changes in the base of support. When initiating motion, anticipatory postural adjustment is needed. For example, when we raise an arm, there is anticipatory activation of trunk muscles prior to activation of the arm muscles6)7). While walking, before toe off, muscles of the lower limb of the opposite side and the trunk are activated8–10). Assessing the ability to control COG during motion is usually accomplished by administering the standing-on-one-leg test, the get-up-and-go test11)12) and the functional reach test13), and by measuring duration of 10-m gait and maximum stride. When perturbation of COG exceeds the threshold area of the base of support within which COG is under control, the next phase, postural response over the whole body, acts to prevent falling by returning the COG to within the threshold. Nashner et al.14)15) have developed a novel method for qualitatively evaluating this postural response. They examined postural response to postural sway induced by a movable platform, and found that 2 comprehensive muscle activation patterns are characteristic of this response. When a subject stands on a normal platform and postural disturbance is elicited by horizontal perturbation of the platform, muscle activity begins in the ankle joint muscles and radiates to other muscles on the dorsal or ventral aspect in a distal-toproximal sequence. This pattern was later termed “ankle strategy” by Horak16). In contrast, when a subject stands on a platform that is shorter than their foot, trunk and thigh muscles that are antagonistic to those used in ankle strategy are activated in a proximal-to-distal sequence. This pattern was termed “hip strategy” by Horak16). When it is difficult to return COG to within the threshold area using these reflexes, a stepping reflex is activated to prevent falling17). These postural responses have been termed “totally automatic postural responses”.
Advancement of osteoporosis due to aging not only decreases the strength of bones and aggravates damage from fracture when falling but also deforms standing postural alignment18). Deformation of the spine (e.g., kyphosis) and the joints of the lower limbs has a strong affect on postural alignment. Postural deformation changes the spatial position of COG of the body mass so greatly that it is assumed that effects of postural deformations on standing balance is related to risk of falling among elderly persons. Thus, precise characterization of the effects of postural deformation on each phase of standing balance described above may be applicable to prevention of falls. Staffel19) and Wiles20) have compiled well-known postural classification systems. However, their classification systems are so complex that they are rarely used in clinical settings. Recently, in Japan, Nakata's21)22) classification system has become popular for characterizing postural deformation in elderly persons. As shown in Fig. 1, Nakata classified postural deformation into 5 groups (extension type, S-character type, flexion type, hands-on-knees type and normal) based on the degree and characteristics of deformation in the spine and lower limbs. The extension type is caused mainly by degeneration of intervertebral disk in the lumbar spine; the spine straightens and declines backward. The S-character type is mainly caused by compression fractures of the thoracic spine, and is characterized by excessive anterior curvature of the thoracic spine and excessive curvature of the lumbar spine. The flexion type is caused by degeneration of intervertebral disk in the lumbar spine and compression fracture of the thoracic spine, and is typical of kyphosis. The hands-on-knees type is such an aggravated form of kyphosis that patients find it difficult to maintain standing without placing hands on knees. Nakata also reported that deformation of the spine is compensated for by joint angles in lower limbs to maintain standing posture. The joint angle of the knee increases in the following sequence of deformation type: S-character, extension, flexion and hands-on-knees. Nakata's classification is useful when considering the role of postural deformation in falling due to impaired standing balance over the whole body, because his classification is based not only on deformation of the spine but also on substitution of lower limbs. Nakata's classification also has the advantage of simplicity.
Fig. 1.
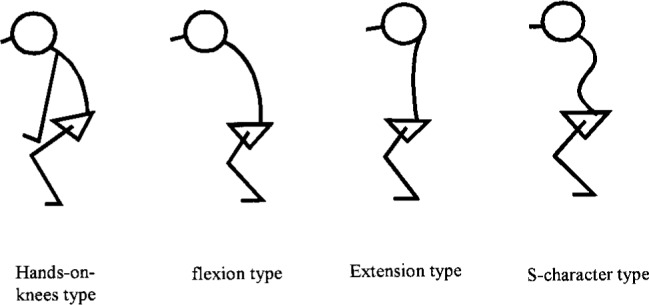
Nakata's classification of postural deformation in the elderly.
In this study, we examined the relationship between postural deformation (using Nakata's classification) and each phase of standing balance in elderly persons, to assess the physical mechanisms involved in falling caused by postural deformation, and to determine indicators for intervention into physical activities of elderly persons to prevent falling.
Methods
Subjects
The subjects were fifty community-dwelling elderly persons (77.7 ± 6.4 years old, fifty females) who could walk safely without a cane. To exclude the effect of sex on data, all subjects consisted of females. None of the subjects had histories of uncorrectable visual defects, vertigo, motor paresis or sensory deficits. Subjects were recruited through various means of advertisement in their community hall. Participation in this study was strictly voluntary. Informed consent was obtained, and subjects signed a document stating that they were never forced to continue with their participation and that they could cease participating at any time.
Classification of postural deformation
In order to classify the subjects into the 5 deformation types of Nakata's classification, we examined photographic images of the whole body in the sagittal plain and spinal curvature while statically standing. Subjects were asked to stand in their most relaxed posture. Markers were fitted to the subjects, and photographic images of the whole body in the sagittal plane were taken from the right side, to measure flexion joint angle of the knee. The sagittal images were stored in a personal computer, and flexion joint angle was measured using image analysis software (NIH Image). Spinal curvature was measured using a spinal mouse (Index Co., Ltd), a novel non-invasive device used to measure spinal curvature, equipped with 2 parallel wheels connected to a load cell. By rolling these wheels from the seventh cervical spine to the third sacral vertebra, the concavoconvex profile of the spine and spatial position of each vertebra was determined. Spatial position of each vertebra was recorded in a personal computer for later calculation of spinal curvature. Curvature of the thoracic spine (thoracic curvature) and the lumbar spine (lumbar curvature) and tilt angle of the trunk (trunk tilt) were calculated. Thoracic curvature was calculated as summation of relative angles between adjacent vertebrae from Th1 to Th12. The larger the calculated value of thoracic curvature, the more kyphotic the thoracic spine is. Lumbar curvature was calculated as summation of relative angles between adjacent vertebrae from Th12 to S1. The larger the calculated value of lumbar curvature, the more lordotic the lumbar spine is. Trunk tilt was calculated as relative angle between the line drawn from Th1 to S1 and the vertical line.
Nakata classified postural deformation into 5 types according to the character of spine deformation and substitution of lower limbs, as seen on sagittal images. However, Nakata did not give clear criteria for classification. Therefore, we attempted to define criteria for classification using the spinal mouse data and joint angles of lower limbs, from sagittal images of the whole body. The resulting classification chart is shown in Fig. 2. In both flexion type and hands-on-knees type, trunk tilt is greater than 10 degrees. Hands-on-knees type is distinguished from flexion type according to whether subjects put their hands on their knees in relaxed stance. In both S-character type and extension type, joint angle of the knee is greater than 10 degrees. S-character type is distinguished from extension type according to whether the value obtained by subtracting lumbar curvature from thoracic curvature is greater than 30 degrees.
Fig. 2.
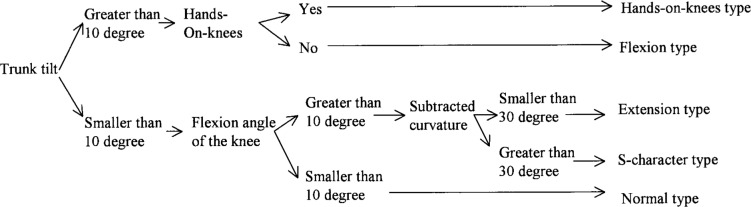
Criteria of Nakata's classification of postural deformation, based on values measured using spinal mouse and joint angles in the lower leg measured from saggital image.
Measurements of standing balance
In order to assess static standing balance, COP was measured during static stance using a force plate (ANIMA Co., Ltd.). Subjects stood on the force plate with their eyes open and their arms relaxed at their sides, and COP was measured for 30 sec. COP was also measured for subjects with eyes closed, for 30 sec. Each excursion of COP was calculated, and the ratio of excursion of COP with eyes closed to that with eyes open was also calculated. This ratio is termed “Romberg's ratio”.
In order to assess the ability to control posture during active motion or change of the base of support, we measured the period the subject could endure standing on one leg, maximal length of stride, 10-m gait time, and functional reach. In the first of these tests, subjects stood on one leg with their eyes open and their arms relaxed at their side for as long as possible. The period the subject could endure this posture was measured for each leg, and the results were averaged. Maximal length of stride was measured for each side and averaged, and the averaged value was normalized by height. For measurement of 10-m gait time, subjects were asked to walk at their usual speed for 20 m. The time required to walk the intermediate 10 m was recorded as 10-m gait time. For measurement of functional reach, subjects raised an arm parallel to the ground and in line with the shoulders, and then moved the raised arm as far forward as possible. The distance that the distal finger moved was measured for both sides and averaged.
The postural response to postural sway was measured using the method of Nashner and Horak16)17). Postural sway was induced by fore-aft horizontal perturbation of the original platform with subjects standing on the force plate. Forward perturbation of the platform causes backward sway of the body, inducing a postural response that moves COG forward. Backward perturbation of the platform induces the reverse response. Trials consisted of 5 forward and 5 backward perturbations of the platform. Prior to each perturbation, subjects looked at a fixed marker in front of them. On the monitor, COP was confirmed to be consistent with that of initial stance in the previous trial. The sequences of trials were randomized, so that subjects could not anticipate the direction of the next perturbation. There was an intermission of 1 minute between each of the 10 trials. The velocity of perturbation of the platform was 100 mm/sec, and the distance of perturbation was 50 mm in each direction. To assess postural responses, COP was measured and electromyography (EMG) of lower limbs was performed during trials. Postural responses caused by this method involve symmetrical muscle activity of the legs. Therefore, surface EMG was measured for the tibia anterior (TA), rectus femoris (RF), gastrocnemius(GAS) and interior hamstrings (HAM) of the right leg. Each subject was fitted with surface electrodes (Vitrode disposable electrodes; NIHON KODEN Co., Ltd.) spaced 2 cm apart on each muscle. Impedance of the skin was adjusted so that it was lower than 5 kohm. EMG signals were bandpass filtered (20–2000 Hz) and full-wave rectified using an amplifier (RMP-6004M; NIHON KODEN Co., Ltd.). They were then low-pass filtered with a time constant of 10 ms. Excursion of COP was also measured from force plate data, without filtration. All EMG and COP data were sampled at 1000 Hz using a PowerLab system (PowerLab/8s, AD Instruments Co., Ltd.), and recorded on the hard disk of a personal computer for later analysis. From the COP data, maximal excursion of COP from initial stance (COP excursion) and period required to reach maximal excursion after onset of perturbation of the platform (COP recovery time) were measured. From the EMG data, period from onset of perturbation of the platform to activation of each muscle was measured. All EMG and COP data were analyzed using wave analyzing software (Chart v4.0.4; AD Instruments Co., Ltd.).
Latencies of muscle activation elicited by postural response varied so much among individuals that it is meaningless to compare latencies between different subjects. However, change in postural strategy alters latencies of muscles and sequences of muscle activation, as described by Horak et al.16)17). When the ankle strategy is elicited, muscle activations radiate from distal to proximal muscle. In contrast, when the hip strategy is elicited, activations of muscles antagonistic to those activated in the ankle strategy radiate from proximal to distal muscle. Thus, changes in the value obtained by subtracting TA latency from RF latency and the value obtained by subtracting GAS latency from HAM latency is useful to indicate changes in postural strategy. So we calculated the value obtained by subtracting TA latency from RF latency and the value obtained by subtracting GAS latency from HAM latency when the platform moved forward. Averaged values of the parameters were analyzed for each direction.
Statistical analysis
Significance of differences in measured values among the 5 deformation types was assessed using one-way ANOVA. Scheffe's test was used as a post-hoc test. A p value less than 0.05 indicated statistical significance.
Results
Distribution of subjects
According to the Nakata classification criteria, there were 16 normal subjects, 8 subjects with hands-on-knees type deformation, 9 with flexion type, 10 with extension type, and 7 with S-character type, as shown in Table.1. The values for age, sex, tilt angle of the trunk, thoracic curvature and lumbar curvature are also shown in Table 1.
Table 1. Distribution of subjects among the 5 types of Nakata's classification.
| The number of subjects | Age (years old) | Tilt angle (deg) | Thoracic curvature (deg) | Lumber curvature (deg) | Subtracted curvature (deg) | |
|---|---|---|---|---|---|---|
| Hands-on-knees type | 8 | 81.9 ± 3.8 | 48.1 ± 16.8 | 11.5 ± 18.6 | 52.8 ± 12.4 | −41.3 ± 26.1 |
| Flexion type | 9 | 81.0 ± 3.8 | 27.7 ± 17.0 | 16.0 ± 19.0 | 22.8 ± 17.0 | −6.9 ± 33.6 |
| Extension type | 10 | 79.0 ± 6.8 | 4.1 ± 4.8 | 18.4 ± 10.8 | 13.6 ± 15.4 | 4.9 ± 23.8 |
| S-character type | 7 | 74.9 ± 7.5 | 6.5 ± 5.1 | 45.8 ± 21.6 | −9.0 ± 6.3 | 54.8 ± 18.7 |
| Normal type | 16 | 79.2 ± 6.1 | 5.9 ± 3.5 | 29.4 ± 15.0 | −4.2 ± 13.7 | 33.6 ± 23.5 |
Relationship between postural deformation and static standing balance
Romberg's ratio and excursion of COP with eyes open and closed are shown in Table 2. There was no statistically significant difference in any of these values among the 5 types. Thus, we did not observe any effect of postural deformation on the ability to stabilize COP in static stance.
Table 2. Comparison of measured values related to static stance.
| COP excursion with eyes opened (msec) | COP excursion with eyes closed (msec) | Romberg's ratio | |
|---|---|---|---|
| Hands-on-knees type | 53.7 ± 27.4 | 104.4 ± 66.2 | 1.9 ± 0.9 |
| Flexion type | 61.9 ± 22.7 | 105.4 ± 44.0 | 1.7 ± 0.5 |
| Extension type | 61.2 ± 25.5 | 126.9 ± 96.4 | 1.9 ± 0.6 |
| S-character type | 56.1 ± 26.2 | 90.7 ± 44.1 | 1.6 ± 0.4 |
| Normal type | 38.8 ± 9.8 | 62.4 ± 21.3 | 1.6 ± 0.4 |
Relationship between postural deformation and the ability to control posture during motions
Relationships between postural deformation and the ability to control posture during motions were shown in Table 3.
Table 3. Comparison of measured values related to ability to control posture during motion.
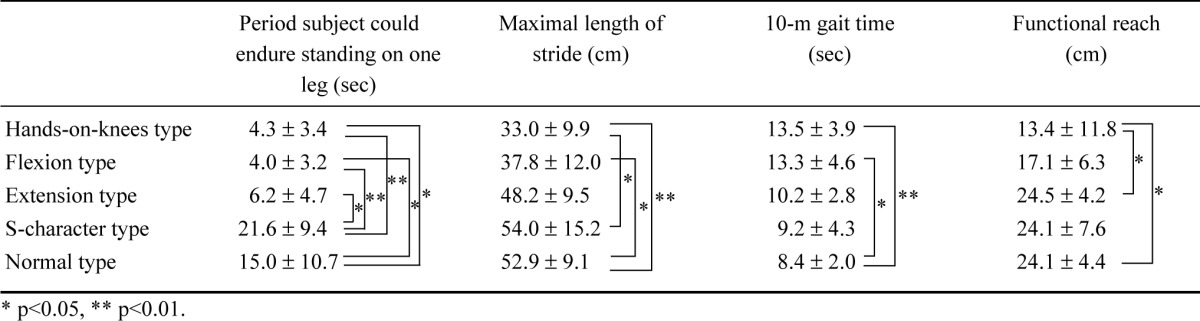 |
For hands-on-knees type and flexion type, the period that subjects could endure standing on one leg was significantly shorter than for normal type and S-character type. For extension type, this period was also significantly shorter than for S-character type.
For hands-on-knees type and flexion type, maximal length of a stride was significantly shorter than for normal type. This length for hands-on-knees type was also significantly shorter than for S-character type.
For hands-on-knees type and flexion type, the 10-m gait time was significantly longer than for normal type.
Functional reach for hands-on-knees type was significantly shorter than for normal type and extension type.
Subjects with particularly serious hands-on-knees type and flexion type deformations were especially impaired in their ability to control posture during motion or change in the base of support.
Relationship between postural deformation and postural responses caused by perturbations
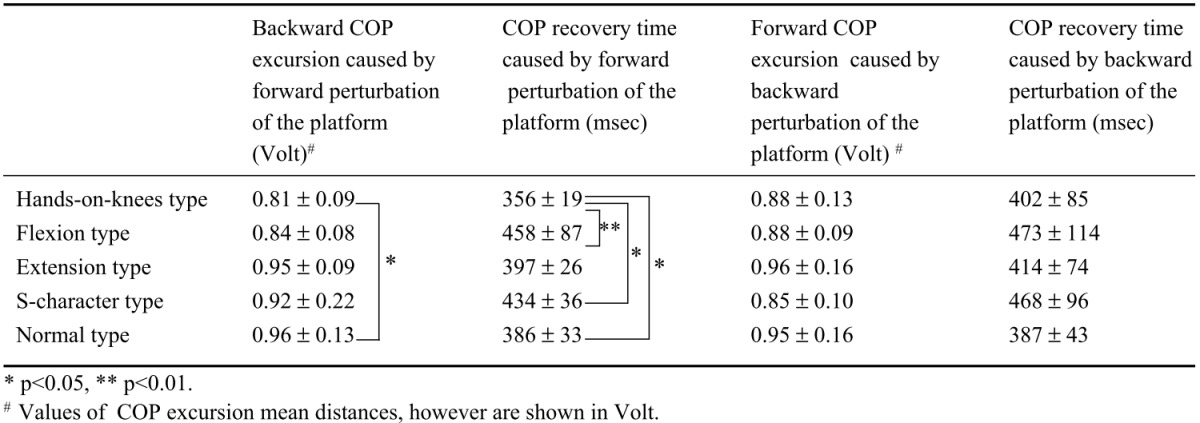
COP excursion and COP recovery time during postural response caused by fore-aft perturbation of the platform are shown in Table 4. For hands-on-knees type, backward COP excursion caused by forward perturbation of the platform was significantly smaller than for normal type. For handson-knees type and flexion type, COP recovery times caused by forward perturbation of the platform were shorter than for normal type, S-character type and flexion type. There was no significant difference in COP excursion or COP recovery time caused by backward perturbation of the platform among the 5 types.
Table 4. Comparison of measured values related to postural response caused by perturbation of the platform.
 |
There was no significant difference in the value obtained by subtracting TA latency from RF latency or the value obtained by subtracting GAS latency from HAM latency among the 5 types, as shown in Table 5.
Table 5. Subtracted values of latency, as recorded by EMG when the platform moved forward.
| RF latency-TA latency (msec) | HAM latency-GAS latency (msec) | |
|---|---|---|
| Hands-on-knees type | −13 ± 73 | −61 ± 124 |
| Flexion type | −2 ± 77 | −56 ± 176 |
| Extension type | −13 ± 57 | 35 ± 21 |
| S-character type | 15 ± 27 | −16 ± 75 |
| Normal type | 3 ± 13 | 10 ± 38 |
Thus, except for superior results for hands-on-knees type in postural response caused by forward perturbation of the platform, we did not observe any effect of postural deformation on postural response caused by perturbation of the platform.
Discussion
In this study, we tried establishing criteria for Nakata's classification of postural deformation, and examined the relationship between postural deformation and various aspects of standing balance in elderly persons.
Though normal subjects appeared (to our eyes) to have superior COP excursion in static stance, there were no significant differences in any of the measured parameters of static balance (Table 2). Lynn23) et al. compared postural sway in static stance among kyphosis subjects, osteoporosis subjects and normal subjects. Postural sway of osteoporosis subjects was significantly greater than that of kyphosis subjects or normal subjects, but they did not find a significant difference between kyphosis subjects and normal subjects. Their results were similar to ours.
In contrast to our results for static stance, the ability to control COG during motion was particularly inferior in subjects with the most severe hands-on-knees type deformation (Table 3). As kyphosis progresses, COP derogates forward from the center of the base of support, and the controllable area of COP in deformed postures becomes narrower18).
However, we considered it unlikely that only inferior control of COG during motion in deformed posture would be simply due to inferiority in mechanical output functions. Prior to motion or change of the base of support, anticipatory postural adjustments are required to keep COG within controllable space8–10). Therefore, we hypothesized that postural deformation may affect anticipatory postural adjustments.
Recently, computational models have been developed to analyze postural control24–28). As first described by Gufinkel24), in the central nervous system, there exists internal representation of body dynamics and sensory dynamics, as a mirror of actual body and sensory dynamics. In postural control, afferent inputs from proprioceptive organs in the neck and the joints, from the labyrinth and from the eyes are integrated as actual afferent signals. In the models of Merfeld25) and Kuo26), expected sensory afferent signals from internal representation are referred to actual afferent signals, and the sensory conflict between them is fed back to modify internal representation. Postural disturbances to the actual body without active motion, as in static stance or postural response caused by postural sway, produce such sensory conflicts. Signals estimating body dynamics from internal representation of body dynamics are integrated with commands from higher centers sent to attain desired posture, with efferent signals traveling to the actual body and copies of these efferent signals traveling to the internal representation of body dynamics.
Our results show weak relationship between postural deformation and static standing as described above. Additionally our result also shows weak relationship between postural deformation and postural response caused by postural sway. It is not surprising that subjects with hands-on-knees type deformation produced superior results in backward postural sway (Table 4), because COP in initial stance prior to perturbation was shifted anteriorly, reducing backward postural sway.
Our results (weak relationship between postural deformation and both static standing balance and postural response caused by body sway) indicate that total feedback control for posture in the central nervous system was not strongly affected by postural deformation. Therefore, we speculate that the internal representation monitors actual body deformation, and that the feedback system functions to correct posture at the level of reflex or response.
In motion, in addition to feedback system, the signal of desired posture passes through a feedforward trajectory generator to induce efferent signals directly26). It has been suggested that anticipatory postural adjustments accompanying motion or change of base of support are mainly generated by this feedforward system. Our results (various degrees of impaired control of posture in active motion due to postural deformation) indicate that the effectiveness of this feedforward system in controlling posture is affected by postural deformation. We speculate that the efferent signals for postural control generated in this feedforward system were not properly modified to account for postural deformation, and thus were not effective in controlling balance.
In our measurements of postural responses, we found that COP excursion was smaller and COP recovery time was shorter in hands-on-knees subjects than in normal subjects when backward postural sway was induced by forward perturbation of the platform (Table 4). As mentioned previously, it is not surprising that the hands-onknees type produced these superior results, because COP in initial stance prior to perturbation is shifted anteriorly in hands-on-knees type, reducing backward postural sway. So we initially hypothesized that COP excursion and COP recovery time of hands-on-knees type would be impaired during forward postural sway caused by backward perturbation of the platform, because COP in initial stance is shifted anteriorly, increasing forward postural sway. However, other than the above-mentioned difference in COP between hands-on-knees type and normal type, there were no differences in COP or EMG during postural response among the 5 types (Tables 4, 5). Thus, postural deformation had little effect on postural response. It has been reported that elderly persons tend to activate the hip strategy as a postural response more often than younger persons29)30). Lynn et al.23) reported that kyphosis subjects tend to activate the hip strategy more often than normal subjects. They concluded that kyphosis subjects had inferior dynamic postural balance because they activated the hip strategy within the range of perturbation that induces the ankle strategy in normal subjects17). In our study, differences in strategy were not detected in timing of muscle activation. However, the method Lynn et al. used to perturb subjects was different from ours. Furthermore, in the present study, because both strategies were sometimes used simultaneously, and the muscles used in these strategies were therefore activated together, even if there were slight differences in timing of muscle activation between the strategies, it might be not detectable in EMG latencies. The present finding that forward COP sway of the hands-on-knees type was not greater than that of normal type suggests that activation of the hip strategy in kyphosis subjects is not due to impaired dynamic balance. The relationship between postural deformation and activation of the hip strategy suggests adaptation to postural deformation in order to decrease postural sway. If this assumption is correct, it would be more effective to facilitate use of the hip strategy in kyphotic patients than to have them exercise so that they can maintain posture using the ankle strategy.
Conclusions
Postural deformation affected the ability to control COG during motion or change of the base of stance, but not during static balance or postural response. One possible explanation for this selectivity in effects of postural deformation on postural control is that postural deformation specifically affects the feedforward system that maintains postural control. As many studies have found31–34), falls involving elderly persons occur most often during motion in activities of daily living. Furthermore, the methods used to evaluate control of COG during motion are very simple and safe, as described in this paper. Together, these findings suggest that monitoring postural balance of elderly persons during motion can produce data useful in preventing falls.
Acknowledgment
This study was supported in part by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS).
References
- 1). Rawsky E: Review of the literature on falls among the elderly. Journal of Nursing Scholarship 30: 47-52, 1998. [DOI] [PubMed] [Google Scholar]
- 2). Mano Y, Nakane R: Factors of impaired gait ability and falls. Clin Rehabil 7: 243-247, 1998. [Google Scholar]
- 3). Rubenstein LZ, Josephason KR, Robbins AS: Falls in the nursing home. Ann Intern Med 121: 442-451, 1994. [DOI] [PubMed] [Google Scholar]
- 4). Uchiyama Y: Equilibrium function and falls in the elderly. Journal of Physical Therapy 18 (9): 858-864, 2001. (in Japanese). [Google Scholar]
- 5). Shumway CA, Horak FB: Balance rehabilitation in the neurologic patient: course syllabus. Seattle, NERA, 1992. [Google Scholar]
- 6). Belen'kii VY, Gurfikel VS, Paltsev YI: Elements of control of voluntary movements. Biofizia 12: 135-141, 1967. [PubMed] [Google Scholar]
- 7). Cordo P, Nashner L: Properties of postural adjustments associated with rapid arm movements. J Neurophysiol 47: 287-302, 1982. [DOI] [PubMed] [Google Scholar]
- 8). Bouisset S, Zattara M: A sequence of postural movements precedes voluntary movement. Neurosci Lett 22: 263-270, 1981. [Google Scholar]
- 9). Mouchino L, Aurenty R, Masion J, Pedotti A: Coordination between equilibrium and head-trunk orientation during leg movement-a new strategy built up by training. J Neurophysiol 67: 1187-1598, 1992. [DOI] [PubMed] [Google Scholar]
- 10). Alstermark B, Wessberg J: Timing of postural adjustment in relation to forelimb target-reaching in cats. Acta Physiol Scand 125: 337-340, 1985. [DOI] [PubMed] [Google Scholar]
- 11). Mathias S, Nayak U, Issacs B: Balance in elderly patients: the Get-up and go test. Arch Phys Med Rehabil 67: 387-389, 1986. [PubMed] [Google Scholar]
- 12). Podsiadlo D, Richardson S: The timed Up&Go: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: 142-148, 1991. [DOI] [PubMed] [Google Scholar]
- 13). Duncan PW, Weiner DK, Chander J, Studenski S: Functional reach: a new clinical measure of balance. J Gerontol 45: 192-195, 1990. [DOI] [PubMed] [Google Scholar]
- 14). Nashner LM: Fixed patterns of rapid postural responses among leg muscles during stance. Exp Brain Res 30: 13-24, 1977. [DOI] [PubMed] [Google Scholar]
- 15). Nashner LM, Woollacott M: The organization of rapid postural adjustments of standing humans: an experimental-conceptual model. In: Talbott RE, Humphrey DR. (eds) Posture and Movement. NY, Raven Press, 1979, pp 243-257. [Google Scholar]
- 16). Horak F, Nashner L: Central programming of postural movements: adaptation to altered support surface configurations. J Neurophysiol 55: 1369-1381, 1986. [DOI] [PubMed] [Google Scholar]
- 17). Horak FB: Effects of neurological disorders on postural movement strategies in the elderly. In: Vellas B, Toupet M, Rubenstein L, et al. (eds) Falls, Balance and Gait Disorders in the Elderly. Paris, Elsevier, 1992, pp 137-152. [Google Scholar]
- 18). Lewis C, Bottomley J: Muscleskeletal changes with age. In: Lewis C. (ed) Aging: Health Care's Challenge. 2nd ed., FA Davis, Philadelphia, 1990, pp 145-146. [Google Scholar]
- 19). Staffel F: Die menschlichen Haltungs Typen. J. F. Bergmann, Wiesbagen, 1889. [Google Scholar]
- 20). Wiles P: Postural deformities of anteroposterior curves of the spine. Lancet 5929: 911-919, 1937. [Google Scholar]
- 21). Nakata K, Iwaya C, Sekiya H: Posture of the elderly: classification and mechanism. Orthopedics Sup 12: 2-6, 1987. (in Japanese). [Google Scholar]
- 22). Nakata K, Iwaya C, et al. : Aberrant posture: Posture of the elderly. Bone Joint Ligament 2: 1441-1449, 1989. (in Japanese). [Google Scholar]
- 23). Lynn SG, Sinaki M, Westerlind KC: Balance characteristics of persons with osterporosis. Arch Phys Med Rehabil 78: 273-277, 1997. [DOI] [PubMed] [Google Scholar]
- 24). Gurfinkel VS, Levik YS, Popov KE, Smetanin BN, Dhlikov VY: Body schema in control of postural activity. In: Gurfinkel VC, et al. (eds). Stance and Motion: Facts and Theories. New York, Plenum Press, 1988, pp 185-193. [Google Scholar]
- 25). Merfeld DM, Young LR, Oman CM, Shelhamer MJ: A multidimensional model of the effect of gravity on spatial orientation of the monkey. J Vestib Res 3: 141-161, 1993. [PubMed] [Google Scholar]
- 26). Kuo A: An optimal control model for analyzing human postural balance. IEEE Trans Biomed Eng 42: 87-101, 1995. [DOI] [PubMed] [Google Scholar]
- 27). Oman CM: A heuristic mathematical model for the dynamics of sensory conflict and motion sickness. Acta Otolayngol 392: 1-44, 1982. [PubMed] [Google Scholar]
- 28). Johasson R, Magnusson M: Optimal coordination and control of posture and locomotion. Math Biosci 103: 203-244, 1991. [DOI] [PubMed] [Google Scholar]
- 29). Horak F, Shupert C, Milka A: Components of postural dyscontrol in the elderly: a review. Neurobiol Aging 10: 727-745, 1989. [DOI] [PubMed] [Google Scholar]
- 30). Manchester D, Woollacott M, Zederbauer HN, Marin O: Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol 44: M118-127, 1989. [DOI] [PubMed] [Google Scholar]
- 31). Kanemura N, Kobayashi R, Yoshimura O, et al. : Analysis of risk factors fro falls in the elderly with dementia. J Phys Ther Sci 12: 27-31, 2000. [Google Scholar]
- 32). Tinetti ME, Speechley M, et al. : Risk factors for falls among elderly persons living in the community. N Engl J Med 319: 1701-1707, 1988. [DOI] [PubMed] [Google Scholar]
- 33). Connell BR, Wolf SR: Environmental and behavioral circumstances associated with falls at home among healthy elderly individuals. Arch Phys Med Rehabil 78: 179-186, 1997. [DOI] [PubMed] [Google Scholar]
- 34). Bath PA, Morgan K: Differential risk factor profiles for indoor and outdoor falls in older people living at home in Nottingham, UK. Eur J Epidemiol 15: 65-73, 1999. [DOI] [PubMed] [Google Scholar]


