Abstract
Purpose: This study aimed to clarify the effects of therapeutic ultrasound on intramuscular local blood circulation (and oxygen dynamics) using near-infrared spectroscopy (NIRS). Participants: The participants were 11 healthy males. Methods: All participants performed all three trials; (1) the ultrasound (US group), (2) without powered ultrasound (placebo group), and (3) rest (control group). Ultrasound was applied at 3 MHz, 1.0 W/cm2, and 100% duty cycle for 10 minutes. Evaluation index were oxygenated, deoxygenated, and total hemoglobin (Hb) concentrations in the intramuscular and skin surface temperature (SST). The experimental protocol was a total of 40 minutes, that is, 10 minutes before trial (rest), 10 minutes during the trial (ultrasound, placebo, and control), and 20 minutes after trial (rest). The NIRS and SST data collected before and after the trial were divided into 5 minutes intervals for further analysis. Results: Oxygenated and total hemoglobin levels were significantly higher in the US group than in the placebo and control groups for the 20 minutes after ultrasound (p < 0.01). The SST was significantly higher in the US group than in the control for 15 minutes after ultrasound (p < 0.05), while it was significantly lower in the placebo group than in the US and control groups for 20 minutes after the trials (p < 0.01). Conclusion: The effects of ultrasound were maintained for 20 minutes after the trial on intramuscular blood circulation and oxygen dynamics. These effects were caused by a combination of thermal and mechanical effects of the ultrasound.
Keywords: ultrasound, local blood circulation, oxygen dynamics
Ultrasound is widely used as a component of physical therapy in clinical practice. In particular, therapeutic ultrasound in physical therapy has a number of uses including treating musculoskeletal disorders such as pain, muscle spasm, joint stiffness, and tissue injury (muscle, tendon, and ligament)1–6). Therefore, it is now recognized as a major physical therapeutic method1–6). Essential treatment parameters for therapeutic ultrasound include frequency, intensity, treatment mode (i.e., duty cycle), treatment time, and treatment area1). The frequency of therapeutic ultrasound ranges from 1 to 3 MHz, with 3 MHz used specifically for the treatment of superficial tissues, and 1 MHz aimed to treat deeper tissues1). In addition, the combination of intensity and duty cycle in ultrasound produces thermal and/or nonthermal (i.e., mechanical) effects1). The physiological effects of thermal therapeutic ultrasound include increased tissue temperature, hyperdynamic tissue metabolism, increased local blood flow, increased extensibility of collagen fibers, and reduced viscosity of fluid elements in the tissue1). However, in terms of local blood flow the current evidence is conflicting. Some researchers stated that therapeutic ultrasound increased the blood flow in the skin, muscle and artery7–11), while others reported that there was no change in the blood flow12–15). This is in agreement with the conclusion of a systematic review done by Baker et al.16), who summarized previous research regarding the effects of therapeutic ultrasound, and concluded that it remained unclear if ultrasound has such effects on the local blood flow in practice. Thus, the effects of therapeutic ultrasound on hemodynamics remain controversial. These previous studies used plethysmography, laser Doppler, and ultrasonography to measure the effects on local blood flow. The plethysmography measures vasodilator potency, laser Doppler measures blood flow at the skin surface, and ultrasonography measures blood flow in deep blood vessels and inside large vessels. However, no studies have reported the effect on tissue hemoglobin (Hb) concentration levels. Changes in blood Hb concentration reflect the blood circulation and oxygen dynamics. From the above, it can be seen that the effects of therapeutic ultrasound on local blood circulation and oxygen dynamics in intramuscular tissue remain unclear. Therefore, the scientific basis for this treatment is insufficient. The current hypothesis is that therapeutic ultrasound improves pain, muscle stiffness, muscle fatigue, tissue injury, and wound healing by promoting blood circulation, which improves nutrition and oxygen supply9, 17).
In this study, we used near-infrared spectroscopy (NIRS) to assess changes in intramuscular hemodynamics and oxygen dynamics to determine the existence of any favorable changes in blood tissue Hb concentration. In recent years, NIRS has often been used to evaluate hemodynamics and oxygen consumption within local muscle tissue18, 19). NIRS allows the noninvasive measurement of relative changes in concentrations of oxygenated Hb (oxy-Hb) and deoxygenated Hb (deoxy-Hb) in the tissues20). Further, total-Hb, which is the sum of oxy-Hb and deoxy-Hb, reflects the blood volume in muscle tissue18, 19). The evaluation indexes in the present study were intramuscular oxy-Hb, deoxy-Hb, and total Hb concentration. NIRS is based on the relative transmissivity of tissues and absorption feature of Hb in the near-infrared region21). The maximum measurement depth of NIRS is reported to be roughly half the distance between the light source and detector (i.e., 20 to 30 mm below the skin surface)22, 23).
Our previous studies have revealed that therapeutic ultrasound on the upper fiber of the trapezius muscle reduced the muscle stiffness and increased the range of motion24, 25). However, we have not attempted to distinguish the relationship between the above effects and local blood circulation, therefore the effects on local blood circulation remain unclear. This study aimed to clarify whether therapeutic ultrasound affects intramuscular (i.e., upper fiber of the trapezius muscle) local blood circulation and oxygen dynamics.
Methods
Participants
The participants were 11 healthy males with a mean age of 25 years (range: 20–39 years). Mean body height was 169.7 ± 4.5 cm; body weight, 67.5 ± 11.2 kg; and body mass index, 22.8 ± 3.0. All the participants were right handed. The trials and measurement site was defined as the upper fiber of the right trapezius muscle, specifically the midpoint on the C7 spinous process to the acromial end of the clavicle. All the participants were instructed not to consume caffeine or alcohol and avoid smoking and performing any intense activities for at least 24 hours before testing.
This study was approved by the Koriyama Tohto Academy Educational Foundation Ethical Committee (No. R-0907). All the participants signed a consent form after being informed of the study purpose and content, and all risks involved.
Design
The participants performed 3 trials randomly. We compared the following 3 trials in a randomized trial using the same participants: (1) ultrasound (US group), (2) without powered ultrasound (placebo group), (3) and rest (control group). The experiment order was randomized, and each condition was tested 24 hours apart.
Instruments and Measurements
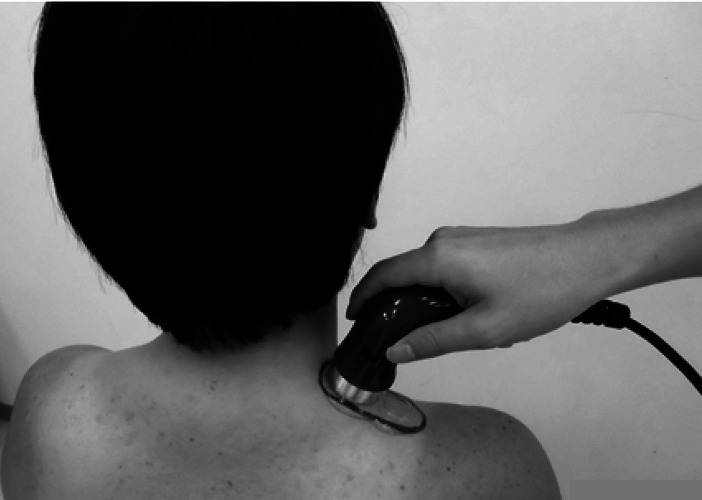
We used an ultrasound unit (EU-940, Ito Co., Ltd. Japan) with an effective radiating area of 6.0 cm2, and a beam nonuniformity ratio of 3.2: 1 (reported by the manufacturer). Ultrasound was applied at 3 MHz, 1.0 W/cm2 intensity, and 100% duty cycle (i.e., continuous mode) for 10 minutes. The above output setting followed the setup used in our previous study24, 25). To denote the ultrasound treatment site, an area twice the size of the ultrasound transducer head was marked on the top of the upper fiber of the right trapezius muscle (Fig. 1). We moved the transducer in a stroking method to approximately twice the length of the ultrasound transducer head (Fig. 1). Ultrasound transmission gel was used as the conducting medium. The ultrasound transmission gel was sufficiently applied to the skin after it was warmed to mean 33°C for performing placebo. The placebo treatment was unpowered ultrasound and otherwise performed using the same method and conditions as those in the US treatment. The control group was instructed to rest.
Fig. 1.
Ultrasound application on the upper fiber of the trapezius muscle
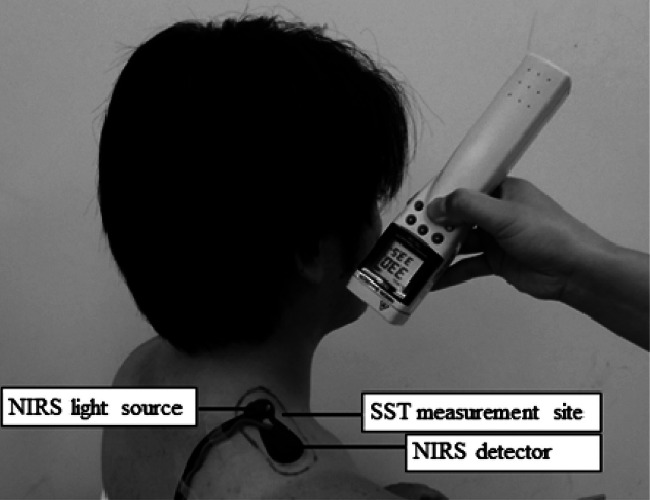
The measurements index was oxy-Hb, deoxy-Hb, and skin surface temperature (SST). The total-Hb was determined by calculation (i.e., total-Hb = oxy-Hb + deoxy-Hb). The measurement instruments were a near-infrared ray scanning spectrophotometer (NIRO-200, Hamamatsu Photonics Co., Ltd. Japan) and an infrared thermometer (THI-700L, Tasco Japan Co., Ltd. Japan), which were used to measure Hb concentration and SST, respectively. The NIRS probe was attached to the skin at the upper fiber of the trapezius muscle by an elastic tape with a distance of 40 mm between the light source and the detector (Fig. 2). The NIRS measurement was performed using a sampling time of 1 second.
Fig. 2.
Near-infrared spectroscopy and skin surface temperature measurements
The NIRS probe was attached to the skin on the upper fiber of the trapezius muscle (i.e., ultrasound application site), and the SST was also measured on the upper fiber by using an infrared thermometer.
Procedures
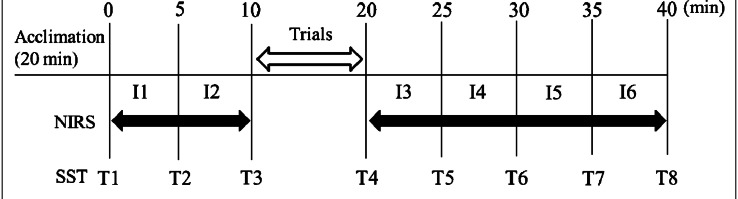
The experimental protocol was executed after a 20 minutes acclimation period and 10 minutes before the trial (rest); 10 minutes during the trial (for each condition); and 20 minutes after the trial (rest). The data collected before and after the trials were divided into 5 minutes intervals for further analysis (Fig. 3). The NIRS data were analyzed by averaging each 5 minutes interval (Fig. 3). I1 (i.e., interval of measurement start) was after the offset processing, and the calculated difference between each section was compared between the groups. SST data were analyzed and compared between the groups using the absolute values measured at 5 minutes intervals (Fig. 3).
Fig. 3.
Experimental protocol
The NIRS measured changes in Hb during 10 minutes rest (I1 & I2) set before the trial in US, placebo, and control groups. Then, the trials for US and placebo groups were performed for 10 minutes. After the trials, the NIRS measurement was performed continuously for 20 minutes, and it was divided into four intervals; from I3 to I6. SST was measured three (T1–T3) and five times (T4–T8) before and after the trial, respectively.
The participant assumed a sitting position with both upper limbs on the top of both thighs, with the upper fiber of the trapezius muscle exposed. The experiment was conducted in a controlled environment with the temperature maintained between 24°C to 26°C and 40% to 60% humidity. All of the participants were allowed a set acclimation time of 20 minutes to adapt to the laboratory environment.
Statistical analysis
Statistical analysis was performed using 2-way analysis of variance (ANOVA) with 3 × 6 repeated measurements on the change in each Hb index for comparisons between all the groups. The SST was analyzed by 2-way ANOVA with 3 × 8 repeated measurements. Tukey post hoc multiple comparison (Tukey-HSD) test was performed to clarify the Hb concentration in the 3 groups.
The level of significance was set at α = 0.05. All the analyses were performed using SPSS for Windows ver. 21.
Results
Difference (Δ) in changes of hemoglobin concentration
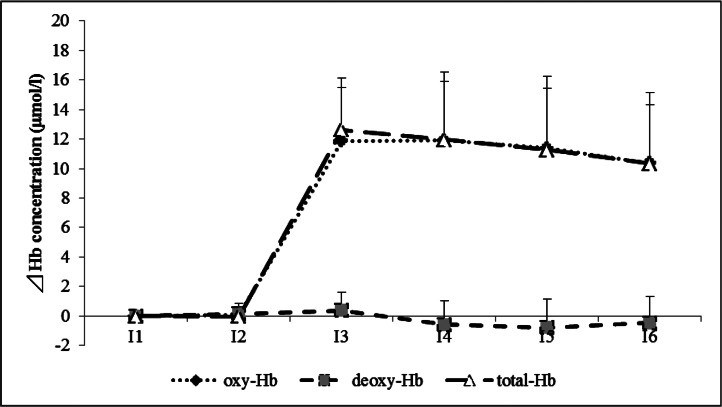
The results demonstrate the presence of a significant interaction in Δ oxy-Hb (F(2, 10) = 51.96, p < 0.01) and Δ total-Hb (F(2, 10) = 52.91, p < 0.01). Δ Deoxy-Hb did not differ significantly between the 3 groups. Fig. 4 shows the changes in the Hb concentration pattern as seen in the US group. Tukey-HSD test revealed that Δ oxy-Hb and Δ total-Hb were significantly higher in the US group than in the placebo and control groups from I3 to I6 (p < 0.01; Table 1).
Fig. 4.
Changes in Δ hemoglobin concentration of ultrasound group
The patterns of chronological changes in oxy-Hb, deoxy-Hb, and total-Hb in the US group are shown. The US group was increased oxy-Hb and total-Hb in I1 compared to I3–I6.
Table 1. Deference in changes of hemoglobin concentration in each trial.
| US | placebo | control | ANOVA | ||
|---|---|---|---|---|---|
| oxy-Hb | I1 | 0 | 0 | 0 | |
| I2 | 0.1 ± 0.6 | 0 ± 0.7 | 0.5 ± 0.7 | ||
| I3 | 11.9 ± 3.6* | 2.4 ± 2.3 | −0.3 ± 1.2 | F = 51.96 | |
| I4 | 11.9 ± 4.6* | 1.8 ± 2.2 | −0.2 ± 1.4 | p < 0.01 | |
| I5 | 11.4 ± 4.9* | 1.0 ± 2.4 | −0.3 ± 1.3 | ||
| I6 | 10.4 ± 4.8* | 0.3 ± 2.3 | −0.6 ± 1.5 | ||
| deoxy-Hb | I1 | 0 | 0 | 0 | |
| I2 | 0.1 ± 0.5 | −0.3 ± 0.7 | −0.3 ± 0.4 | ||
| I3 | 0.4 ± 1.3 | −0.7 ± 2.2 | −0.1 ± 0.5 | n. s. | |
| I4 | −0.6 ± 1.6 | −1.0 ± 2.3 | −0.1 ± 0.5 | ||
| I5 | −0.8 ± 1.9 | −0.7 ± 2.4 | −0.2 ± 0.5 | ||
| I6 | −0.5 ± 1.8 | −0.5 ± 2.3 | 0.1 ± 0.5 | ||
| total-Hb | I1 | 0 | 0 | 0 | |
| I2 | 0 ± 0.9 | −0.3 ± 0.4 | 0.1 ± 0.8 | ||
| I3 | 12.6 ± 3.5* | 2.1 ± 3.1 | −0.2 ± 1.2 | F = 52.91 | |
| I4 | 12.0 ± 3.9* | 1.2 ± 3.0 | −0.2 ± 1.4 | p < 0.01 | |
| I5 | 11.3 ± 4.1* | 0.5 ± 2.9 | −0.3 ± 1.3 | ||
| I6 | 10.3 ± 4.0* | 0.1 ± 2.5 | −0.2 ± 1.6 | ||
Unit: µmol/l (mean ± s.d.)
p < 0.01 US vs. placebo, control
n. s.: no significant
The chronological changes in oxy-Hb, deoxy-Hb, and total-Hb in the US, placebo, and control groups are shown. From I3 to I6, oxy-Hb and total-Hb were significantly higher in the US group than in the placebo and control groups (p < 0.01). No significant differences were observed between the placebo and control groups.
Changes in skin surface temperature
The results demonstrate the presence of a significant interaction in SST (F(2, 14) = 165.39, p < 0.01). Tukey-HSD test revealed that the SST from T4 to T7, and from T4 to T8 were significantly higher in the US group than in the control group (p < 0.05; Table 2) and the placebo group (p < 0.01; Table 2), respectively. The mean increase in SST from T1 to T4 was 3.1°C in the US group. Although the SST increased with ultrasound, it decreased rapidly afterwards, and the SST tended to return to baseline levels by T8 (20 minutes after ultrasound exposure). The SST was significantly lower in the placebo group than the control group from T4 to T8 (p < 0.01; Table 2).
Table 2. Changes of skin surface temperature in each trial.
| US | placebo | control | ANOVA | |
|---|---|---|---|---|
| T1 | 33.2 ± 0.6 | 33.6 ± 0.5 | 33.5 ± 0.5 | |
| T2 | 33.2 ± 0.4 | 33.5 ± 0.4 | 33.5 ± 0.5 | |
| T3 | 33.2 ± 0.5 | 33.5 ± 0.4 | 33.6 ± 0.5 | |
| T4 | 36.3 ± 0.9* | 30.1 ± 0.9‡ | 33.6 ± 0.5 | F = 165.39 |
| T5 | 35.4 ± 0.6* | 31.4 ± 0.7‡ | 33.6 ± 0.5 | p < 0.01 |
| T6 | 34.4 ± 0.5* | 32.6 ± 0.5‡ | 33.7 ± 0.4 | |
| T7 | 34.2 ± 0.4‡‡ § | 32.9 ± 0.5‡ | 33.7 ± 0.4 | |
| T8 | 33.9 ± 0.4§ | 33.1 ± 0.5‡ | 33.8 ± 0.5 |
Unit: degree. C (°C) (mean ± s.d.)
p < 0.01 US vs. placebo, control
p < 0.01 placebo vs. control
p < 0.01 US vs. placebo
p < 0.05 US vs. control
The chronological changes in SST in the US, placebo, and control groups are shown. From T4 to T7, SST was significantly higher in the US group than in the control group. And, the SST from T4 to T8 was significantly higher in the US than in the placebo group. However, immediately after the irradiation, SST decreased rapidly towards T8, and it tended to be back to the levels of temperature observed in T1. The SST in the placebo group was significantly lower than that in the control group.
Discussion
In this study, we investigated the effects of therapeutic ultrasound on intramuscular local blood circulation and oxygen dynamics using NIRS. Furthermore, we included SST as an assessment criterion to determine how changes in local blood circulation are related to tissue temperature during therapeutic ultrasound. We compared changes in local blood circulation and SST in the US, placebo, and control groups for 20 minutes after the trials.
The present study revealed that the total-Hb was significantly higher during the 20 minutes after the trial period in the US group than in the placebo and control groups. The NIRS measures of all the Hb components within the capillaries of local tissues within the penetrable distance of NIRS18, 19, 21–23). Considering that the measurements in the present study were taken from the upper fiber of the trapezius muscle, which is a superficial muscle, these measurements are an adequate reflection of intramuscular local blood circulation and oxygen dynamics, even when factoring in the minimal effect of subcutaneous tissue depth. Our results indicate that therapeutic ultrasound improves intramuscular local blood circulation. The oxy-Hb, which is a component of total-Hb, increased in the US group, whereas no change was detected in deoxy-Hb. This suggests that the changes in total-Hb were due to the increases in oxy-Hb. This is objective evidence supporting the notion that therapeutic ultrasound effectively improves intramuscular oxygen dynamics. These results suggest that therapeutic ultrasound provided a continuous increase of intramuscular local blood circulation and oxygen dynamics for at least 20 minutes after the conclusion of the trials in our study.
The observed changes in SST indicate that the effects of therapeutic ultrasound are due to the thermal effect of ultrasound. In a previous study done with the same methods in our study (i.e., same frequency, intensity, duty cycle, and treatment time), Draper et al.26) reported temperature increases of 5.8°C in the tissues at 0.8 and 1.6 cm depths after the application of ultrasound. In our study, SST increased by mean 3.1°C in the US group after the trial. According to the previous study observed the relationship of temperature changes between the skin surface and muscle (20 and 40 mm depths), there was a consistent pattern of changes between them since both temperatures in the skin and muscle were increased with the thermal stimulation27). The pattern of temperature changes in the skin surface and muscle tissue showed in our study and reported by Draper et al., respectively, were similar, thus it is hypothesized that the increases in the skin temperature caused by ultrasound application in our study indicates increases in the temperature of the muscle tissue as well. Given these data, we conclude that ultrasound leads to vasodilation of local tissue from the skin surface to muscle due to the thermal effect, affecting blood circulation and oxygen dynamics. The mechanism of thermal effects leading to vasodilation can be attributed to the direct reflective activation of vascular smooth muscles via skin temperature receptors, suppression of the sympathetic nervous system through indirect activation of local spinal reflexes, and increases in the local release of inflammatory chemical mediators, and the compound effect would result in dermovascular dilation28, 29).
Although these increases indicate that ultrasound exerts a thermal effect, SST rapidly decreased immediately after the application of ultrasound. At 20 minutes after ultrasound, the SST tended to return to baseline levels, suggesting that even though SST exhibited rapid decreases, the increases in local blood circulation and oxygen dynamics were maintained. Draper et al. observed the changes in temperature of muscle tissue at 12 mm depth after the application of ultrasound using the setup of 3 MHz and 1.5 W/cm2, and reported that it took 18 minutes to return the deep tissue temperature to the basal level from the 5°C increased condition30). This result was close to the pattern of surface skin temperature changes in the present study, therefore, it would indicate that there is a consistent relationship between the skin surface and deep muscle temperatures. In other words, the thermal effects in the skin and muscle caused by ultrasound tend to be decreased simultaneously with time. Given this, while it has been widely documented that the main cause of increased blood flow is due to the thermal effect of ultrasound, the data in our study demonstrates the greater presence of a maintained mechanical effect on the increased blood circulation. However, the thermal effect cannot be obviously ignored, and its residual effect combined with the direct mechanical effect on vascular smooth muscle and the muscular autonomic nervous system was also confirmed by the result of SST.
The mechanical effects of ultrasound lead to increased cellular permeability, promoting tissue metabolism1, 31). In addition, decreases in the stiffness of soft tissue24, 25) around blood vessels led to the reduction of intramuscular pressure, thus alleviated direct pressure on blood vessels. These combined mechanical and thermal effects of ultrasound suggest a reduction in vasoconstrictor activity and elevated vasodilator activity, activating intramuscular circulation. In turn, this dilates intramuscular arterioles and capillaries, increasing the flow of oxy-Hb-rich arterial blood and accelerating intramuscular oxygen distribution and blood circulation. In contrast, the SST in the placebo group decreased during the 20 minutes after the trial period. This decrease could be due to the cooling of the ultrasound gel through vaporization and the affect of ultrasound transducer head metal. Tissue cooling generally leads to a secondary increase in blood flow32). However, no changes in blood circulation were observed in the placebo group and no cooling effect was observed. Hence, no placebo effect of local blood circulation was observed, and its clinical significance is considered low. In contrast, increases in local blood circulation and oxygen dynamics were observed during the 20 minutes period after application in the US group. This is suggestive of the direct effect of ultrasound on tissues from the skin surface to muscle. These results indicate the effectiveness and continuous effect of therapeutic ultrasound on intramuscular blood circulation and oxygen dynamics. This can be provided as new knowledge which is not observed in previous studies7–16).
Clinically, blood circulation insufficiency gives rise to hypoxic conditions in tissues, the production and release of algesic substances, and tissue fibrosis, thereby causing pain, muscle spasms, and joint contracture. Therefore, increasing blood flow to affected sites and promoting tissue oxygenation are clinically important for improvements of the above conditions; moreover, it would also enhance muscle fatigue recovery, tissue repair, and wound healing1, 5, 9, 15, 16). Our results can be used as fundamental data and scientific evidence of the therapeutic effects of ultrasound on a variety of patients and conditions with blood circulation insufficiency. Furthermore, our results endorse the active execution of pain management and range-of-motion exercises through therapeutic exercise in the 20 minutes window after ultrasound24, 25), when increases in intramuscular blood circulation are confirmed to occur. This window represents a treatment opportunity in which the combined effect of multiple therapeutic avenues is greatest. Our results suggest that therapeutic ultrasound is possibly an effective treatment together with therapeutic exercise, with high clinical significance. Furthermore, our results suggest that increases in SST after ultrasound promote intramuscular blood circulation and may be useful for future clinical assessments.
Given that we only measured SST in the present study, we did not directly confirm temperature changes in the deeper tissues and instead discuss changes based on the relevant literature26, 30). In the future, we plan to measure deep tissue temperature and assess its relationship with intramuscular blood circulation.
Reference
- 1). Knight LK, Draper OD: Therapeutic Modalities. The Art and Science 2th ed, Chap 14. Therapeutic ultrasound, Philadelphia Lippincott Williams & Wilkins, 2013, pp. 252-282. [Google Scholar]
- 2). Gam NA, Johannsen F: Ultrasound therapy in musculoskeletal disorders: a meta-analysis. Pain. 1995, 63: 85-91. [DOI] [PubMed] [Google Scholar]
- 3). Windt D, Heijden G, Berg S, Winter R, Bouter L: Ultrasound therapy for musculoskeletal disorders: a systematic review. Pain. 1999, 81: 257-271. [DOI] [PubMed] [Google Scholar]
- 4). Robertson JV, Baker GK: A review of therapeutic ultrasound: Effectiveness studies. Phys Ther. 2001, 81: 1339-1350. [PubMed] [Google Scholar]
- 5). Speed CA: Therapeutic ultrasound in soft tissue lesions. Rheumatology. 2001, 40: 1331-1336. [DOI] [PubMed] [Google Scholar]
- 6). Wong AR, Schumann B, Townsend R, Phelps AC: A Survey of therapeutic ultrasound use by physical therapists who are orthopaedic certified specialists. Phys Ther. 2007, 87(8): 986-994. [DOI] [PubMed] [Google Scholar]
- 7). Paul WD, Imig CJ: Temperature and blood flow studies after ultrasonic irradiation. Am J Phys Med Rehabil. 1955, 34: 370-375. [PubMed] [Google Scholar]
- 8). Abramson DI, Carolyn B, Yvonne B, Samuel T, Habib R, Clara F: Changes in blood flow, oxygen uptake and tissue temperatures produced by therapeutic physical agents: I. effect of ultrasound. J Phys Med Rehabil. 1960, 39(2): 51-62. [PubMed] [Google Scholar]
- 9). Baker RJ, Bell GW: The effect of therapeutic modalities on blood flow in the human calf. J Orthop Sports Phys Ther. 1991, 13(1): 23-27. [DOI] [PubMed] [Google Scholar]
- 10). Noble GJ, Lee V, Noble GF: Therapeutic ultrasound: The effects upon cutaneous blood flow in humans. Ultrasound Med Biol. 2007, 33(2): 279-285. [DOI] [PubMed] [Google Scholar]
- 11). Fabrizio PA, Schmidt JA, Clemente FR, Lankiewicz LA, Levine ZA: Acute effects of therapeutic US delivered at varying parameters on the blood flow velocity in a muscular distribution artery. J Orthop Sports Phys Ther. 1996, 24: 294-302. [DOI] [PubMed] [Google Scholar]
- 12). Paaske WP, Hovind H, Sejrsen P: Influence of therapeutic ultrasonic irradiation on blood flow in human cutaneous, subcutaneous, and muscular tissues. Scand J Clin Lab Invest. 1973, 31(4): 389-394. [DOI] [PubMed] [Google Scholar]
- 13). Wyper DJ, McNiven DR: Effects of some physiotherapeutic agents on skeletal muscle blood flow. J Physiol. 1976, 62: 83-85. [PubMed] [Google Scholar]
- 14). Rubin JM, Etchison RM, Condra AK, Franklin DT, Snoddy MA: Acute effects of ultrasound on skeletal muscle oxygen tension, blood flow and capillary density. Ultrasound Med Biol. 1990, 16: 271-277. [DOI] [PubMed] [Google Scholar]
- 15). Robinson SE, Buono MJ: Effect of continuous-wave ultrasound on blood flow in skeletal muscle. Phys Ther. 1995, 75: 145-150. [DOI] [PubMed] [Google Scholar]
- 16). Baker GK, Robertson JV, Duck AF: A review of therapeutic ultrasound: Biophysical effects. Phys Ther. 2001, 81: 1351-1358. [PubMed] [Google Scholar]
- 17). Houghton PE: Effects of therapeutic modalities on wound healing: A conservative approach to the management of chronic wounds. Phys Ther Rev. 1999, 4: 167-182. [Google Scholar]
- 18). Karasuno H, Morozumi K, Fujiwara T, Goh AC, Yamamoto I, Senga F: Change in intramuscular blood volume induced by continuous shortwave diathermy. J phys ther sci. 2005, 17: 71-79. [Google Scholar]
- 19). Kubo K, Ikebukuro T: Acute and chronic effects of hyperbaric oxygen therapy on blood circulation of human muscle and tendon in vivo. J Strength Cond Res. 2012, 26(10): 2765-2770. [DOI] [PubMed] [Google Scholar]
- 20). Jobsis FF: Noninvasive, Infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977, 198(4323): 1264-1267. [DOI] [PubMed] [Google Scholar]
- 21). Beekvelt M, Borghuis M, Engelen B, Wevers R, Colier W: Adipose tissue thickness affects in vivo quantitative NIRS in human skeltal muscle. Clin Sci. 2001, 101: 21-28. [DOI] [PubMed] [Google Scholar]
- 22). Cui W, Kumar C, Chance B: Experimental study of migration depth for photons measured at sample surface. Proc Soc Photo Opt Instrum Eng. 1991, 1431: 180-191. [Google Scholar]
- 23). Homma S, Fukkunaga T, Kagaya A: Influence of adipose tissue thickness on near infared spectroscopic signal in the measurement of human muscle. J Biomed Opt. 1996, 1: 418-424. [DOI] [PubMed] [Google Scholar]
- 24). Morishita K, Karasuno H, Utsunomiya M, Yoshikawa A, Fujiwara T, Fujimoto T, Abe K: Effect of the therapeutic ultrasound on soft tissue hardness. J Jpn Soc Phys Ther. 2010, 17: 25-30 [JPN]. [Google Scholar]
- 25). Morishita K, Karasuno H, Fujiwara T, Fujimoto T, Abe K: Effect of the therapeutic ultrasound on muscle hardness and range of motion. J Appl Bio-metrology. 2011, 2: 7-10 [JPN]. [Google Scholar]
- 26). Draper OD, Castel CJ, Castel D: Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995, 22(4): 142-150. [DOI] [PubMed] [Google Scholar]
- 27). Paul W: Temperature of skin, subcutaneous tissue, muscle and core in resting men in cold, and hot conditions. Eur J Appl Physiol. 1992, 64: 471-476. [DOI] [PubMed] [Google Scholar]
- 28). Crockford WG, Hellon FR, Parkhouse J: Thermal vasomotor response in human skin mediated by local mechanism. J Physiol. 1962, 161: 10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Wessman MS, Kottke FJ: The effects of indirect heating on peripheral blood flow, pulse rate, blood pressure and temperature. Arch Phys Med Rehabil. 1967, 48: 567-576. [PubMed] [Google Scholar]
- 30). Draper OD, Ricard DM: Rate of temperature decay in human muscle following 3 MHz ultrasound: The stretching window revealed. J Athl Train. 1995, 30(4): 304-307. [PMC free article] [PubMed] [Google Scholar]
- 31). Dinno AM, Crum AL, Jisen W: The effect of therapeutic ultrasound on electrophysiological parameters of frog skin. Ultrasound Med Biol. 1989, 15(5): 461-470. [DOI] [PubMed] [Google Scholar]
- 32). Knight LK, Draper OD: Therapeutic Modalities. The Art and Science 2th ed, Philadelphia Lippincott Williams & Wilkins, 2013, pp. 216-225. [Google Scholar]