Abstract
Immobilization is often associated with decreased muscle elasticity. This condition is known as muscle contracture; however, the mechanism remains unclear. The purpose of this study was to clarify the mechanism governing muscle contracture in rat soleus muscle by identifying changes in ankle joint mobility, insoluble collagen concentration and type I and type III collagen isoforms following 1- and 3-week immobilizations. Following a 1-week immobilization, range of motion (ROM) of dorsiflexion declined to 90% of the control value; additionally, ROM dropped to 67.5% of the control value after a 3-week immobilization. This finding suggested that ankle joint mobility decreases in conjunction with extended periods of immobilization. Insoluble collagen concentration in soleus muscles, which was unchanged after 1 week of immobilization, increased 3 weeks after immobilization. These results may be indicative of collagen fibers with strong intermolecular cross-links contained in the muscle was made increased relatively by 3 weeks of immobilization. Therefore, the change in intermolecular cross-links may be significant in terms of progress of muscle contracture with longer periods of immobilization. On the other hand, the ratio of type III to type I collagen isoforms in muscular tissue increased following a 1-week immobilization; moreover, this ratio remained constant after 3 weeks of immobilization. These data suggested that muscle immobilization may induce type III collagen isoform expression. The increase in the ratio of type III to type I collagen isoforms do not change in parallel with the increase in the limitation in ROM; however, this phenomenon probably is not closely related to the progress of muscle contracture. The change of collagen isoform in immobilized muscle may be involved in the mechanism governing the progression of muscle fibrosis.
Keywords: immobilization, muscle contracture, collagen
Elastic behavior, which is a primary property of skeletal muscle, is determined by components of the muscle fiber and intramuscular connective tissue1–4). Clinically, in cases involving bed rest for prolonged periods, immobilization of a joint with a plaster cast or orthosis decreases muscle elasticity and reduces the range of joint motion. This condition, known as muscle contracture, is thought to result from changes in intramuscular connective tissue5–8); at present, the mechanism of muscle contracture is obscure. Additionally, muscle contracture is perhaps the most frequent complication encountered by physical therapists.
In terms of changes in intramuscular connective tissue with respect to the mechanism underlying muscle contracture, earlier investigations have consisted predominantly of morphological and biochemical analyses9–13). In a morphological assessment, Jarvinen et al.9) reported that the number of circular and rectangular-oriented collagen fibers increased at the contacts of two adjacent muscle fibers in the endomysium 3 weeks after immobilization of rat calf muscles. Previous studies by our group10) noted that collagen fibril arrangement in the endomysium 1 and 2 weeks after immobilization in rat soleus muscles was longitudinal to the axis of the muscle fibers, whereas 4, 8 and 12 weeks after immobilization, fibril arrangement was circumferential. Such changes in collagen fibril arrangement indicate decreased collagen fiber movement in the endomysium of immobilized muscle. Additionally, we surmised that the increase in this circumferential component of collagen fibrils following muscle immobilization may reflect an increase in intermolecular cross-links and/or cross-links between adjacent collagen fibrils.
The biochemical investigation of Sugama et al.12) revealed that salt soluble collagen and the pepsin solubilization rate of insoluble collagen were diminished significantly during 3- and 7-week immobilizations of rat soleus muscle. These results, which are suggestive of enhanced intermolecular cross-links corresponding to a stronger molecular structure, support our hypothesis. However, comparisons between the Sugama et al.12) article and our previous report10) are difficult due to differences in the methodology, i.e., immobilization position in rat ankle joint. Therefore, evaluation of changes in intermolecular cross-links in our immobilization model is necessary.
On the other hand, collagen isoforms are thought to affect the mechanical properties of muscle14,15). Collagen types I and III are the primary isoforms of skeletal muscle; furthermore, each isoform possesses distinct mechanical properties. Type I collagen is typically associated with tissue stiffness (i.e., resistance to stretch), whereas the abundance of type III collagen correlates with tissue compliance (i.e., ability to elongate readily)16,17). Han et al.13) examined the lack of change in type I or III collagen mRNA expression in immobilized rat soleus muscle following a 1-day immobilization. In the same study, type I collagen mRNA expression declined in soleus muscle following 3 days of immobilization; however, it returned to control levels after 7 days. In contrast, type III collagen mRNA diminished in soleus following both 3- and 7-day immobilizations. Jarvinen et al. 9) performed immunohistochemical analysis of immobilized rat skeletal muscles, whereas the current investigation revealed that type III collagen increased in the perimysium and endomysium of immobilized muscles. Several previous studies9,13) determined the change in collagen isoforms in immobilized muscle although an identical tendency was not demonstrated. Changes in collagen isoforms in muscle may influence the mechanism governing muscle contracture; however, few reports appear in the literature regarding the quantitative alteration of type I and type III collagen isoforms in immobilized muscular tissue.
The objective of this study was to clarify the mechanism governing muscle contracture in rat soleus muscle by identifying changes in ankle joint mobility, insoluble collagen concentration and type I and type III collagen isoforms following 1- and 3-week immobilizations.
Materials and Methods
Animals
Twenty 8-week-old male Wistar rats (weight, 240–265 g) were used in this study. Rats were divided randomly into experimental (n=10) and control (n=10) groups. Animals of the experimental group were anesthetized with pentobarbital sodium (40 mg/kg); subsequently, their bilateral ankle joints were fixed in full plantarflexion with plaster casts; that is, the soleus muscles were immobilized in a shortened position. The plaster cast, which was fitted from above the knee joint to the distal foot, was changed weekly due to loosening consequent to muscle atrophy. The ankle joint was immobilized for either 1 or 3 weeks; each experimental group consisted of five rats. In addition, five untreated control rats were tested weekly.
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation at Seijoh University (Approval no. 2005B0001).
Measurement of range of motion of ankle joint dorsiflexion
At 1 and 3 weeks after immobilization, rats were anesthetized with pentobarbital sodium (40 mg/kg). Following body weight measurement, the range of motion (ROM) of dorsiflexion of the ankle joint was determined with a goniometer10).
Measurement of ROM was defined as the angle (0–180 degrees) of the straight line connecting the 5th metatarsal and the malleolus lateralis of the fibula to the line connecting the malleolus lateralis of the fibula and the center of the knee joint when the ankle was passively dorsiflexed maximally and the knee joint was flexed 90 degrees.
Muscle sample
After ROM measurement in each immobilization period, bilateral soleus muscles were excised and weighed. Subsequently, muscle samples were frozen in isopentane cooled with dry ice and stored in a freezer at −80°C until biochemical analyses were performed.
Evaluation of insoluble collagen concentration
Extraction of insoluble collagen in muscle tissue: The left soleus muscles of experimental and control rats of each immobilization period were minced with a razor, after which specimens were placed in 1.0 M NaCl in 0.05 M Tris-HCl buffer that was 10 times the wet weight and homogenized. Homogenates were stirred for 24 hr at 4°C and centrifuged at 15,000 g for 60 min at 4°C. Next, sediments were introduced to 0.5 M acetic acid that was 5 times the wet weight, stirred for 24 hr at 4°C, and centrifuged at 15,000 g for 60 min at 4°C. Furthermore, sediments were added to 0.5 M acetic acid containing pepsin (1 mg/ml) that was 5 times the wet weight and stirred for 24 hr at 4°C; subsequently, sediments were centrifuged at 15,000 g for 60 min at 4°C. Finally, sediments were collected as insoluble collagen.
Determination of amount of collagen: In general, hydroxyproline is thought to be exclusive to collagen; thus, the concentration of collagen was determined via measurement of hydroxyproline content. Collagen content was estimated via the modified technique of Reddy et al.18)
Samples, which were lyophilized for 24 hr, were hydrolyzed in 6 N HCl for 15 hr at 110°C. Next, the samples were hydrolyzed in alkali for 20 min at 90°C. The hydrolyzed specimens were then mixed with buffered chloramine-T reagent; subsequent oxidation occurred at room temperature. The chromophore developed upon the addition of Ehrlich's aldehyde reagent, after which the absorbance of each sample was measured at 540 nm with a spectrophotometer. Absorbance values were plotted against the concentration of standard hydroxyproline; the presence of hydroxyproline in unknown sample extracts was determined from the standard curve. The hydroxyproline concentration of samples was calculated as the content per wet weight (µg/mg wet weight).
Analysis of type I and type III collagen isoforms
Preparation of collagen: Collagen type of right soleus muscles was analyzed. Changes in the ratio of type III to type I collagen isoforms in muscle tissue were examined with polyacrylamide gel electrophoresis involving the modified technique of Sykes et al.19).
Specimens were minced with a razor and washed overnight in 0.05 M Tris-HCl buffer supplemented with 0.9% NaCl, pH 7.4, at 4°C. Subsequently, samples were rinsed with 0.5 M acetic acid, homogenized in 0.5 M acetic acid containing pepsin (1 mg/ml), digested for 24 hr at 4°C while stirring and centrifuged at 15,000 g for 1 hr at 4°C. The precipitates were suspended twice in the same solution and centrifuged under identical conditions. The resulting supernatants were combined and lyophilized.
Polyacrylamide gel electrophoresis: The lyophilized samples were dissolved in 0.01 M phosphate-buffered saline (PBS) and boiled for 2 min at 80°C in a buffer containing 0.005% bromophenol blue, 20% glycerol, 4% sodium dodecyl sulfate (SDS) and 126 mM Tris-HCl (pH 6.8). Electrophoresis was performed employing polyacrylamide gel of 3–10% gradient concentration with twelve parallel tracks. Samples were introduced to the sample wells; subsequently, the gels were run at 20 mA for 40 min in running buffer (25 mM Tris, 192 mM glycine and 0.1% SDS). Following termination of the electric current, 20% 2-mercaptoethanol was added to the sample wells, after which it was allowed to diffuse into the gel for 1 hr before the electric current was re-applied. Electrophoresis was resumed for 1 hr. Upon completion of electrophoresis, the gel was rinsed with ultra pure water and stained with a coomassie brilliant blue R solution at room temperature. The stained gel was bathed until the background was sufficiently reduced.
Analysis of collagen type confirmed bands corresponding to the α1 (I) chain, α2 chain and α1 (III) chain from the electrophoresis image. The type I collagen isoform is comprised of two α1 (I) chains and a single α2 chain, whereas the type III collagen isoform is comprised of three α1 (III) chains. As a result, the ratio of type III to type I collagen isoforms was calculated: α1 (III) / {α1 (I) +α2}×100 (%).
Data analysis
Comparisons of control and experimental groups for each immobilization period were conducted with the non-paired Student's t-test. In all statistical analyses, a level of p<0.05 was required for significance.
Results
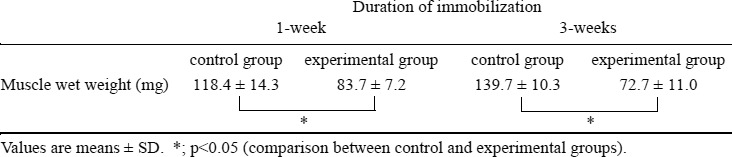
Muscle wet weight
Mean ± SD values for the soleus muscle wet weight was as follows: at 1 week, control group, 118.4 ± 14.3 mg and experimental group, 83.7 ± 7.2 mg; at 3 weeks, control group, 139.7 ± 10.3 mg and experimental group, 72.7 ± 11.0 mg (Table 1). After 1 and 3 weeks of immobilization, the soleus muscle wet weight of the experimental group had decreased significantly (p<0.01) in comparison with control animals.
Table 1. Muscle wet weight.
 |
Range of motion
Figure 1 presents data corresponding to ROM of dorsiflexion for the control and experimental groups at various times during immobilization. Mean ± SD values for ROM of dorsiflexion in the experimental group were 143.0 ± 4.5 degrees at 1 week and 106.0 ± 5.5 degrees at 3 weeks after immobilization. After 1 and 3 weeks of immobilization, ROM of dorsiflexion of the experimental group had decreased in comparison with control animals.
Fig. 1.
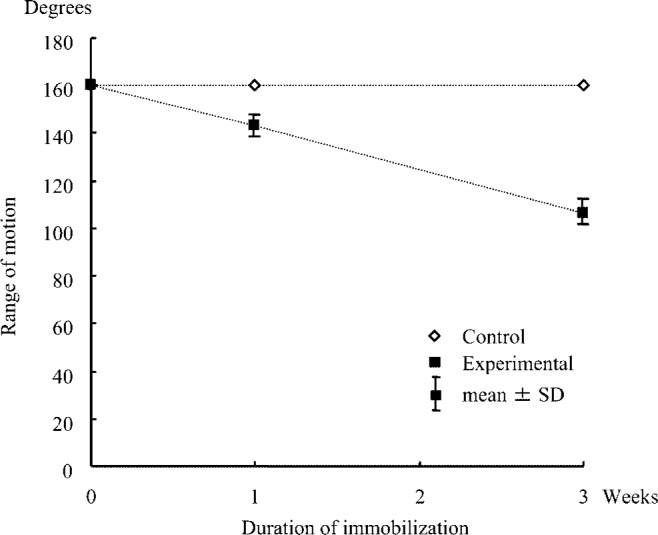
Change in range of motion of ankle joint during dorsiflexion.
Insoluble collagen concentration
Mean ± SD values for hydroxyproline concentration were as follows: at 1 week, control group, 0.77 ± 0.34 µg/mg wet weight and experimental group, 0.90 ± 0.37 µg/mg wet weight; at 3 weeks, control group, 0.47 ± 0.22 µg/mg wet weight and experimental group, 1.00 ± 0.53 µg/mg wet weight. After the 1-week immobilization, no significant difference was observed between the experimental and control groups in terms of hydroxyproline concentration. Following the 3-week immobilization, however, the experimental group exhibited a meaningful increase (p<0.01) relative to the control group (Fig. 2).
Fig. 2.
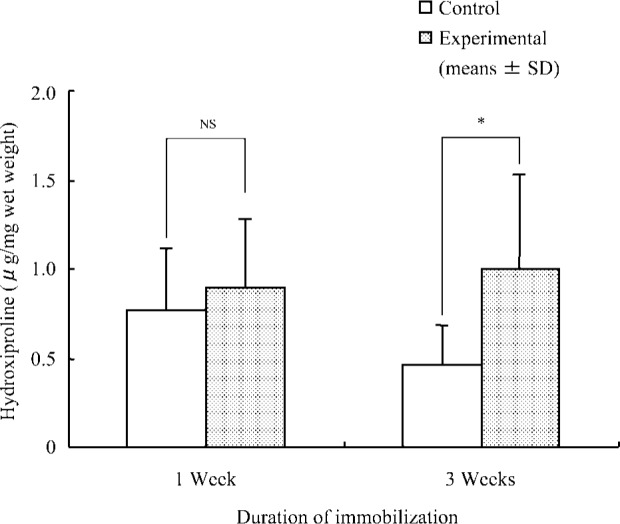
Concentration of hydroxyproline in insoluble collagen in soleus muscle.
*; p<0.05 (comparison between control and experimental groups), NS; Not significant.
Ratio of type III to type I collagen isoforms
A representative example of polyacrylamide gel electrophoresis of the control and experimental groups subjected to 1-week immobilization appears in Fig. 3.
Fig. 3.
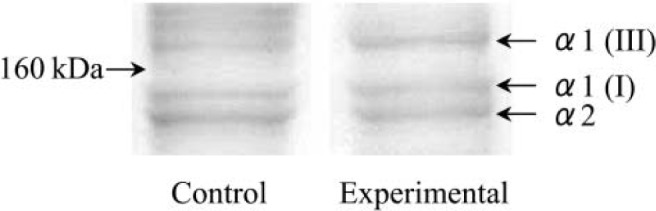
Polyacrylamide gel electrophoresis of intramuscular collagen of control and experimental groups 1 week after immobilization.
Mean ± SD values for the ratio of type III to type I collagen isoforms were as follows: at 1 week, control group, 68.7 ± 8.1% and experimental group, 92.1 ± 17.1%; at 3 weeks, control group 70.4 ± 14.6% and experimental group, 100.5 ± 20.2%. After 1 and 3 weeks of immobilization, the ratio of type III to type I collagen isoforms of the experimental group increased significantly (p<0.01) in comparison with the control group (Fig. 4).
Fig. 4.
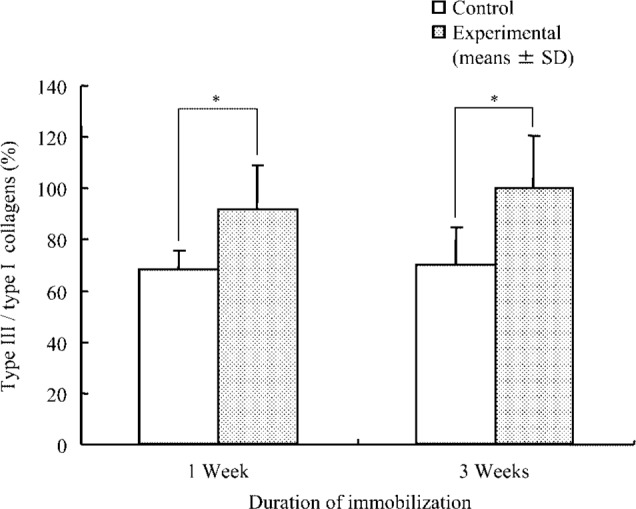
Ratio of type III to type I collagen isoforms in soleus muscle.
The ratio of type III collagen to type I collagen = {α1 (III) / (α1 (I) + α2)}×100 (%)
*; p<0.05 (comparison between control and experimental groups).
Discussion
The current findings demonstrated declines in ROM of dorsiflexion to 90% and 67.5% of the control value following 1- and 3-week immobilizations, respectively. These data suggested that ankle joint mobility decreases with prolonged periods of immobilization.
Several studies20–22) documented those tissues believed to be responsible for the limitation in ROM of the joint following immobilization. A recent review23) proposed that the myogenic limitation predominates during the first 90 days of immobility, whereas the limitation is mainly arthrogenic beyond 90 days of immobility. Okamoto et al.22) reported that the limitation in ROM of joints at 1 month after immobilization is derived from soft tissue (skin and muscle) changes and that the arthrogenic limitation increases at 2–3 months after immobilization. Therefore, the limitation in ROM after 1- and 3-week immobilizations in this investigation was thought to be attributable to myogenic change. The limitation in joint ROM due to myogenic change is referred to as muscle contracture; this mechanism may be related to alteration of intramuscular connective tissue.
Collagen, a major organic constituent of intramuscular connective tissue, is important with respect to maintenance of the structure and the mechanical function of skeletal muscles1,13). Woo et al.24) noted that cross-link formation in the collagen fiber reduces the flexibility of the tissue. In general, enhancement of intermolecular cross-links affects collagen solubility and induces an increase in insoluble collagen12,25).
In terms of hydroxyproline concentration of insoluble collagen, no significant difference was apparent between the experimental and control groups after 1 week of immobilization. After 3 weeks of immobilization, however, the experimental group exhibited a meaningful increase in comparison with the control group. On the other hand, the soleus muscles wet weight in the experimental group decreased with prolonged periods of immobilization. The hydroxyproline concentration of samples was calculated as the content per wet weight in this study, therefore, increase in the insoluble collagen concentration following 3 weeks immobilization may be shown that collagen fibers with strong intermolecular cross-links contained in the muscle was made increased relatively. This change in collagen fibers may be important with respect to the progress of muscle contracture associated with extended periods of immobilization.
Our study used 8-week-old young rats; on the other hand, the accumulation of collagen in rat skeletal muscle in youth is preceded by a strong biosynthetic activity of collagen14). In fact, insoluble collagen concentration in control group at 3 weeks was decreased in comparison with 1 week. Therefore, as the animal to use for an experiment, it may be chosen stable age of collagen metabolism.
Collagen isoforms are thought to influence the mechanical properties of muscle14,15). Collagen types I and III are the primary isoforms in skeletal muscle; furthermore, each isoform possesses distinct mechanical properties16,17). Each collagen isoform differs in terms of property, function and morbid condition, e.g., restoration processes of organization. Collagen type I is typically associated with high tensile strength and elastic stiffness, and collagen type III is prominent in loose connective tissues having higher compliance. Collagen type III is especially characteristic of embryonic and young tissues and is the other main collagenous component in intramuscular connective tissue. Additionally, the expression of a specific collagen isoform is recognized in the pathogenesis of various disease processes11,26,27). Miller et al.17) noted a decline in the proportion of type I collagen and an increase in the proportion of type III collagen in the soleus muscle of rats following hindlimb unloading. Jarvinen et al.9) reported an increase in type III collagen in the perimysium and endomysium of immobilized rat calf muscles. The relative proportion of type I and III collagen may be important in that the diameter of the fibres is known to decrease in proportion to increases in the amount of type III collagen14). The change of the ratio of type III to type I collagen may indicate the condition and the function of muscle. Our study is the first to present that the ratio of type III to type I collagen isoforms increased after 1- and 3-week immobilizations. Therefore, it is possible that atrophied muscles following hindlimb unloading or immobilization induce an increase in the type III collagen isoform.
The increase in the ratio of type III to type I collagen isoforms occurred 1 week after immobilization with concomitant ROM restriction; however, the increase did not parallel progression in the limitation in ROM. The ratio of type III to type I collagen isoforms in immobilized muscular tissue does not change in parallel with the increase in the limitation in ROM; consequently, muscle immobilization may lead to induction of type III collagen isoform expression. Therefore, type III collagen isoform expression in muscular tissue probably is not closely related to the progress of muscle contracture.
Previous studies7,12,17) indicated that total collagen content in muscular tissue increases after immobilization or hindlimb unloading. This phenomenon is thought to contribute to the progression of muscle fibrosis; however, it is not clear as to whether this phenomenon influences decreased muscle elasticity. Therefore, an increase in the type III collagen isoform in immobilized muscle may be related to the mechanism underlying the progression of muscle fibrosis.
In conclusion, the current results suggested that muscle contracture, which occurred after a 1-week immobilization, progresses during prolonged periods of immobilization. Consequently, insoluble collagen concentration increased after only 3 weeks of immobilization. This change may suggest that collagen fibers with strong intermolecular cross-links contained in the muscle was made increased relatively, that is, enhancement of intermolecular cross-links may play a progressive role in muscle contracture during extended periods of immobilization. Muscle immobilization may be responsible for the increase in the ratio of type III to type I collagen isoforms. However, this change is not closely related to the progress of muscle contracture; on the other hand, it may be related to the mechanism governing the progression of muscle fibrosis.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Grant no. 18500428).
References
- 1). Gould RP: The microanatomy of muscle. In: Bourne GH. (ed) The Structure and Function of Muscle. Vol. 2, Part. 2, Academic Press, 1973, pp186-243. [Google Scholar]
- 2). Borg TK, Caulfield JB: Morphology of connective tissue in skeletal muscle. Tissue Cell 12: 197-207, 1980. [DOI] [PubMed] [Google Scholar]
- 3). Williams PE, Goldspink G: Connective tissue changes in surgically overloaded muscle. Cell Tissue Res 221: 465-470, 1981. [DOI] [PubMed] [Google Scholar]
- 4). Joza L, Thoring J, et al. : Quantitative alterations in intramuscular connective tissue following immobilization: an experimental study in the rat calf muscles. Exp Mol Pathol 49: 267-278, 1988. [DOI] [PubMed] [Google Scholar]
- 5). Stolov WC, Fry LR, et al. : Adhesive forces between muscle fibers and connective tissue in normal and denervated rat skeletal muscle. Arch Phys Med Rehabil 54: 208-213, 1973. [PubMed] [Google Scholar]
- 6). Melichna J, Gutmann E: Stimulation and immobilization effects on contractile and histochemical properties of denervated muscle. Pflugers Arch 352: 165-178, 1974. [DOI] [PubMed] [Google Scholar]
- 7). Williams PE, Goldspink G: Connective tissue changes in immobilized muscle. J Anat 138: 343-350, 1984. [PMC free article] [PubMed] [Google Scholar]
- 8). Jozas L, Kannus P, et al. : The effect of tenotomy and immobilization on intramuscular connective tissue. J Bone Joint Surg 72B: 293-297, 1990. [DOI] [PubMed] [Google Scholar]
- 9). Jarvinen TAH, Joza L, et al. : Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An imunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil 23: 245-254, 2002. [DOI] [PubMed] [Google Scholar]
- 10). Okita M, Yoshimura T, et al. : Effect of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil 25: 159-166, 2004. [DOI] [PubMed] [Google Scholar]
- 11). Karpakka J, Vaananen K, et al. : The effect of preimmobilization training and immobilization on collagen synthesis in rat skeletal muscle. Int J Sports Med 11: 484-488, 1990. [DOI] [PubMed] [Google Scholar]
- 12). Sugama S, Tachino K, et al. : Effect of immobilization on solubility of soleus and gastrocnemius muscle collagen—Biochemical studies on collagen from soleus and gastrocnemius muscles of rat—. J Jpn Phys Ther Assoc 2: 25-29, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Han XY, Wang W, et al. : mRNA levels for α-subunit of prolyl 4-hydroxylase and fibrillar collagens in immobilized rat skeletal muscle. J Appl Physiol 87: 90-96, 1999. [DOI] [PubMed] [Google Scholar]
- 14). Kovanen V: Effect of aging and physical training on rat skeletal muscle. Acta Physiol Scand 577: 1-56, 1989. [PubMed] [Google Scholar]
- 15). Kovanen V, Suominen H, et al. : Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech 17: 725-735, 1984. [DOI] [PubMed] [Google Scholar]
- 16). Burgeson RE: The collagen of skin. Curr Probl Dermatol 17: 61-75, 1987. [PubMed] [Google Scholar]
- 17). Miller TA, Lesniewski LA, et al. : Hindlimb unloading induces a collagen isoform shift in the soleus muscle of the rat. Am J Physiol Regulatory Integrative Comp Physiol 281: R1710-R1717, 2001. [DOI] [PubMed] [Google Scholar]
- 18). Reddy GK, Enwemeka CS: A simple method for the analysis of hydroxyproline in biological tissues. Clin Biochem 29: 225-229, 1996. [DOI] [PubMed] [Google Scholar]
- 19). Sykes B, Puddle B, et al. : The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun 72: 1472-1480, 1976. [DOI] [PubMed] [Google Scholar]
- 20). Trudel G: Differentiating the myogenic and arthrogenic components of joint contracture. An experimental study on the rat knee joint. Int J Rehabil Res 20: 397-404, 1997. [DOI] [PubMed] [Google Scholar]
- 21). Trudel G, Uhthoff HK: Contractures secondary to immobility: Is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil 81: 6-13, 2000. [DOI] [PubMed] [Google Scholar]
- 22). Okamoto M, Okita M, et al. : Effects of immobilization period on restriction of soft tissue and articulation in rat ankle joint. Rigakuryohogaku 31: 36-42, 2004. (in Japanese). [Google Scholar]
- 23). Harburn KL, Potter PJ: Spasticity and contracture. Phys Med Rehabil State of the Art Rev 7: 113-132, 1993. [Google Scholar]
- 24). Woo SL, Matthews JV, et al. : Connective tissue response to immobility. Correlative study of biomechanical and biochemical measurements of normal and immobilized rabbit knees. Arthritis and Rheumatism 18: 257-264, 1975. [DOI] [PubMed] [Google Scholar]
- 25). Fujii K: Aging of the collagen in human joint components; changes in the reducible cross-links and solubilities. J Jpn Orthop Ass 49: 145-155, 1975. [Google Scholar]
- 26). Fujimoto M, Trudel G, et al. : High collagen type I and low collagen type III levels in knee joint contracture. Acta Orthop Scand 73: 335-343, 2002. [DOI] [PubMed] [Google Scholar]
- 27). Stephens HR, Duance VC, et al. : Collagen types in neuromuscular diseases. J Neurol Sci 53: 45-92, 1982. [DOI] [PubMed] [Google Scholar]


