Abstract
Aim: To facilitate establishment of an effective thermotherapy for osteoarthritis (OA), we investigated the effects of the thermal environment on articular chondrocyte metabolism in vitro. Methods: Chondrocytes were isolated from porcine knee joints, and cultured at 32°C, 37°C and 41°C. Cell proliferation and viability were assessed at Days 2, 4 and 8. In addition, TdT-mediated dUTP nick end labeling (TUNEL) assay was performed at Day 3 to determine the proportion of apoptotic chondrocytes. Analysis of genes specific for factors related to the cartilage extracellular matrix (ECM), cartilage destruction, and cartilage protection was performed at Day 2. Furthermore, evaluation of heat stress tolerance, and heat shock protein 70 (HSP70) mRNA expression and protein synthesis was performed at Day 2 and 3, respectively. Results: Cell proliferation was more at 37°C than at 32°C and 41°C. Cell viability and the number of TUNEL-positive cells were not affected until Day 8 and 3, respectively. The expression of the ECM-related genes was up-regulated at higher temperature. The expression of MMP13, a type II collagen destructive enzyme, and that of TIMP1 and TIMP2, which are MMP inhibitors, were up-regulated at higher temperatures. Finally, the chondrocytes cultured at 41°C may acquire heat stress tolerance, in part, due to the up-regulation of HSP70, and may inhibit apoptosis induced by various stresses, which is observed in OA. Conclusions: The thermal environment affects articular chondrocyte metabolism, and a heat stimulus of approximately 41°C could enhance chondrocyte anabolism and induce heat stress tolerance.
Keywords: Thermal environment, articular chondrocyte metabolism, thermotherapy
Osteoarthritis (OA) is a highly prevalent degenerative disease1), characterized by progressive articular cartilage destruction, which results in pain and decreased physical function. While many approaches for treating various aspects of OA have been studied, there exists no established effective treatment, because severely degenerated articular cartilage cannot be regenerated, and the specific factors related to OA development are complex2–4).
Physical therapy is often used as a first-line treatment in the management of OA symptoms. Since it has been reported that exercise intervention has a therapeutic effect on pain and physical function5,6), exercise therapy has been recommended in OA treatment guidelines7,8). On the other hand, the therapeutic effect of physical therapy modalities for OA treatment is still under debate7,8). In particular, clear evidence of the therapeutic effect of thermotherapy has not been shown9,10). However, in a previous study, application of a heat stimulus to treat OA within an experimental animal model, showed inhibition of OA development11,12). In addition, when women with chronic knee pain were treated with long-term thermotherapy in a randomized controlled trial, their pain was found to decrease; this reduction in pain and improvement of physical function was attributed to the combination of thermotherapy and exercise therapy, rather than to the exercise therapy alone13). These reports suggest that thermotherapy could be an effective treatment for OA if the temperature of the heat stimulus and the duration of exposure are optimized and if the target region for heat stimulus application is selected appropriately. However, the knowledge used as the basis for setting up each condition, which in many cases is based on empirical rules and not on objective data, makes it difficult to properly setup conditions. Furthermore, existing thermotherapy research for OA treatment has considered the effects on pain, blood flow, and physical function, but, there is little research which has taken into consideration the influence on the articular cartilage, which is a primary point of pathological OA processes, or the articular chondrocytes which play a key role in the metabolic activities of the articular cartilage.
Therefore, the purpose of this study was to facilitate the establishment of an effective thermotherapy for OA by clarifying the effects of the thermal environment on articular chondrocyte metabolism. In particular, in this study we focused on the effects of the thermal environment on cell proliferation, cell death, gene expression, and heat stress tolerance for articular chondrocytes.
Materials and Methods
The protocols described for the following experiments did not require approval of the animal research committee.
Chondrocyte isolation and expansion
Knee joints of 6-month-old pigs were purchased from a meat processor. Articular cartilage plugs were aseptically harvested from the femoral condyle using a biopsy punch, and chondrocytes were isolated as previously described14). The isolated cells were suspended in Dulbecco's modified Eagle medium/Ham's F12 (Nacalai Tesque Inc., Kyoto, Japan) containing 10% fetal bovine serum (Hyclone, Logan, USA), 50 U/mL penicillin (Nacalai Tesque Inc.), and 50 µg/mL streptomycin (Nacalai Tesque Inc.), were seeded in culture dishes. The chondrocytes were expanded in a CO2 incubator (5% CO2, 37°C, 95% humidity) to obtain a sufficient quantity of cells (2–3 passages). To investigate the effects of the thermal environment on chondrocyte metabolism, the chondrocytes were cultured at the following three conditions: 32°C as normal intra-articular temperature15,16), 37°C as internal body temperature, and 41°C as the threshold temperature for mammalian cell survival17,18).
Cell proliferation, viability, and apoptosis
To assess cell proliferation and viability at the three different culturing temperatures, the expanded chondrocytes were divided into 27 culture dishes at 8.5 × 103 cells/cm2, and then pre-cultured overnight at 37°C in a CO2 incubator. After pre-culture, the dishes were transferred into three distinct CO2 incubators set at 32°C, 37°C, and 41°C, 9 dishes for each. The cells were trypsinized from the culture dishes at Days 2, 4, and 8 (3 dishes for each day: n = 3). The number of live chondrocytes was counted using a hemocytometer, and cell viability was measured by using a trypan blue dye exclusion test. This experiment was repeated again using cells harvested from another pig to confirm the accuracy and reproducibility of the results. Additionally, a TdT-mediated dUTP nick end labeling (TUNEL) assay was performed to analyze the number of apoptotic chondrocytes according to the manufacturer's protocol (In Situ Apoptosis Detection Kit; Takara Bio Inc., Shiga, Japan). Briefly, the expanded chondrocytes were sub-cultured into 2-well Lab-Tek chamber slides (Nalge Nunc International, Naperville, USA) at 7.2 × 103 cells/cm2. After pre-culture as described above, the cells were cultured at 32°C, 37°C, and 41°C for 3 days. Subsequently, the cells were fixed in 4% paraformaldehyde for 15 min. After digestion with a permeabilization buffer, the slides were treated with TdT enzyme and incubated at 37°C for 60 min, then further incubated at 37°C for 30 min with an anti-FITC HRP Conjugate and propidium iodide, which were used as counter stains. More than 3,000 TUNEL-positive and TUNEL-negative cells were counted from more than 6 views (1272.8 µm × 1272.8 µm/view) that were randomly captured using a confocal laser scanning microscope system (FluoView FV10i; OLYMPUS CO., Tokyo, Japan). The proportion of TUNEL-positive cells/view was then calculated (n ≥ 6).
Gene expression analysis
Analysis of genes related to cartilage extracellular matrix (ECM) and cartilage-destroying/protecting factors was performed. The 9 culture dishes of expanded chondrocytes were transferred into incubators at 32°C, 37°C, and 41°C (3 dishes for each: n = 3), and incubated for 2 days. Total RNA was extracted using the RNeasy Mini Kit according to the manufacturer's protocol (Qiagen Inc., Valencia, USA). Extracted total RNA was dissolved in water and tested for purity using a NanoDrop2000 (Thermo Scientific, Wilmington, USA). The gene expression level was quantified by real-time reverse transcription-polymerase chain reaction (RT-PCR). Total RNA (300 ng) was reverse-transcribed to synthesize cDNA, and then real-time PCR was performed using the Applied Biosystems7500 Real-Time PCR System (Life Technologies Corporation, Carlsbad, USA). cDNA templates were amplified with PowerSYBR Green PCR Master Mix (Life Technologies Corporation) in 25-µL reactions with 0.2 µM gene-specific primers (Table 1) and de-ionized water. The PCR reaction was performed for 10 min at 95°C, followed by 40 amplification cycles (15 s at 95°C, 60 s at 60°C). The following target genes were examined: the ECM-related markers collagen type IIA1 (COL2A1), aggrecan, SRY (sex-determining region Y)-box 9 (SOX9), and collagen type IA1 (COL1A1); the cartilagedestroying factors matrix metalloproteinase (MMP)-1 and MMP-13; and the MMP-inhibitory factors tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP2. Beta-actin was used as the control housekeeping gene. This experiment was repeated again using cells harvested from another pig to confirm the accuracy and reproducibility of the results.
Table 1. Primer sequences for real-time RT-PCR.
| Sense (5′–3′) | Antisense (5′–3′) | Length (bp) | |
|---|---|---|---|
| COL2A1 | GCTATGGAGATGACAACCTGGCTC | CACTTACCGGTGTGTTTCGTGCAG | 256 |
| COL1A1 | CAGAACGGCCTCAGGTACCA | CAGATCACGTCATCGCACAAC | 101 |
| aggrecan | GAATTTCCTGGCGTGAGAAC | GGGGATGTTGCGTAAAAGAC | 107 |
| SOX9 | GTACCCGCACCTGCACAAC | GACTGCTGAATGAGAGCGAGA | 68 |
| MMP13 | CACCCGTGACCTTATCTTCATC | GCTGCGCTTATCCTTTTAACC | 132 |
| MMP1 | GCCAAATGGACTTCAAGCTG | AGCCAAAGGATCTGTGGATG | 137 |
| TIMP1 | ACCACCTGCAGTTTTGTGG | AGTTTGCAGGGGATGGATG | 134 |
| TIMP2 | GAACGACATCTACGGCAACC | TTCTTTCCTCCGATGTCCAG | 150 |
| HSP70 | TCACCATCACCAACGACAAG | TCATGTTGAAGGCGTACGAC | 147 |
| Beta-actin | AAGCCAACCGTGAGAAGATG | TCCATCACGATGCCAGTG | 124 |
The data obtained by real-time PCR were analyzed using the comparative threshold cycle method. Briefly, the amount of target was normalized to that of beta-actin, the value of the calibration sample (the cells cultured at 32°C) was set to 1, and the values for each of the other conditions were shown relative to that of the calibration sample. Before using the comparative threshold cycle method for quantitation, we performed a validation experiment, and the obtained absolute value of which the slope of log input amount versus delta threshold cycle was less than 0.1.
Heat shock protein 70 synthesis
The 18 culture dishes of expanded chondrocytes were transferred into incubators maintained at temperatures of 32°C, 37°C, and 41°C, and incubated for 2 or 3 days for the analysis of heat shock protein 70 (HSP70) mRNA expression and HSP70 protein synthesis, respectively (3 dishes for each: n = 3). The HSP70 mRNA expression was analyzed by the method described above. HSP70 protein synthesis was evaluated using western blotting. Cell lysates were prepared in SDS-sample buffer (70 mM Tris-HCl, pH 6.8, 11.2% glycerol, 3% SDS, 0.01% bromophenol blue, and 5% 2-mercaptoethanol). Equal amounts of protein (2 µg) were loaded on 10% SDS-polyacrylamide gels and blotted on a polyvinylidene fluoride microporous membrane using a semi-dry transfer system (Bio-Rad, Hercules, USA). The primary antibodies used were as follows: rabbit anti-HSP70 polyclonal antibody (StressGen Biotech. Victoria, Canada), and mouse anti-beta-actin monoclonal antibody (BioVision, Mountain View, USA). Blots were probed with HRP-conjugated donkey anti-rabbit IgG (GE Healthcare, Little Chalfont, England) for HSP70, and with sheep antimouse IgG (GE Healthcare) for beta-actin, and then visualized using an ECL method (GE Healthcare). Protein levels were quantified using Image Lab software (Bio-Lad). The expression level of HSP70 was normalized to that of betaactin, and was calculated by comparing to the expression level of 32°C samples.
Heat stress tolerance
Heat stress tolerance of the cells cultured at 32°C and 41°C was analyzed. 37°C condition was excluded in this experiment, since the expression of the HSP70 on the mRNA and the protein level didn't differ from 32°C. The chondrocytes cultured at 32°C or 41°C for 7 days were sub-cultured into 2-well Lab-Tek chamber slides at 1.2 × 104 cells/cm2. After pre-culture as previously described, the cells were exposed to excessive heat stress at 48°C for 1 h on a hot plate and then incubated at 37°C for 1 day. Subsequently, a TUNEL assay was performed as described above. The cells were counted from 20 random views (1272.8 µm × 1272.8 µm/view), and then the cell number/view and the proportion of the TUNEL-positive cell/view were calculated (n = 20).
Statistical analysis
All values are reported as means ± standard deviation (SD). Statistical significance was determined using unpaired Student's t test or one-way analysis of variance (ANOVA) with the post-hoc multiple comparison Tukey-Kramer test. The differences observed were considered to be significant if the p value was less than 0.05.
Results
The effects of thermal environment on cell proliferation, viability, and apoptosis
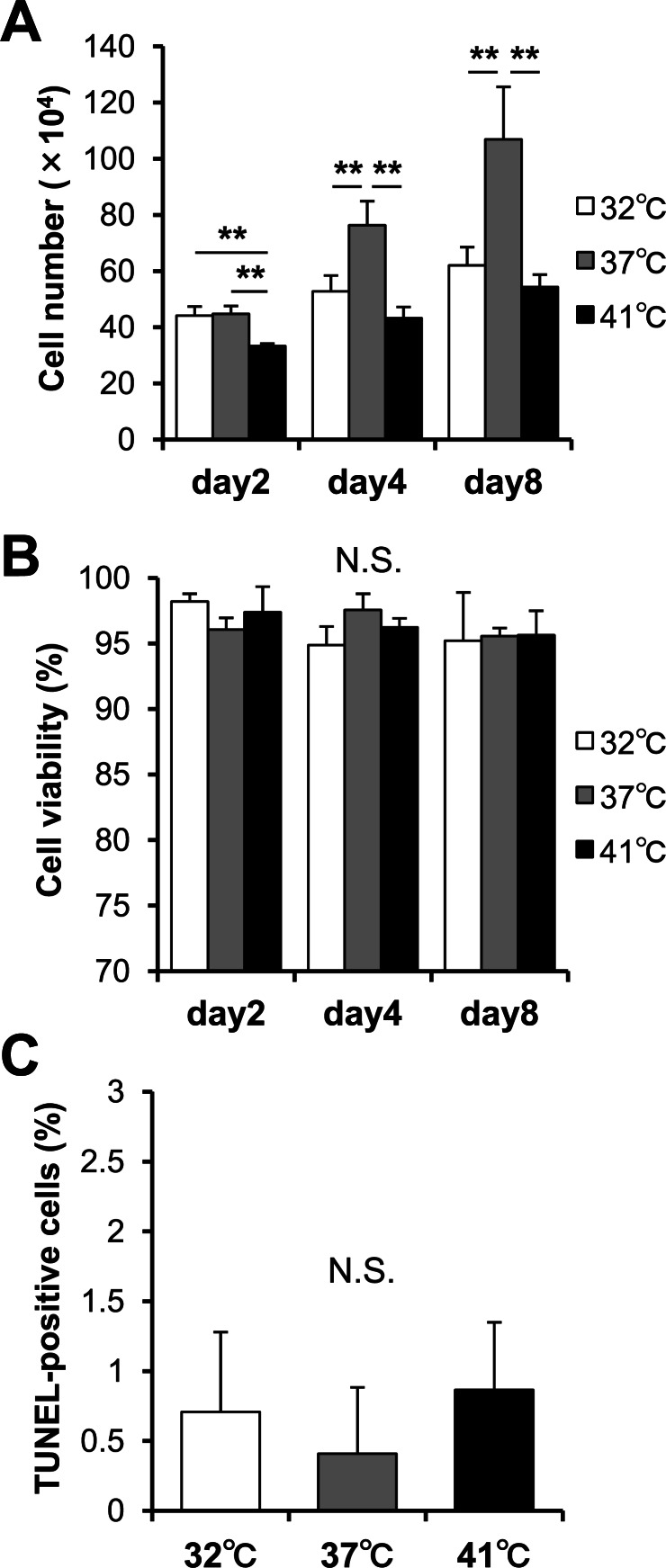
The cell proliferation, viability, and apoptosis induction at three different culturing temperatures were assessed. The cell number increased at each temperature, however, the cells were more proliferative at 37°C than at 32°C and 41°C (Fig. 1A). There were no significant differences between 32°C and 41°C at Day 4 and 8, although 41°C showed a lower number of cells than 32°C at Day 2. There were no significant differences in the cell viabilities (Fig. 1B) and the proportion of TUNEL-positive cells (Fig. 1C) among the three examined temperatures. Under these experimental conditions, the viability remained high (more than 94.5%), and the proportion of TUNEL-positive cells remained low (less than 1%).
Fig. 1.
Effects of the thermal environment on cell proliferation, viability, and apoptosis. (A) Cell number and (B) cell viability when cultured at 32°C, 37°C, and 41°C for 2, 4 and 8 days (n = 3). (C) The proportion of TdT-mediated dUTP nick end labeling (TUNEL)-positive cells. The TUNEL assay was performed to determine the proportion of apoptotic chondrocytes cultured at 32°C, 37°C, and 41°C for 3 days (n ≥ 6). All values represent the means and standard deviations (SD). **P < 0.01. N.S.; not significant.
Gene expression analysis
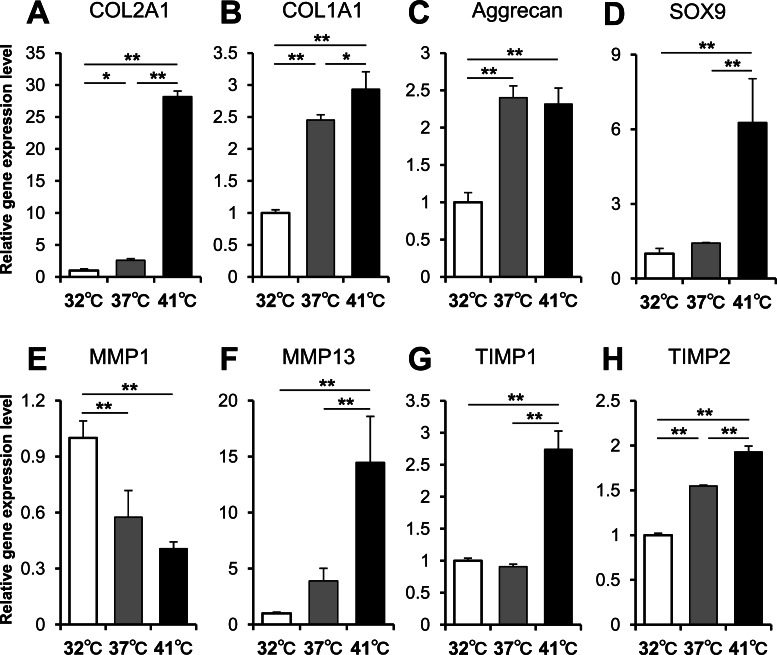
The expression of genes related to the ECM, cartilage-destroying factors, and cartilage-protecting factors were analyzed at each temperature by real time PCR. The expression of genes related to the ECM tended to increase in a temperature-dependent manner. Specifically, COL2A1 mRNA expression at 41°C was approximately 28 times the expression at 32°C (Fig. 2A). COL1A1 mRNA expression was also higher at higher temperatures, although increase in expression was apparently less than that of COL2A1 (Fig. 2B). Additionally, aggrecan mRNA expression was up-regulated at 37°C and 41°C (Fig. 2C), and SOX9 mRNA expression at 41°C was up-regulated approximately 6 times that of the 32°C samples (Fig. 2D). Interestingly, MMP1 and MMP13, which are cartilage-destroying factors, showed different trends with respect to each other. The expression of MMP1 mRNA was higher at lower temperatures (Fig. 2E), while expression of MMP13 mRNA was higher at higher temperatures (Fig. 2F). The mRNA expression of both TIMP1 (Fig. 2G) and TIMP2 (Fig. 2H), which are MMP-inhibitory factors, was up-regulated at 41°C and was up-regulated in a temperature-dependent manner.
Fig. 2.
Gene expression analysis. Relative mRNA expression of (A) COL2A1, (B) COL1A1, (C) aggrecan, (D) SOX9, (E) MMP1, (F) MMP13, (G) TIMP1, and (H) TIMP2 cultured at 32°C, 37°C, and 41°C for 2 days are shown (n = 3). Values represent the means and SD. *P < 0.05. **P < 0.01.
HSP70 synthesis and heat stress tolerance
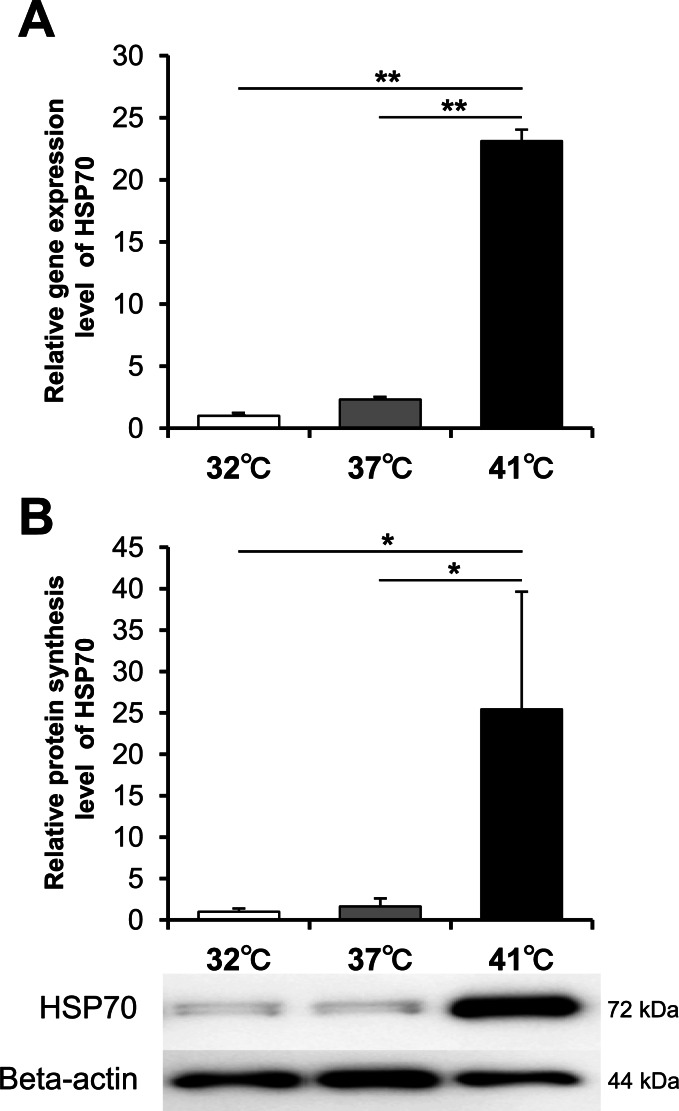
The HSP70 mRNA and the HSP70 protein were analyzed by real-time RT-PCR and Western blotting, respectively. A significant increase in mRNA (Fig. 3A) and protein levels (Fig. 3B) at 41°C was observed, although a significant difference was not detected between 32°C and 37°C.
Fig. 3.
Heat shock protein 70 synthesis. (A) Relative mRNA expression of heat shock protein 70 (HSP70) analyzed by real-time PCR and (B) relative protein synthesis level analyzed by western blotting when cultured at 32°C, 37°C, and 41°C for 2 days and 3 days, respectively, are shown (n = 3). Values represent the means and SD. *P < 0.05. **P < 0.01.
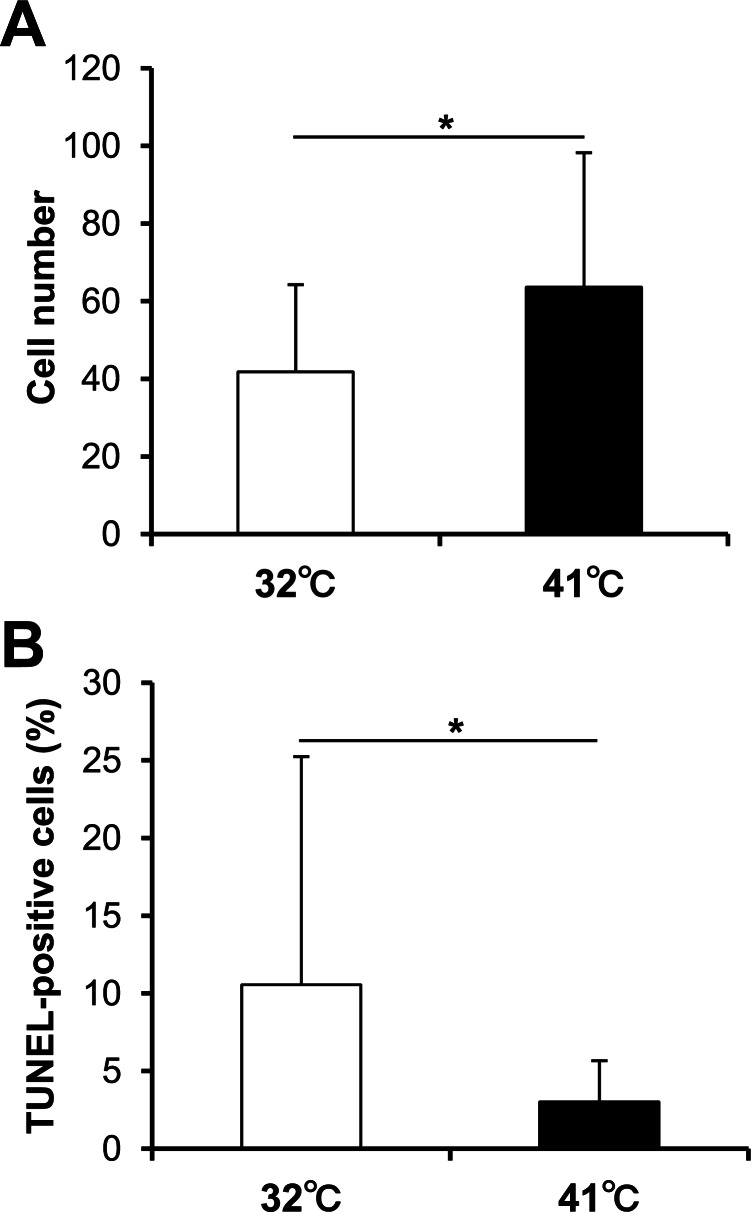
In order to clarify articular chondrocyte heat stress tolerance, the cell death after exposure to an excessive heat stimulus (48°C) was evaluated. There was a significantly larger number of cells/view at 41°C (Fig. 4A). Moreover, the proportion of TUNEL-positive cells was significantly higher at 32°C (Fig. 4B).
Fig. 4.
Heat stress tolerance. The chondrocytes cultured at 32°C or 41°C for 7 days were exposed to excessive heat stimulus at 48°C for 1 h on a hot plate. (A) The cell number and (B) the proportion of TUNEL-positive cells 1 day following heat stimulus were counted (n = 20). Values represent the means and SD. *P < 0.05.
Discussion
We investigated the effects of thermal environment on chondrocyte metabolism from a viewpoint of cell proliferation, cell death, gene expression, and heat stress tolerance.
Our results indicated that approximately 37°C (near internal body temperature) is the most suitable temperature for cell proliferation (Fig. 1A). The more the temperature deviates from the internal body temperature, the more the cellular proliferative potential is inhibited. To the best of our knowledge, there are few published reports demonstrating that mammalian cells cultured at temperatures below 37°C proliferate more than those cultured at 37°C, with all other conditions being identical. There is only one report indicating that oligodendrocyte precursor cells cultured at 31.5°C resulted in an increase of the cell number relative to the cells cultured at 37°C19). Generally, reduced metabolic rate and expression of cold shock response genes, caused by low temperatures lead to reduction in the growth rate20). It has also been reported that cell cycle arrest has occurred when cells were exposed to high temperature (42°C)21,22). Our results are consistent with these previous reports. Although the mature chondrocyte is seldom proliferating in the living body, in a process of cartilage regeneration, there is a possibility that 37°C is suitable when cell growth is required.
In research on the cell death, acute reactions caused by heat stimuli have been well-studied23,24). Wheatley et al.18) reported that mammalian cells seemed to tolerate temperatures of 42°C, whereas 43°C appeared to be a critical threshold, which rapidly led to cell death. Hojo et al.25) have reported that heat stimulus at 41°C for 15 or 30 min increased the viability of chondrocytes, and the borderline temperature that determines the increase or decrease of their metabolism would be between 41°C and 43°C. Moreover, they have indicated that these effects vary, not only according to temperature, but also according to the duration of exposure. However, the influence of chronic exposure is still unclear24). In this study, we demonstrated that the cell viability of the cartilage chondrocytes did not change in the temperature of the range of 32–41°C until Day 8 of culture (Fig. 1B). The proportion of TUNEL-positive cells at Day 3 was also low at < 1% of the total cells (Fig. 1C). Therefore, at temperatures from 32–41°C, chondrocytes may not experience cell death, even if exposed for a long period of time.
The expression of the genes related to cartilage ECM were up-regulated in a temperature-dependent manner (Fig. 2). This suggested that the production of cartilage ECM was enhanced by heat stimulus. Interestingly, MMP1 and MMP13 showed an opposite expression trend. MMP1 tended to be down-regulated while MMP13 tended to be up-regulated in a temperature-dependent manner. Although this up-regulation of MMP13, which is a specific degradation factor of type II collagen protein, indicates the induction of cartilage degeneration, both TIMP1 and TIMP2, which are cartilage-protective factors, were also up-regulated in a temperature-dependent manner. It has been reported that TIMP proteins combine with MMP proteins and inactivate the function of the MMP proteins26). Therefore, it is necessary to consider the relative amounts of both MMP and TIMP proteins. However, these amounts cannot be assessed at the protein level, but only at the mRNA level in this study.
The MMP1 mRNA expression, which is of a different MMP family, was higher at 32°C in our study conditions, however, it has been reported that the mRNA expression of MMP1, induced by heat stimulus, was up-regulated in a temperature-dependent manner in human skin fibroblasts and epidermal keratinocytes27,28). Although the detailed mechanism was unknown, these results imply that the influence of the thermal environment on MMP1 mRNA expression may differ depending on the cell species.
The cells cultured at 41°C were more tolerant to heat stress than those cultured at 32°C (Fig. 4A, B). It was assumed that the acquisition of this heat tolerance was related to induction of the HSP family, mainly HSP70. HSPs are a vital set of chaperone proteins that respond to thermal stress by assisting denatured proteins in the cytosol to refold into their native, functional conformations, thereby restoring the homeostasis of the cell29). It has been reported that the stress tolerance by HSPs shows tolerance to not only heat stress30), but also to oxidant stress31), and mechanical stress32). Moreover, these effects were confirmed not only in the articular cartilage11,12) but also in various organs such as skeletal muscles33–35), liver36), and blood vessels37). Therefore, the heat stimulus at 41°C might reduce the apoptosis of chondrocytes, which has been reported as a part of OA pathology, through the induction of HSPs.
We identified five limitations of this study. First, our results were obtained from in vitro experiments. Since the same result may not be obtained in vivo, it is necessary to verify these findings using animal experiments, and to also further confirm in a clinical study. Second, the heat stress tolerance acquired by the heat stimulus did not verify whether the tolerance is actually shown to stress other than the excessive heat stress used in this study, even though it has generally been reported that tolerance is shown to various other stressors. Third, we used 6-month-old pigs in this study, which are skeletally immature and not osteoarthritic, and it is likely that juvenile chondrocytes will react differently to temperature than adult chondrocytes. Fourth, our results assessing the metabolic change were obtained from basically mRNA level, not protein level. Since mRNA expression level does not always correlate with protein synthesis level, we should have also confirmed the effects of the thermal environment on articular chondrocyte metabolism in the protein level. Finally, the effects on other cell types that exist in the constituted synovial joint were not investigated. It has been reported that the metabolic reaction and the thermotolerance will differ among cell types38–40). To establish a safe and effective thermotherapeutic approach, it is essential to investigate its influence on chondrocytes and also all other cell types.
Conclusions
We investigated the effects of the thermal environment on articular chondrocyte metabolism in vitro to facilitate the establishment of an effective thermotherapy for OA. Several points were suggested by our results. First, articular chondrocyte cell death is not promoted even when cells are cultured at 32–41°C for a long period of time in a monolayer culture. Second, cell proliferation is promoted at 37°C, which is near internal body temperature, and is inhibited at temperatures higher or lower than 37°C. Third, in an environment with temperature higher than 32°C, which is the normal intra-articular temperature, the expression of the genes related to the ECM is up-regulated, and the ECM production may be enhanced. Fourth, although the expression of MMP13, the main destructive enzyme of type II collagen, is up-regulated in a temperature-dependent manner, the expression of TIMP1 and TIMP2, which are MMP inhibitors, are also up-regulated in a similar manner. Therefore, it cannot be simply concluded that a high-temperature environment promotes cartilage destruction. Finally, the chondrocytes cultured at 41°C may acquire heat stress tolerance, in part, due to the up-regulation of HSP70, and thus may inhibit the apoptosis of the chondrocytes induced by various stresses, which is observed in OA. Taken together, thermal environment could affect articular chondrocyte metabolism, and heat stimulus at approximately 41°C may enhance chondrocyte anabolism and induce heat stress tolerance.
Acknowledgements
This study was supported in part by Grant-in-Aid for JSPS Research Fellows (number 820130600018), JSPS KAKENHI Grant-in-Aid for Scientific Research (A) (number 25242055), and JSPS KAKENHI Grant-in-Aid for Challenging Exploratory Research (number 25560258).
Declaration of interest
The authors declare no competing interests associated with manuscript.
References
- 1). Muraki S, Oka H, Akune T, Mabuchi A, En-Yo Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H, Yamamoto S, Nakamura K, Kawaguchi H, Yoshimura N: Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of japanese population-based cohorts: The road study. Osteoarthritis Cartilage. 2009, 17: 1137-1143. [DOI] [PubMed] [Google Scholar]
- 2). Kim HK, Moran ME, Salter RB: The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am. 1991, 73: 1301-1315. [PubMed] [Google Scholar]
- 3). Hayes DW, Jr., Brower RL, John KJ: Articular cartilage. Anatomy, injury, and repair. Clin Podiatr Med Surg. 2001, 18: 35-53. [PubMed] [Google Scholar]
- 4). Hunziker EB: Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002, 10: 432-463. [DOI] [PubMed] [Google Scholar]
- 5). Fransen M, McConnell S: Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008: CD004376. [DOI] [PubMed] [Google Scholar]
- 6). Uthman OA, Van Der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM, Foster NE: Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013, 347: f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P: OARSI recommendations for the management of hip and knee osteoarthritis: Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010, 18: 476-499. [DOI] [PubMed] [Google Scholar]
- 8). Jevsevar DS: Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013, 21: 571-576 [DOI] [PubMed] [Google Scholar]
- 9). Brosseau L, Yonge KA, Robinson V, Marchand S, Judd M, Wells G, Tugwell P: Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev. 2003: CD004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Moriyama H, Iijima H, Kanemura N, Murata K, Hata Y, Nishihara K, Ozawa J, Takayanagi K, Gomi T, Tobimatsu Y: Efficacy of thermotherapy on musculoskeletal disorders. J Phys med. 2009, 20: 260-268. (Japanese) [Google Scholar]
- 11). Tonomura H, Takahashi KA, Mazda O, Arai Y, Shin-Ya M, Inoue A, Honjo K, Hojo T, Imanishi J, Kubo T: Effects of heat stimulation via microwave applicator on cartilage matrix gene and hsp70 expression in the rabbit knee joint. J Orthop Res. 2008, 26: 34-41. [DOI] [PubMed] [Google Scholar]
- 12). Fujita S, Arai Y, Nakagawa S, Takahashi KA, Terauchi R, Inoue A, Tonomura H, Hiraoka N, Inoue H, Tsuchida S, Mazda O, Kubo T: Combined microwave irradiation and intraarticular glutamine administration-induced hsp70 expression therapy prevents cartilage degradation in a rat osteoarthritis model. J Orthop Res. 2012, 30: 401-407. [DOI] [PubMed] [Google Scholar]
- 13). Kim H, Suzuki T, Saito K, Kim M, Kojima N, Ishizaki T, Yamashiro Y, Hosoi E, Yoshida H: Effectiveness of exercise with or without thermal therapy for community-dwelling elderly japanese women with non-specific knee pain: a randomized controlled trial. Arch Gerontol Geriatr. 2013, 57: 352-359. [DOI] [PubMed] [Google Scholar]
- 14). Ito A, Aoyama T, Yamaguchi S, Zhang X, Akiyama H, Kuroki H: Low-intensity pulsed ultrasound inhibits messenger RNA expression of matrix metalloproteinase-13 induced by interleukin-1beta in chondrocytes in an intensity-dependent manner. Ultrasound Med Biol. 2012, 38: 1726-1733. [DOI] [PubMed] [Google Scholar]
- 15). Oosterveld FG, Rasker JJ: Treating arthritis with locally applied heat or cold. Semin Arthritis Rheum. 1994, 24: 82-90. [DOI] [PubMed] [Google Scholar]
- 16). Sanchez-Inchausti G, Vaquero-Martin J, Vidal-Fernandez C: Effect of arthroscopy and continuous cryotherapy on the intra-articular temperature of the knee. Arthroscopy. 2005, 21: 552-556. [DOI] [PubMed] [Google Scholar]
- 17). Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE: Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977, 123: 463-474. [DOI] [PubMed] [Google Scholar]
- 18). Wheatley DN, Kerr C, Gregory DW: Heat-induced damage to HeLa-S3 cells: correlation of viability, permeability, osmosensitivity, phase-contrast light-, scanning electron- and transmission electron-microscopical findings. Int J Hyperthermia. 1989, 5: 145-162. [DOI] [PubMed] [Google Scholar]
- 19). Imada S, Yamamoto M, Tanaka K, Seiwa C, Watanabe K, Kamei Y, Kozuma S, Taketani Y, Asou H: Hypothermiainduced increase of oligodendrocyte precursor cells: possible involvement of plasmalemmal voltage-dependent anion channel 1. J Neurosci Res. 2010, 88: 3457-3466. [DOI] [PubMed] [Google Scholar]
- 20). Al-Fageeh MB, Smales CM: Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J. 2006, 397: 247-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Read RA, Fox MH, Bedford JS: The cell cycle dependence of thermotolerance. I. CHO cells heated at 42 degrees C. Radiat Res. 1983, 93: 93-106. [PubMed] [Google Scholar]
- 22). Mackey MA, Dewey WC: Cell cycle progression during chronic hyperthermia in S phase CHO cells. Int J Hyperthermia. 1989, 5: 405-415. [DOI] [PubMed] [Google Scholar]
- 23). Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ: Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003, 19: 267-294. [DOI] [PubMed] [Google Scholar]
- 24). Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, Dewhirst MW: Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011, 27: 320-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Hojo T, Fujioka M, Otsuka G, Inoue S, Kim U, Kubo T: Effect of heat stimulation on viability and proteoglycan metabolism of cultured chondrocytes: Preliminary report. J Orthop Sci. 2003, 8: 396-399. [DOI] [PubMed] [Google Scholar]
- 26). Kafienah W, Al-Fayez F, Hollander AP, Barker MD: Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach. Arthritis Rheum. 2003, 48: 709-718. [DOI] [PubMed] [Google Scholar]
- 27). Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, Eun HC, Chung JH: Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Invest Dermatol. 2004, 123: 1012-1019. [DOI] [PubMed] [Google Scholar]
- 28). Li WH, Lee YM, Kim JY, Kang S, Kim S, Kim KH, Park CH, Chung JH: Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J Invest Dermatol. 2007, 127: 2328-2335. [DOI] [PubMed] [Google Scholar]
- 29). Morimoto RI, Kroeger PE, Cotto JJ: The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. EXS. 1996, 77: 139-163. [DOI] [PubMed] [Google Scholar]
- 30). Beckham JT, Wilmink GJ, Mackanos MA, Takahashi K, Contag CH, Takahashi T, Jansen ED: Role of hsp70 in cellular thermotolerance. Lasers Surg Med. 2008, 40: 704-715. [DOI] [PubMed] [Google Scholar]
- 31). Terauchi R, Takahashi KA, Arai Y, Ikeda T, Ohashi S, Imanishi J, Mazda O, Kubo T: Hsp70 prevents nitric oxide-induced apoptosis in articular chondrocytes. Arthritis Rheum. 2003, 48: 1562-1568. [DOI] [PubMed] [Google Scholar]
- 32). Mcardle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ: Overexpression of hsp70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004, 18: 355-357. [DOI] [PubMed] [Google Scholar]
- 33). Okita M, Nakai K, Kataoka H, Toyoda N, Nakano J, Origuchi T, Yoshimura T: Effects of heat stress on prevention of disuse muscle atrophy in rat soleus muscle. Rigakuryohogaku. 2004, 31: 63-69. (Japanese) [Google Scholar]
- 34). Fujino H, Kohzuki H, Takeda I, Tasaki H, Kondo H, Ishida T, Kajiya F: Protective effects of a pre-conditioning exercise on soleus muscle atrophy by tail suspension. Rigakuryohogaku. 2005, 32: 400-405. (Japanese) [Google Scholar]
- 35). Watanabe Y, Yoshikawa S, Kataoka H, Kataoka N, Sakamoto J, Nakano J, Okita M: Effects of differences in heating methods on inhibiting the progression of disuse muscle atrophy in rat soleus muscle. -comparison of a warm water bath and electrical heating plate. Rigakuryohogaku. 2006, 33: 355-362. (Japanese) [Google Scholar]
- 36). Li L, Zhang T, Zhou L, Xing G, Chen Y, Xin Y: Schisandrin B attenuates acetaminophen-induced hepatic injury through overexpression of heat shock protein 27 and 70 in mice. J Gastroenterol Hepatol. 2013, 10.1111/jgh.12425 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37). Chen Y, Ross BM, Currie RW: Heat shock treatment protects against angiotensin II-induced hypertension and inflammation in aorta. Cell Stress Chaperones. 2004, 9: 99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Flour MP, Ronot X, Vincent F, Benoit B, Adolphe M: Differential temperature sensitivity of cultured cells from cartilaginous or bone origin. Biol Cell. 1992, 75: 83-87. [DOI] [PubMed] [Google Scholar]
- 39). Shui CX, Scutt A: Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and MG-63 cells in vitro. J Bone Miner Res. 2001, 16: 731-741. [DOI] [PubMed] [Google Scholar]
- 40). Morrissey JJ, Higashikubo R, Goswami PC, Dixon P: Mild hyperthermia as a potential mechanism to locally enhance cell growth kinetics. J Drug Target. 2009, 17: 719-723. [DOI] [PubMed] [Google Scholar]