Abstract
This study investigates the effects of Ca on bone in the ovariectomized mice. Twenty-six female ICR mice aged 5 weeks were used. They were ovariectomized (OVX) or sham-operated (SHAM) and fed standard mouse diet (SF) or special low calcium diet (L.Ca), respectively. All animals were sacrificed at day 100 after operation. Mechanical strength of the left femur and tibia was measured by the three-point bending strength test. The bones were dried, weighed and burned to ash. Correlation between mechanical strength and ash content was found. A specimen of the right tibia was prepared. Mechanical strength, ash content and ratio of dry bone weight to body weight of the femur and tibia in OVX and L.Ca mice were significantly less than in SHAM and SF mice. SHAM/SF mice and OVX/L.Ca group showed highest and lowest values in all cases. The values for the femur and tibia in OVX/SF mice were lower than in SHAM/SF group and in OVX/L.Ca group were less than in OVX/SF mice. Correlation coefficients for mechanical strength and ash content were 0.704 and 0.776 for the femur and tibia. Ca is thus related to inhibition of bone loss and maintenance of bone mass and effective prevention of osteoporosis.
Keywords: low calcium diet, ovariectomized mice, mechanical strength, ash content
Osteoporosis increase with aging. Fractures are major problems which associate with osteoporosis and hamper activities of daily livings (ADL). In osteoporosis, there are many risk factors that are very complicated. Women cannot avoid the menopause and thus are liable to osteoporosis owing to rapid reduction in bone mass. Lack of moderate exercise or insufficient Ca intake limit peak bone mass and thus lead to osteoporosis1). Though eating habits have improved and nutriments are sufficient, required Ca intake has remained stable at 90% for 20 years. Regional differences, eating habits and difficulty of absorption should be considered. Ca may thus be useful for preventing bone loss and osteoporosis. The relation of bone mineral density, osteoporosis and risk of fracture has been reported2,3–8). The diagnosis and treatment of osteoporosis have improved, but prevention and treatment not only by chemical methods but physical activity and dietetics should be considered. Rehabilitation of osteoporosis is a developing ways and the main prevention and treatment is rehabilitation including osteoporosis education in general because it is necessary to understand the disease and its treatment. Besides, there is no guarantee that chemical methods such as hormones or medicine will not produce ill effects. Thus it is important to prevent osteoporosis and to keep ADL by rehabilitation.
It is well known that Ca is necessary and important for bone but Ca intake tends to be insufficient actually. Therefore, it was investigated what effects on bone, mainly mechanical strength, are caused by Ca intake using OVX mice9,10) and low Ca diet for its basic data.
Materials and Methods
In this study, twenty six female ICR mice aged 5 weeks (27.7 ± 1.8g) were used. They were either ovariectomized (OVX) (group A, C) or sham-operated (SHAM) (group B, D) under pentobarbitlum sodium (1 ml/kg), and assigned randomly to 4 groups. Groups A and B were fed standard mouse diet MF (SF, Ca-1.1 g/100 g) and groups C and D, special low calcium diet (L.Ca, Ca-8.1 mg/100 g) for 100 days (Table 1). During this time, all the mice were allowed ad libitum feeding and drinking water. They were kept in separate cages (20.5 × 31 × 13 cm) under the following conditions: temperature, 23 ± 1 °C, the humidity, 50 ± 5% and 12 hour day-night cycle. There was no difference in physical activities conditions of mice. All animals were sacrificed at day 100 after operation. Femur and tibia were dissected out and soft tissue was removed.
Table 1. Subjects in this experiment.
| group | number | operation | diet |
|---|---|---|---|
| A | 7 | OVX | standard |
| B | 6 | SHAM | standard |
| C | 7 | OVX | low calcium |
| D | 6 | SHAM | low calcium |
OVX: ovariectomy, SHAM: sham-operation.
A materials testing machine (computer control system autograph AGS-1000A, SHIMADZU, Co, Japan) was used to measure three-point bending strength of the left femur and tibia. Speed of the compression head was 0.5 mm/min and pushed the center of specimens vertically. Calculated values were revised based on body weight because the femur and the tibia are loaded bones and the point of measurement is the cortex bone area said to respond to mechanical stress. The left femur and tibia were dried, weighed and burned at 600°C for 6 hours to ash. The right femur and tibia were fixed in formalin for 3 days, demineralize in K-CX solution for 24 hours and embedded in paraffin for morphology study. Sections (3–4 µm) were prepared and stained by Hematoxylin-Eosin.
This study was carried out in accordance with Guide for Animal Experimentation, Hiroshima University and the Committee of Research Facilities of Laboratory Animal Science, Hiroshima University School of Medicine.
For statistic analysis, two-way analysis of variance and correlation analysis for finding the correlation coefficient between mechanical strength and ash content were used. All values were expressed as means ± standard deviation (SD). p less than 0.05 was considered significant.
Results
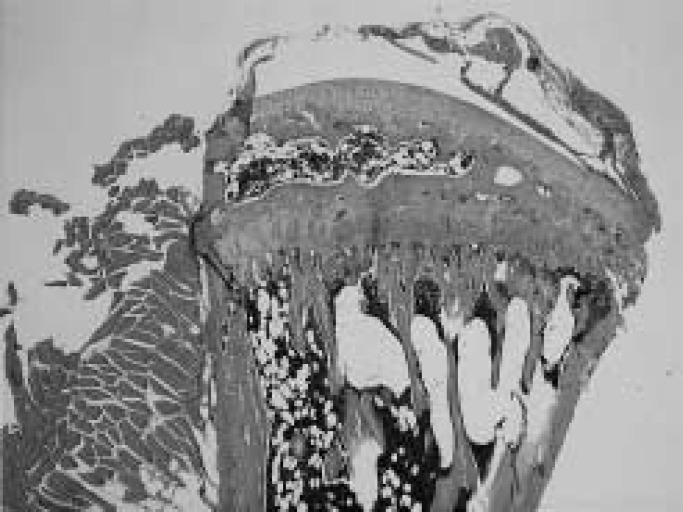
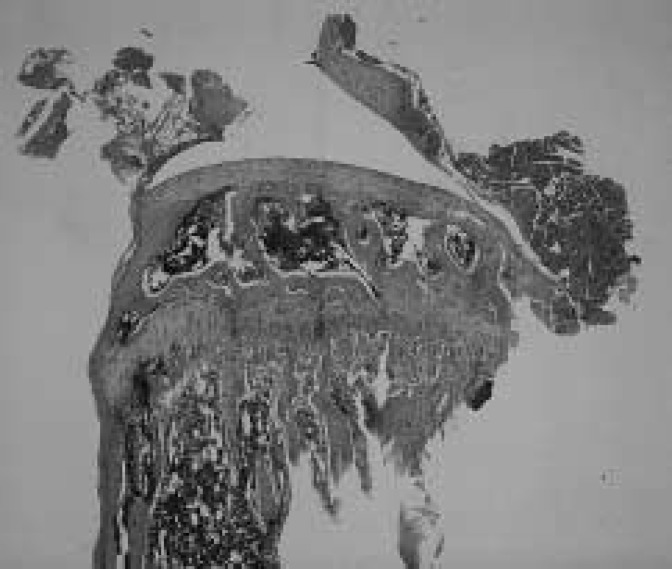
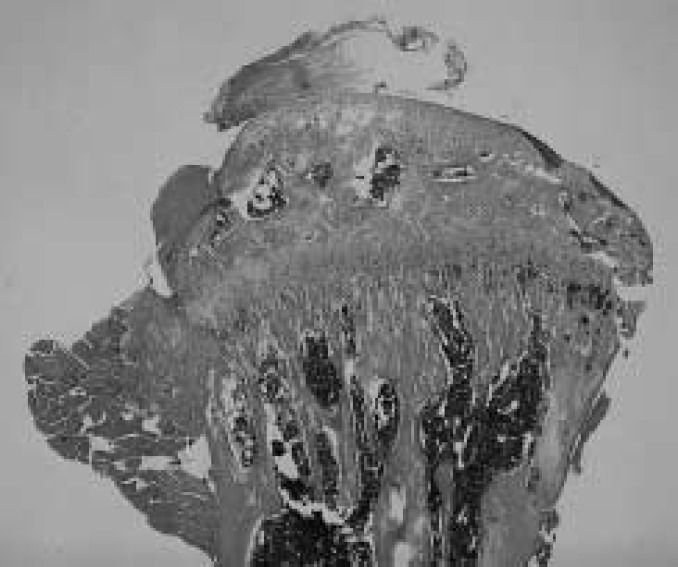
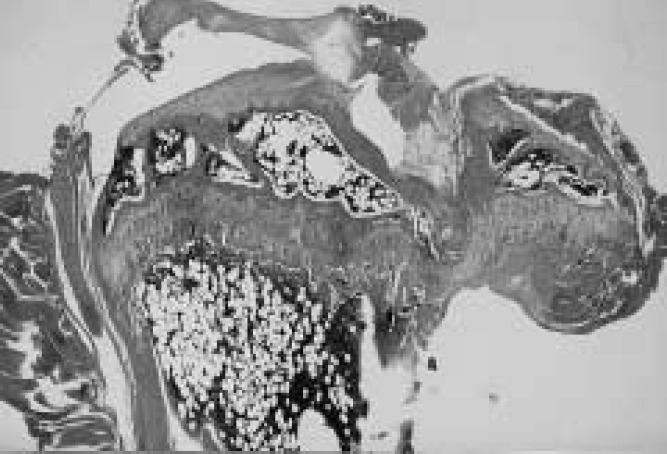
Results of measured parameters were shown in Table 2. Final body weight of OVX mice was significantly more than of SHAM animals. Mechanical strength of the femur and tibia in OVX mice was significantly less than in SHAM animals, in L.Ca group was less than in SF mice. Interaction was shown in mechanical strength of the femur. Ash content of the femur and tibia in OVX and L.Ca mice was significantly less than in SHAM or SF groups. The dry bone weight of SHAM was higher than in OVX essentially the same for L.Ca group and SF group. Group C showed the lowest mechanical strength, ash content and dry bone weight. All parameters indicated the effect of OVX and Ca intake. The correlation coefficient between mechanical strength and ash content was 0.704 and 0.776 for the femur and tibia, respectively. Table 3 shows result of two-way ANOVA. Figures 1–4 illustrate specimens of the proximal tibia (light microscope × 5, Hematoxylin-Eosin stain) and indicates denseness and coarseness in the primary spongiosa. Trabecular changed. Trabecular in Fig.2 is dense and there are no gaps. Figs. 1 and 4 show respective specimens of the A and D group, and gaps among trabecular remarkably. A few trabecular are present in Fig. 3.
Table 2. Effects of OVX and Ca on measured parameters.
| group | body weight (g) | mechanical strength/body weight (10−3kgf/g) |
ash content (mg) |
dry bone weight/body weight (mg/g) |
|||
|---|---|---|---|---|---|---|---|
| femur | tibia | femur | tibia | femur | tibia | ||
| A | 50.3 ± 4.6* | 43.0 ± 6.3* | 33.2 ± 4.0* | 37.8 ± 2.5* | 25.2 ± 4.8* | 1.45 ± 0.14* | 1.10 ± 0.10* |
| B | 40.0 ± 4.1 | 57.7 ± 9.6 | 45.5 ± 5.9 | 40.8 ± 3.0 | 30.0 ± 2.9 | 1.83 ± 0.12 | 1.38 ± 0.07 |
| C | 43.0 ± 3.8*# | 34.1 ± 8.1*# | 30.4 ± 4.4*# | 26.0 ± 2.2*# | 22.1 ± 3.2*# | 1.19 ± 0.07* | 1.03 ± 0.07* |
| D | 35.1 ± 2.3# | 48.0 ± 7.0# | 38.6 ± 4.7# | 28.0 ± 1.5# | 23.4 ± 1.2# | 1.75 ± 0.10 | 1.38 ± 0.06 |
Mean ± SD.
A: OVX/SF, B: SHAM/SF, C: OVX/L.Ca, D: SHAM/L. Ca.
Significantly different from SHAM group (p<0.05).
Significantly different from SF group (p<0.05).
Results of ANOVA. Mechanical strength of the femur showed the interaction.
Table 3. p- and F-value in ANOVA (OVX and Ca) for parameters.
| body weight (g) | maximum load/body weight (10−3kgf/g) | ash content (mg) | dry bone weight/body weight (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| femur | tibia | femur | tibia | femur | tibia | ||
| OVX | <0.0001/28.757 | <0.0001/27.784 | 0.0484/ 4.418 | 0.0002/20.311 | 0.0005/17.735 | 0.0017/13.081 | 0.0002/20.271 |
| food | 0.0294/ 5.502 | <0.0001/61.355 | 0.0493/ 0.728 | <0.0001/62.631 | 0.0005/17.502 | <0.0001/41.569 | 0.0009/15.400 |
| OVX• food | 0.0633/ 3.867 | 0.0003/15.265 | 0.4612/ 0.564 | 0.3314/ 0.991 | 0.5821/ 0.313 | 0.5914/ 0.298 | 0.3437/ 0.943 |
p-/F-values are shown. Degree of freedom is one.
Fig. 1.
The proximal tibia of OVX/SF (A) group. Trabecular is remained barely but a gap is remarkable.
Fig. 4.
The proximal tibia of SHAM/L.Ca (D) group. Trabecular is sparse and a gap is found here and there.
Fig. 2.
The proximal tibia of SHAM/SF (B) group. Trabecular is dense and a gap is not found.
Fig. 3.
The proximal tibia of OVX/L.Ca (C) group. There are few trabecular and this area becomes a hollow.
Discussion
The most serious problem in osteoporosis is fracture. In this study, mechanical strength in relation to fractures was significantly small value in the OVX and L.Ca groups. For combined OVX with L.Ca (group C), these values were lowest in all cases except for body weight. Deficiency of estrogen causes bone loss. Ca intake may maintain bone mass and prevent bone loss since mechanical strength and ash content in group A were not less than in group C and in group D decreased even in SHAM mice. Thus, bone loss occurs by low Ca intake with or without OVX. Thus Ca prevents bone loss. The relationship of Ca to bone was demonstrated by the correlation between mechanical strength and ash content.
Osteoporosis occurs in men and women though there are differences in the degree. In women, bone loss is rapid because menopause is unavoidable. Ca intake and the regulation of Ca are controlled by organs such as the kidney, intestine, bone, Ca metabolic hormones and cytokines. Differences in Ca ingestion may arise from Ca intake in the intestine or kinds food. Vitamin D intake that influences Ca absorption in the intestine11–13), hormones unbalance by aging2) and suppression of decline in Ca absorption in the intestine by exercise14,15) are significant factors for this. Ca intake maintains bone mass and inhibits bone loss and decreases risk of fracture. Ca intake could be effective for pre/postmenopausal women4).
Risk factors of osteoporosis are various, but Ca intake may be primary care for prevention. Although Ca is not contained foods very much and it is difficult to absorb Ca, necessary Ca intake per day according to the Ministry of Health and Welfare (600 mg/day) is much less than in the USA (800–1500 mg/day) after adolescent. Ca intake is thus important. For the prevention of osteoporosis, bone mass should be as high as possible. Risk factors may be lessened by life style modification and thus peak bone mass may be increased by guidelines on nutrition and moderate exercise, all quite important from childhood. Ca have a great influence on bone mass.
References
- 1). Bonjor J, et al. : Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 73: 555-563, 1991. [DOI] [PubMed] [Google Scholar]
- 2). Nordin BEC, Wilkinson R, et al. : Calcium absorption in the elderly. Calcif Tissue Res 21: 442-447, 1976. [PubMed] [Google Scholar]
- 3). Matkovic V, et al. : Bone status and fracture rates in two regions of Yugoslavia. Am J Clin Nutr 32: 540, 1979. [DOI] [PubMed] [Google Scholar]
- 4). Comming RG: Calcium intake and bone mass: quantitative review of the evidence. Calcif Tissue Int 47: 194-201, 1990. [DOI] [PubMed] [Google Scholar]
- 5). Riis B, et al. : Does calcium supplementation prevent postmenopausal bone loss? —A double-blind, controlled clinical study—. N Engl J Med 316: 173, 1987. [DOI] [PubMed] [Google Scholar]
- 6). Dawson-Hughes B, et al. : A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J 323, 878, 1990. [DOI] [PubMed] [Google Scholar]
- 7). Aloia JF, et al. : Calcium supplementation with and without hormone replacement therapy to prevent postmenopausal bone loss. Ann Intern Med 120: 97, 1994. [DOI] [PubMed] [Google Scholar]
- 8). Matkovic V: Calcium and peak bone mass. J Int Med 231: 151-160, 1992. [DOI] [PubMed] [Google Scholar]
- 9). Kalu DN: The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15: 175-192, 1991. [DOI] [PubMed] [Google Scholar]
- 10). Higuchi Y, Ichikawa F, et al. : A basic study on making rat osteoporosis model by ovariectomy and 0.5% calcium diet. In: Takahashi H. (ed) Handbook of Bone Morphometry. Nishimura-shoten, Niigata, 1997, pp 177-178. [Google Scholar]
- 11). Yamamoto M, et al. : Vitamin D deficiency and calcium transport in the rat. J Clin Invest 74: 507-513, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Chapuy MC, et al. : Vitamin D3 and calcium to prevent hip fractures in elderly women. Engl J Med 327: 1637-1642, 1992. [DOI] [PubMed] [Google Scholar]
- 13). Baylink D, et al. : Decreased bone formation, mineralization, and enhanced resorption in calcium-deficient rats. Am J Physiol 225: 269-276, 1973. [DOI] [PubMed] [Google Scholar]
- 14). Pohlman RL, et al. : Morphometry and calcium contents in appendicular and axial bones of exercised ovariectomized rats. Am J Physiol 248: 1212-1217, 1985. [DOI] [PubMed] [Google Scholar]
- 15). Dawson B, Hughes B, et al. : Calcium absorption on and low calcium intakes in relation to vitamin D receptor genotype. J Bone Miner Res 10 (Suppl ): S162, 1995. [Google Scholar]