Abstract
We investigated the histopathological and immunohistochemical effects of loading on cartilage repair in rat full-thickness articular cartilage defects. A total of 40 male 9-week-old Wistar rats were studied. Full-thickness articular cartilage defects were created over the capsule at the loading portion in the medial condyle of the femur. Twenty rats were randomly allocated into each of the 2 groups: a loading group and a unloading group. Twenty rats from these 2 groups were later randomly allocated to each of the 2 groups for evaluation at 1 and 2 weeks after surgery. At the end of each period, knee joints were examined histopathologically and immunohistochemically. In both groups at 1 and 2 weeks, the defects were filled with a mixture of granulation tissue and some remnants of hyaline cartilage. The repair tissue was not stained with toluidine blue in both groups. Strong staining of type I collagen was observed in the repair tissue of both groups. The area stained with type I collagen was smaller in the unloading group than in the loading groups, and the stained area was smaller at 2 weeks than at 1 week. In the staining for type II collagen, apparent staining of type II collagen was observed in the repair tissue of both groups at 1 week. At 2 weeks, there was a tendency toward a higher degree of apparent staining in the loading group than in the unloading group. Accordingly, these results indicated that loading and unloading in the early phase of cartilage repair have both merits and demerits.
Keywords: Articular cartilage, full-thickness defect, mechanical loading
Articular cartilage functions as a nearly frictionless bearing surface while uniformly transferring loads on underlying bone and preventing high stress concentrations1). The articular cartilage consists of 1 cell type, chondrocytes, which are embedded in an extracellular matrix of mainly type II collagen and proteoglycans1). The articular cartilage contains no blood supply, neural network, or lymphatic drainage2,3). Furthermore, the nutrition supplied to the cartilage depends on the compression and restoration of the articular cartilage by intermittent loading and synovial fluid circulation. Therefore, the articular cartilage is exquisitely sensitive to the mechanical environment, and mechanical loading may be the most important external factor regulating the development and long-term maintenance of the cartilage4). Moderate mechanical loading maintains the integrity of the articular cartilage1,4). Moderate loading of the articular cartilage generates mechanical signals that increase the synthetic activities of chondrocytes while suppressing their catabolic actions5–7). Although joints maintain homeostasis within a physiological range of mechanical loading, both reduced loading and overloading have catabolic effects, particularly for cartilaginous components1,4). Studies show that excessive mechanical stress can directly damage the cartilage extracellular matrix and shift the balance in chondrocytes to favor catabolic activity over anabolism8–13). Consistent with these results, high strain rates were reported to result in significant matrix fluid pressurization and impact-like surface cracking with cell death near the superficial zone in bovine osteochondral explants11). Reduced joint loading also creates catabolic responses within the articular cartilage. Animal models of reduced loading report that a decrease in mechanical stimuli leads to atrophy of the cartilage and ultimately erosion of the articular cartilage14–18).
When the articular cartilage is damaged because of injury or disease, it has a limited capacity to heal. Full-thickness articular cartilage defects that penetrate through cartilage undergo regenerative repair of the hyaline cartilage under restricted conditions. Furthermore, the reparative tissue is not identical to the original tissue, and there is no integration of repair tissue. Consequently, surgical treatment, such as microfracture, mosaicplasty, and autologous chondrocyte implantation, has become popular19). The rehabilitation protocols after surgery are classified according to the position and size of injury, and there is general agreement among many researchers about the period and intensity of muscle strengthening exercise and range of motion19–23). For patients with microfractures, if the chondral defect is located in the medial or lateral compartment of the knee, the patient is only allowed to undertake touchdown weight bearing for the first 6 weeks after surgery19). Full-weight bearing is allowed subsequently19). The reason for this practice is to protect immature reparative tissue and promote differentiation into cartilaginous tissue19–23). However, there is some evidence on graduated weight bearing, and one research has found no differences in treatment outcomes between unloading and loading groups immediately after surgery24). Marder et al.24) found that in 50 patients, there were no differences in results between 2 rehabilitative regimens that differed by weight-bearing status and use of continuous passive motion (CPM) for small full-thickness chondral defects treated by microfracture. To the best of our knowledge, only a few in vivo studies have investigated time-sequential changes in articular cartilage regeneration under different mechanical conditions. Harada et al.25) reported that dynamic compressive strain stimulated regeneration of joint surface structure. Harada et al.25) also suggested that the contact condition of the defect with surface cartilage may have an important role in the hyaline cartilage repair. However, effects of loading for cartilage repair and the underlying mechanism have not been fully clarified. The purpose of the present study was to histopathologically and immunohistochemically evaluate the influence of mechanical loading on the healing process of full-thickness articular cartilage defects in rat knee joints.
Methods
Forty 9-week-old male Wistar rats were evaluated in the present study. The animals were kept under normal conditions for 1 week before the start of the experiments to acclimatize them to the environment. They were housed, 1 or 2 per cage, in a room maintained under a 12-h light-dark cycle, and food and water were given ad libitum. This investigation was approved by the Animal Research Committee of the Kanazawa University Graduate School of Medicine, Kanazawa, Japan (approval No. 112206). All procedures for animal care and treatment were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Kanazawa University.
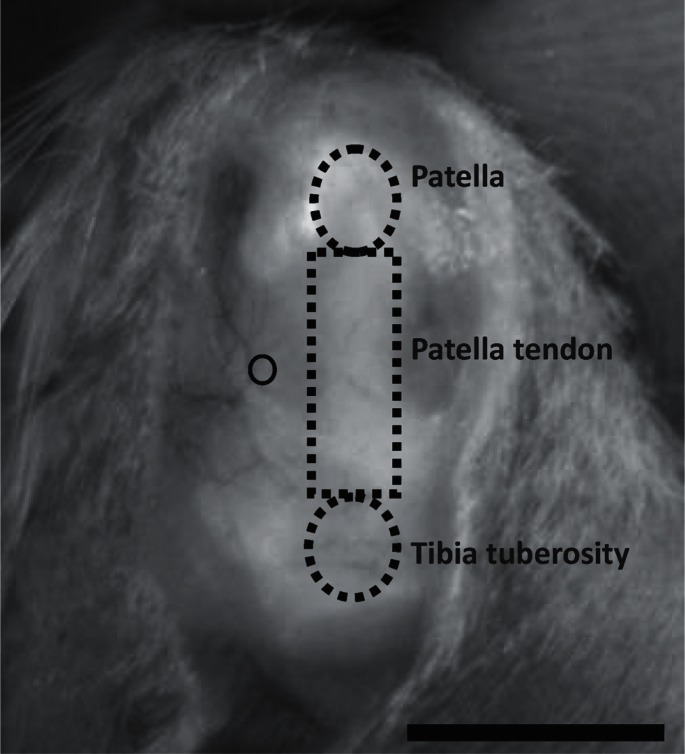
In our previous study, we reported a low-invasive method used to create full-thickness articular cartilage defects of femoral condyles in a rat model26). Consequently, measurements of the defects showed that the full-thickness articular cartilage defects were created at the identical position with a high degree of accuracy and reliability. These results suggested that the low-invasive method designed in that study was useful for creating full-thickness articular cartilage defects. In the present study, full-thickness articular cartilage defects were created as described previously26). The rats were anesthetized by an intraperitoneal injection of sodium pentobarbital at a dose of 40 mg/kg. After shaving the left knees, they were disinfected, and a parapatellar incision was performed to expose the knee joint. In maximum flexion of the knee, full-thickness defects (0.8-mm diameter, 2.0-mm depth) of the articular cartilage were created over the capsule by using a Kirschner wire (0.8-mm in diameter) in the medial condyle (Fig. 1). At the height of the center of the patella tendon, the defect was created at the medial position of the inner margin of the tendon (half the tendon width in length). The wire was marked at a position 2.0 mm from the tip to ensure invasive depth uniformity. After creation of the defect, the skin was sutured. Immobilization of the left knee and intervention in the right knee were not performed in any of the rats. After the surgery, the rats were randomly assigned to 2 groups: a loading group (n = 20) and an unloading group (n = 20). Twenty rats from these 2 groups were later randomly allocated to each of the 2 groups for evaluation at 1 and 2 weeks after surgery. The loading group was allowed to walk immediately after regaining consciousness following anesthesia. The unloading group was subjected to hindlimb suspension for each experimental term; therefore, their knee joints were under a unloading condition. Hindlimb suspension in the present study was performed using the modified Andries Ferreira's tail-suspended method27). This modified method was low-invasive and consisted of the application of a Kirchner wire. The hindlimb was suspended so that it did not touch the floor, and both knee joints bore no weight. During suspension, the rats could move their forelimbs freely for intake of food and water.
Fig. 1.
Position of the full-thickness articular cartilage defect over the capsule
The created defect is in the inside position of the inner margin of the tendon at the height of the center of the patella tendon (circle). Scale bar = 10 mm.
At 1 and 2 weeks after surgery, the rats were sacrificed by an intraperitoneal injection of a lethal dose of sodium pentobarbital. Immediately after death, their left hind limbs were disarticulated at the hip joint. All left knees were fixed in 10% neutral-buffered formalin for 72 h and decalcified with Decalcifying Solution A (Plank-Rychlo Method, Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 72 h. The knees were excised, deacidified in 5% sodium sulfate solution for 72 h, dehydrated in ethanol after washing with water, and embedded in paraffin wax. Sagittal sections (3 µm) were stained with hematoxylin and eosin and with toluidine blue, respectively. A light microscope and a digital camera were used to image and examine the sections (BX-51 and DP-50; Olympus Corporation, Tokyo, Japan).
The presence of type I and type II collagen in the repair tissue were examined immunohistochemically using human monoclonal antibodies against mouse type I and type II collagen that specifically cross-reacted with mouse and rat type I and type II collagen, respectively. The paraffin sections were deparaffinized and hydrated through graded alcohols. The sections were digested with proteinase K (S3020; Dako Japan, Tokyo, Japan) for 5 min, and endogenous peroxidase was inactivated by the addition of 3% H2O2 for 20 min. Protein Block Serum-Free (X0909; Dako Japan, Tokyo, Japan) was used to block nonspecific bindings of immunoglobulins for 15 min. The sections were incubated with the antibody against type I collagen (ab34710; Abcam, Tokyo, Japan; dilution 1:500) or with the antibody against type II collagen (F-57; Cosmobio, Tokyo, Japan; dilution 1:500) overnight at room temperature. After washing with phosphate-buffered saline (PBS), the slides were incubated with a horseradish peroxidase conjugated goat anti-mouse immunoglobulin antibody (K4000; Dako Japan, Tokyo, Japan) for 60 min and then rinsed in PBS. Antibody binding was visualized using Liquid DAB Substrate Chromogen System (K3468; Dako Japan, Tokyo, Japan) for 3 min at room temperature. The sections were counterstained with hematoxylin.
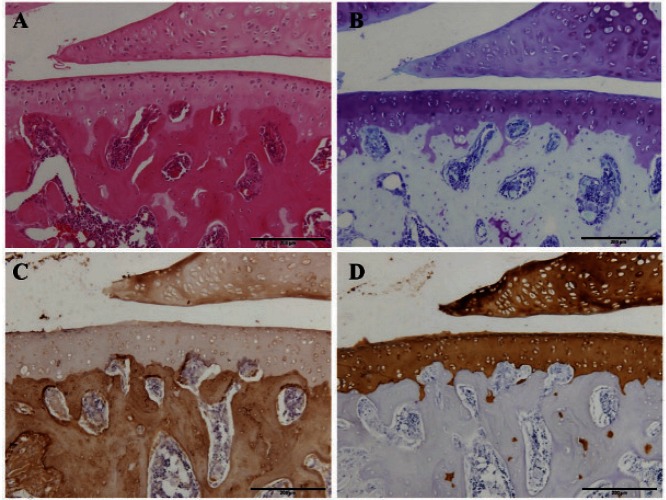
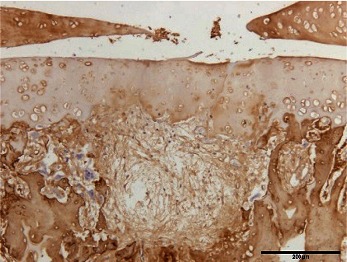
Normal articular cartilage samples that were histopathologically and immunohistochemically stained are shown in Fig. 2. In the toluidine blue and immunohistochemical stainings, the degree of staining was evaluated semiquantitatively by classifying the stains into 3 grades according to the original scale in Table 1.
Fig. 2.
Histopathological and immunohistochemical staining of normal articular cartilage
Hematoxylin and eosin staining (A), toluidine blue staining (B), and immunohistochemical staining for type I collagen (C) and type II collagen (D). Scale bar = 200 µm.
Table 1. The original semiquantitative scale for histopathological and immunohistochemical staining.
| Scale bar = 500 µm | |||
|---|---|---|---|
| Toluidine Blue | Type I collagen | Type II collagen | |
| I | 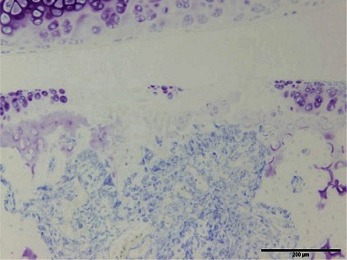 |
 |
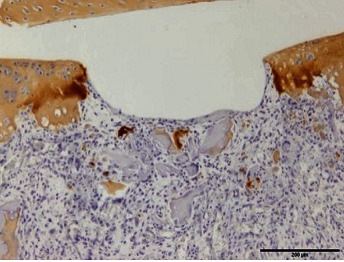 |
| II | 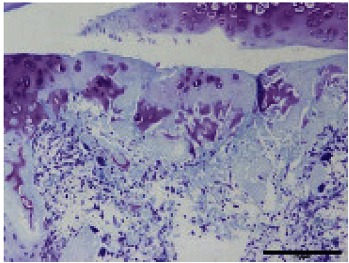 |
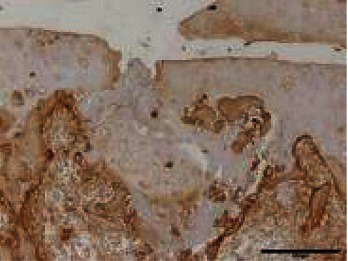 |
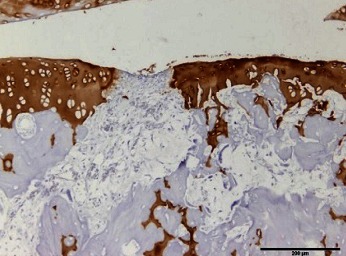 |
| III | 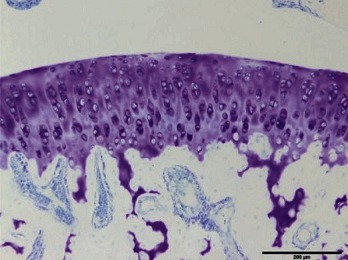 |
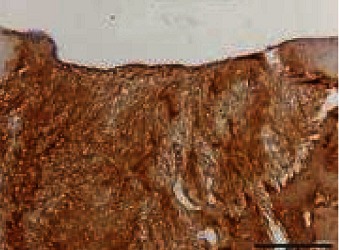 |
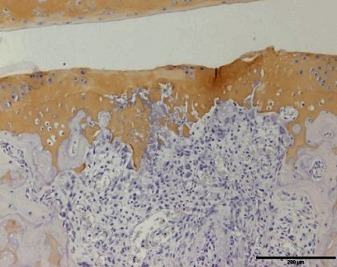 |
I: No or little staining in the repair tissue; II: Partial staining of the repair tissue; III: Complete or almost complete staining of the repair tissue.
Tissue types were distinguished according to the modified criteria from a previous work for cell and matrix appearance and the presence of toluidine blue staining and staining for type I and II collagen28). Samples were judged to be hyaline if they had a homogeneous matrix, abundant toluidine blue staining, abundant type II collagen immunohistochemical staining, little or no type I collagen immunohistochemical staining, and round cells in the lacunae. Fibrocartilage was defined as a cartilage with distinct fibers in the matrix, round or elliptical cells with or without lacunae, abundant or mildly reduced toluidine blue staining, and both type I and type II collagen immunohistochemical staining. Fibrous tissue was defined as tissue having a fibrous matrix with small, irregularly-shaped cells, little or no toluidine blue staining, little or no type II collagen immunohistochemical staining, and abundant type I collagen immunohistochemical staining. Granulation tissue was defined as fibrous tissue with spindle-shaped cells, blood vessels, no type II collagen immunohistochemical staining, and abundant type I collagen immunohistochemical staining.
Results
All animals were conscious and started to move within several hours after the surgery. No rat showed signs of knee infection or swelling or died during the experimental period. Thus, the inflammation was macroscopically and microscopically well controlled.
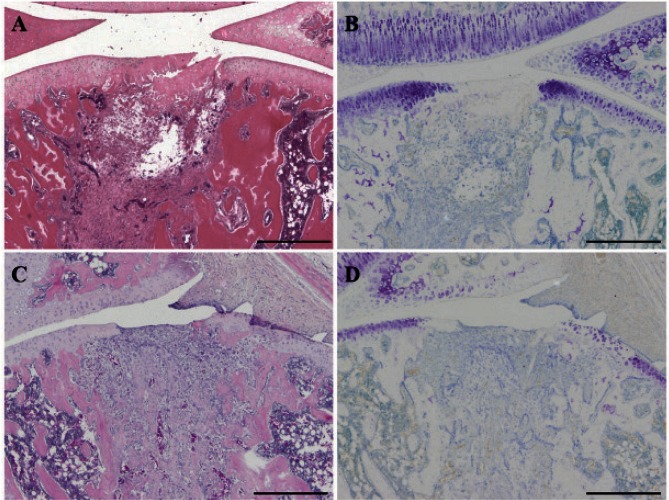
In hematoxylin and eosin staining of the histological examination in both groups at 1 week, the defects were filled with a mixture of granulation tissue and some remnants of hyaline cartilage (Fig. 3-A, C). Aseptic necrosis was observed in the remnants. Invasion of blood vessels and the presence of fibroblasts were observed in the repair tissue (Fig. 3-A, C). In both groups at 2 weeks, similar results were obtained (Fig. 4-A, C).
Fig. 3.
Histopathological staining of the repair tissue at 1 week after surgery
Sagittal sections of full-thickness articular cartilage defects in the loading group (A, B) and unloading group (C, D). The articular cartilage defect is located in the center of the femur. The sections are stained with hematoxylin and eosin (A, C) and toluidine blue (B, D). In the hematoxylin eosin staining, the defects are filled with a mixture of granulation tissue and some remnants of the hyaline cartilage. The invasion of blood vessels and the presence of fibroblasts are observed in the repair tissue. In the toluidine blue staining, both groups tend to show no or little staining. Scale bar = 500 µm.
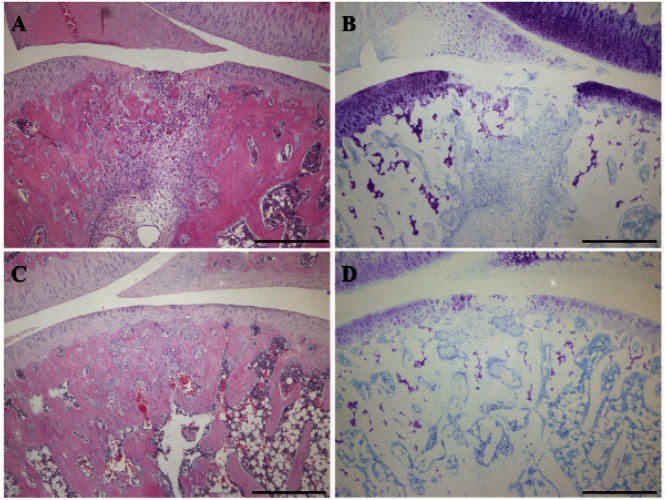
Fig. 4.
Histopathological staining of the repair tissue at 2 weeks after surgery
Sagittal sections of full-thickness articular cartilage defects in the loading group (A, B) and unloading group (C, D). The articular cartilage defect is located in the center of the femur. The sections are stained with hematoxylin and eosin (A, C) and with toluidine blue (B, D). In the hematoxylin and eosin staining, the defects are filled with a mixture of granulation tissue and some remnants of the hyaline cartilage. The invasion of blood vessels and the presence of fibroblasts are observed in the repair tissue. In the toluidine blue staining, both groups tend to show no or little staining. Scale bar = 500 µm.
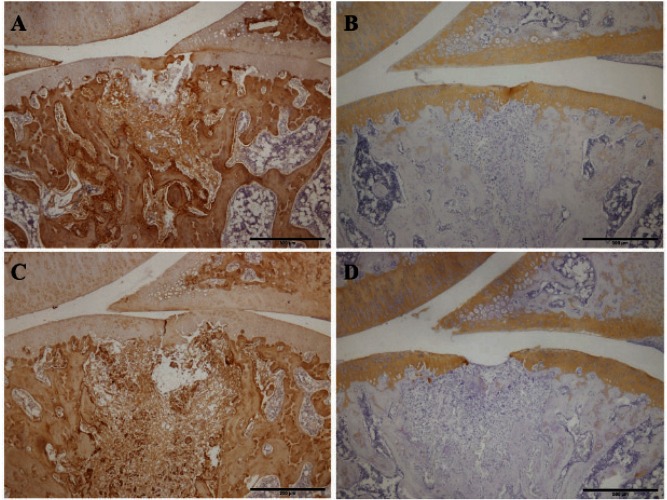
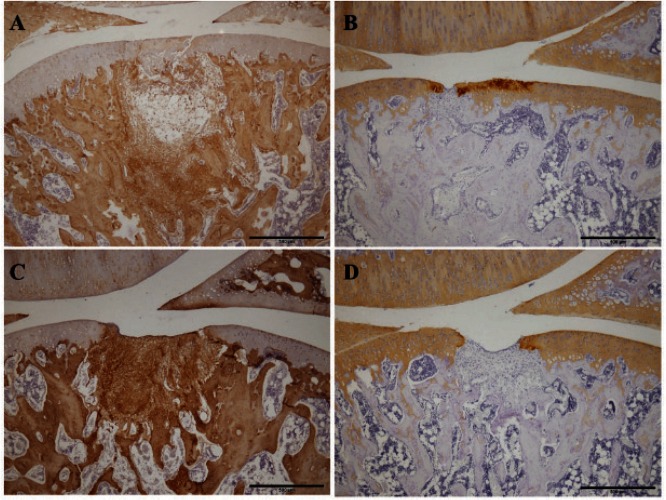
The results from the evaluation of the degree of staining are shown in Table 2. In toluidine blue staining, both groups tended to show no or little staining at 1 week (Fig. 3-B, D). In both groups at 2 weeks, similar results were obtained (Fig. 4-B, D). In the immunohistochemical staining for type I collagen, strong staining was observed in repair tissue in both groups at 1 week (Fig. 5-A, C). At 2 weeks, there was a tendency toward a smaller stained area in the unloading group than in the loading group (Fig. 6-A, C) and a smaller stained area at 2 weeks than at 1 week (Fig. 5-A, C; Fig. 6-A, C). In the staining for type II collagen, partial, almost complete, and complete staining of type II collagen was observed in the repair tissue of both groups at 1 week (Fig. 5-B, D). At 2 weeks, there was a tendency toward a higher degree of apparent staining in the loading group than in the unloading group (Table 2; Fig. 6-B, D).
Table 2. Original semiquantitative scale results of histopathological and immunohistochemical staining.
| Toluidine blue | Type I collagen | Type II collagen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | ||
| Loading group | 1 week | 8 | 1 | 1 | 1 | 7 | 2 | 1 | 1 | 8 |
| 2 week | 9 | 1 | 0 | 0 | 6 | 4 | 0 | 3 | 7 | |
| Unloading group | 1 week | 4 | 6 | 0 | 1 | 3 | 6 | 3 | 3 | 4 |
| 2 week | 9 | 1 | 0 | 0 | 3 | 7 | 7 | 0 | 3 | |
The numerals represent the number of individuals.
Fig. 5.
Immunohistochemical staining of the repair tissue at 1 week after surgery
Sagittal sections of full-thickness articular cartilage defects in the loading group (A, B) and unloading group (C, D). The articular cartilage defect is located in the center of the femur. The sections are immunohistochemically stained for type I collagen (A, C) and type II collagen (B, D). In the staining for type I collagen, strong staining is observed in the repair tissue of both groups. In the staining for type II collagen, partial, almost complete, and complete staining of type II collagen is observed in the repair tissue of both groups. Scale bar = 500 µm.
Fig. 6.
Immunohistochemical staining of the repair tissue at 2 weeks after surgery
Sagittal sections of full-thickness articular cartilage defects in the loading group (A, B) and unloading group (C, D). The articular cartilage defect is located in the center of the femur. The sections are immunohistochemically stained for type I collagen (A, C) and type II collagen (B, D). In the staining for type I collagen, there are tendencies toward smaller stained areas at 2 weeks than at 1 week and smaller stained areas in the unloading group than in the loading group. In the staining for type II collagen, the degree of apparent staining is greater in the loading group than in the unloading group. Scale bar = 500 µm.
Discussion
In general, full-thickness articular cartilage defects induce fibrin clot formation in the area of the chondral defect3,23,29). This clot contains pluripotent marrow-derived mesenchymal stem cells3,23,29). By 2 to 3 weeks after the injury, type I collagen was the primary collagen22), and expression of type II collagen started at 4–6 weeks28,30). These cells are able to differentiate into fibrocytes and chondrocytes, which results in hyaline cartilage or fibrocartilage repair with varying amounts of type I, II, and X collagen content3,23,30). Five genetically distinct collagen types are known to exist in adult cartilage31). Type II collagen is a major structural protein in the cartilage and is also essentially unique to cartilaginous tissues31). Through its high tensile strength, type II collagen provides structural integrity and resiliency to the articular cartilage31). Collagen fibrils are stabilized by covalent cross-links formed between adjacent collagen chains and adjacent collagen molecules31). The tensile strength of the collagen fibers is dependent on formation of intramolecular cross-links31). The results of the immunohistochemical staining in the present study showed that the presence of type II collagen tended to be observed more in the loading group than in the unloading group at 2 weeks after surgery.
It has been reported that mechanical stress-like loading is essential for cartilage metabolism32–35). Many researchers have reported the influence of loading on cartilage metabolism and shown that mechanical stress that is quantitatively appropriate stimulated cartilage metabolism32–35). The appropriate mechanical stress applied to the articular cartilage stimulates expression of transforming growth factor-β1, which promotes matrix metabolism, and Sox 9, which promotes differentiation of mesenchymal stem cells into chondral cells and increases production-type collagen and aggrecan32–35). In addition, previous studies have reported the effects and responses of unloading14–18,36–38). Reduced joint loading also creates catabolic responses within the articular cartilage14–18,36–38). Animal models of reduced loading have been used to show that a decrease in mechanical stimuli leads to atrophy of the articular cartilage and ultimately to erosion of the articular cartilage14–18). Tomiya et al.36) reported that insufficient stresses decreased metabolism and led to full-thickness articular cartilage defects, thinning of articular cartilage, and expansion of subchondral ossification. Kitade et al.37) reported histopathological changes in rat knee-joint components after spinal cord injury. Moriyama et al.38) reported that spinal cord injuries decreased the number of chondrocytes and cartilage thickness.
However, many researchers also have reported the risk of weight bearing in the early phase after surgery22,23,39,40). In a previous study, we reported on the risk of weight bearing in the early phase after articular cartilage defects39). The results of that study indicated that weight bearing in the early phase may make the surface of the repair tissue irregular and discontinuous39). Gill et al.22) suggested that weight bearing or joint loading delays healing and that up to 2 months of no weight bearing may be required to promote early fibrous tissue maturation. Williams et al.23) reported that weight bearing, particularly in the first 6 weeks after surgery, can cause potential propagation or collapse of the subchondral bone and that shear stress or excessive pressure in this early phase can flatten the repair cartilage or displace the mesenchymal cells and clot from the defect. Kuroki et al.40) also reported that the acoustic stiffness of implanted cartilage after autologous osteochondral transplantation decreased up to 12 weeks after surgery. Moreover, in the present study, the results of staining of type I collagen showed that the repair process was advanced in the unloading group and that tissue injury was delayed in the loading group. We presumed that the effects of weight bearing on the articular cartilage defects in the early repair process may have prolonged inflammation and caused delayed tissue repair in the loading group and that nonweight bearing in the early phase of the articular cartilage defects may have helped promote good tissue repair. Accordingly, these results indicated that loading and unloading in the early phase have both merits and demerits for cartilage repair.
In the present study, we have to consider the influence of not only loading but also joint motion on cartilage repair41,42). The beneficial effect of joint motion on the healing of the articular cartilage injuries has been well documented41,42). Salter et al.41,42) reported more rapid and complete metaplasia of healing tissue in articular cartilage defects exposed to CPM. The use of CPM enhances nutrition and metabolic activity of the articular cartilage41,42). In addition, CPM may stimulate differentiation of pluripotential mesenchymal cells into articular cartilage41,42). Consequently, CPM may accelerate healing of both the articular cartilage and periarticular cartilage41,42). In the present study, the intervention of immobilization was not performed in either group; therefore, the effects of cartilage repair by joint motion were the same in both groups.
The timing of expression of type II collagen in the present study was earlier than that observed in previous studies. It has been known that cartilage repair is influenced by species and age and by the position, depth, and size of the defect2). In addition, the defects were filled with a mixture of granulation tissue and some remnants of hyaline cartilage in both groups at 1 and 2 weeks, and aseptic necrosis was observed in the remnants in the present study. We considered them to be the remnants of original articular cartilage that were produced secondarily by the low-invasive method used in the present study26). The remnants of hyaline cartilage may promote expression of type II collagen.
The results of this basic study suggest that clinically, in the field of physical therapy and rehabilitation, it is important to determine the appropriate loading quantity required for optimal cartilage repair and to be aware that the appropriate loading quantity has to increase. Appropriate loading and sufficient joint motion can promote cartilage repair of a defect and prevent deterioration of the cartilage adjacent to the defect. Further studies involving immobilization groups and exercise groups using treadmills are needed to evaluate the appropriate loading quantity and to clarify the influence of mechanical stress on cartilage repair.
References
- 1). Yokota H, Leong DJ, Sun HB: Mechanical Loading: Bone Remodeling and Cartilage Maintenance. Curr Osteoporos Rep. 2011, 9: 237-242. [DOI] [PubMed] [Google Scholar]
- 2). Reinholz GG, Lu L, Yaszemski MJ, O'Driscoll SW: Animal models for cartilage reconstruction. Biomaterials. 2004, 25: 1511-1521. [DOI] [PubMed] [Google Scholar]
- 3). Mankin HJ: Response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982, 64: 460-466. [PubMed] [Google Scholar]
- 4). Sun HB, May LW, May PW: Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010, 1211: 37-50. [DOI] [PubMed] [Google Scholar]
- 5). Ikenoue T, Trindade MC, Lee MS, Lin E, Schurman DJ, Goodman SB, Smith RL: Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003, 21: 110-116. [DOI] [PubMed] [Google Scholar]
- 6). Lee DA, Bader DL: Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997, 15: 181-188. [DOI] [PubMed] [Google Scholar]
- 7). Shelton JC, Bader DL, Lee DA: Mechanical conditioning influences the metabolic response of cell-seeded constructs. Cells Tissues Organs. 2003, 175: 140-150. [DOI] [PubMed] [Google Scholar]
- 8). Torzilli PA, Grigiene R, Borrelli J, Jr, Helfet DL: Effect of impact load on articular cartilage: cellmetabolism and viability, and matrix water content. J Biomech Eng. 1999, 121: 433-441. [DOI] [PubMed] [Google Scholar]
- 9). Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, Nuttall ME, Hung HH, Blake SM, Grodzinsky AJ, Lark MW: Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000, 381: 205-212. [DOI] [PubMed] [Google Scholar]
- 10). Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ: Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001, 19: 1140-1146. [DOI] [PubMed] [Google Scholar]
- 11). Morel V, Quinn TM: Cartilage injury by ramp compression near the gel diffusion rate. J Orthop Res. 2004, 22: 145-151. [DOI] [PubMed] [Google Scholar]
- 12). Chen CT, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM: Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading duration dependent. J Orthop Res. 1999, 17: 870-879. [DOI] [PubMed] [Google Scholar]
- 13). Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G: Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res. 2001, 19: 703-711. [DOI] [PubMed] [Google Scholar]
- 14). Jones MH, Amendola AS: Acute treatment of inversion ankle sprains: immobilization versus functional treatment. Clin Orthop Relat Res. 2007, 455: 169-172. [DOI] [PubMed] [Google Scholar]
- 15). McCarthy C, Oakley E: Management of suspected cervical spine injuries - the paediatric perspective. Accid Emerg Nurs. 2002, 10: 163-169. [DOI] [PubMed] [Google Scholar]
- 16). Haapala J, Arokoski JP, Hyttinen MM, Lammi M, Tammi M, Kovanen V, Helminen HJ, Kiviranta I: Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res. 1999. May: 218-229. [PubMed] [Google Scholar]
- 17). Jurvelin J, Kiviranta I, Tammi M, Helminen JH: Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop Relat Res. 1986. June: 246-252. [PubMed] [Google Scholar]
- 18). Haapala J, Arokoski J, Pirttimaki L, Lyyra T, Jurvelin J, Tammi M, Helminen HJ, Kiviranta: Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000, 21: 76-81. [DOI] [PubMed] [Google Scholar]
- 19). Reinold MM, Wilk KE, Macrina LC, Dugas JR, Cain EL: Current concepts in the rehabilitation following articular cartilage repair procedures in the knee. J Orthop Sports Phys Ther. 2006, 36: 774-794. [DOI] [PubMed] [Google Scholar]
- 20). Steadman JR, Rodkey WG, Rodrigo JJ: Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001. October: 362-369. [DOI] [PubMed] [Google Scholar]
- 21). Steadman JR, Rodkey WG, Briggs KK: Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002, 15: 170-176. [PubMed] [Google Scholar]
- 22). Gill TJ, Asnis PD, Berkson EM: The treatment of articular cartilage defects using microfracture technique. J Orthop Sports Phys Ther. 2006, 36: 728-738. [DOI] [PubMed] [Google Scholar]
- 23). Williams RJ, 3rd, Harnly HW: Microfracture: indica tions, technique, and results. Instr Course Lect. 2007, 56: 419-428. [PubMed] [Google Scholar]
- 24). Marder RA, Hopkins G, Jr, Timmerman LA: Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy. 2005, 21: 152-158. [DOI] [PubMed] [Google Scholar]
- 25). Harada Y, Tomita N, Nakajima M, Ikeuchi K, Wakitani S: Effect of low loading and joint immobilization for spontaneous repair of Osteochondral defect in the knees of weightless (tail suspension) rats. J Orthop Sci. 2005, 10: 508-514. [DOI] [PubMed] [Google Scholar]
- 26). Takahashi I, Hoso M, Matsuzaki T: Analysis of a lowinvasive method to create full-thickness articular cartilage defects in a rat model. J Phys Ther Sci. 2011, 23: 879-882. [Google Scholar]
- 27). Ferreira JA, Crissey JM, Brown M: An Alternant Method to the Traditional NASA Hindlimb Unloading Model in Mice. J Vis Exp. 2011, 49: 2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Anraku Y, Mizuta H, Sei A, Kudo S, Nakamura E, Senba K, Takagi K, Hiraki Y: The chondrogenic repair response of undifferentiated mesenchymal cells in rat full-thickness articular cartilage defects. Osteoarthritis Cartilage. 2008, 16: 961-964. [DOI] [PubMed] [Google Scholar]
- 29). Shapiro F, Koide S, Glimcher MJ: Cell origin and differentiation in the repair full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993, 75: 532-553. [DOI] [PubMed] [Google Scholar]
- 30). Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, Goodnight JL, Mcllwraith CW: Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003, 407: 215-227. [DOI] [PubMed] [Google Scholar]
- 31). Garhunia HK, Pritzker KP: Effect of exercise on articular cartilage. Orthop Clin North Am. 2012, 43: 187-189. [DOI] [PubMed] [Google Scholar]
- 32). Nagase H, Kashiwagi M: Aggrecanases and cartilage matrix degradation. Arthrits Res Ther. 2003, 5: 94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M: Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem. 1999, 125: 966-975. [DOI] [PubMed] [Google Scholar]
- 34). Takahashi I, Nuckolls GH, Takahashi K, Tanaka O, Semba O, Dashner R, Shum L, Slavkin HC: Compressive force promotes sox 9, type II collagen and aggrecan and inhibits IL-1beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci. 1998, 111: 2067-2076. [DOI] [PubMed] [Google Scholar]
- 35). Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B: Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003, 21: 451-457. [DOI] [PubMed] [Google Scholar]
- 36). Tomiya M, Fujikawa K, Ichimura S, Kikuchi T, Yoshihara Y, Nemoto K: Skeletal unloading induces a full-thickness patellar cartilage defect with increase of urinary collagen II CTx degradation marker in growing rats. Bone. 2009, 44: 295-305. [DOI] [PubMed] [Google Scholar]
- 37). Kitade I, Hoso M, Matsuzaki T, Inaoka PT, Kamijo A, Araki Y, Takahashi I: Histopathological changes in knee joint components after spinal cord injury in rats. J Phys Ther Sci. 2012, 24: 31-35. [Google Scholar]
- 38). Moriyama H, Yoshimura O, Kawamata S, Takayanagi K, Kurose T, Kubota A, Hosoda M, Tabimatsu Y: Alternation in articular cartilage of rat knee joints after spinal cord injury. Osteoarthritis Cartilage. 2008, 16: 392-398. [DOI] [PubMed] [Google Scholar]
- 39). Takahashi I, Hoso M, Matsuzaki T: Histopathological Effects of Loading on Cartilage Repair in a Rat Full-thickness Articular Cartilage Defect Model. J Phys Ther Sci. 2012, 24: 1187-1190. [Google Scholar]
- 40). Kuroki H, Nakagawa Y, Mori K, Kobayashi M, Okamoto Y, Yasura K, Nishitani K, Nakamura T: Sequential changes in implanted cartilage after autologous osteochondral transplantation: postoperative acoustic properties up to 1 year in an in vivo rabbit model. Arthroscopy. 2007, 23: 647-654. [DOI] [PubMed] [Google Scholar]
- 41). Salter RB, Bell RS, Keeley FW: The protective effect of continuous passive motion on living articular cartilage in acute septic arthritis: An experimental investigation in the rabbit. Clin Orthop Relat Res. 1981, 159: 223-247. [PubMed] [Google Scholar]
- 42). Salter RB: The biologic concept of continuous passive motion of synovial joints: The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989, 242: 12-25. [PubMed] [Google Scholar]