Abstract
Background
The phylogenetic location of Chinese Spirometra sparganum isolates remains unclear. The aim of this study was to explore the phylogenetic location of the Spirometra sparganum isolates from China.
Methods
The 28S ribosomal DNA (rDNA) D1 sequences of 14 Spirometra sparganum isolates collected from thirteen locations in China were analyzed by using Neighbor-Joining (NJ), maximum parsimony (MP) and Bayesian inference (BI), respectively. To investigate the deep variance of 28S rDNA D1 region among included species, the secondary structure of 28S rDNA D1 region was also calculated using the program RNA structure.
Results
The genus Spirometra as a monophyletic group was evidenced by two inference methods (MP and BI). All sequences within the genus Spirometra had a bulge of a cytosine residue (Bulge C) in the stem 13 of the secondary structure model of 28S rRNA D1 region. Varietal sites in sequences from all thirteen Chinese isolates were appeared in loops. In loops, adenine was the most abundant base (averagely 41.9%) followed by guanine (averagely 30.0%), and cytosine (averagely 15.1%). In stems, the average percentage of G + C (58.3%) was higher than the percentage of A + T (41.7%).
Conclusion
The ‘Bulge C’ in the stem 13 of the 28S rDNA D1 secondary structure could be as a suitable mark to identify the Spirometra species.
Keywords: Spirometra, Diphyllobothrium, Molecular phylogeny, Secondary structure, 28S ribosomal DNA
Introduction
Tapeworms of the genus Spirometra belong to the class Cestoda, order Pseudophyllidea, family Diphyllobothriidae. The plerocercoid larvae (spargana) of Spirometra can lodge in the subcutaneous tissues and sometimes invades the abdominal cavity, eye, and central nervous system of human causing a serious parasitic zoonosis: sparganosis; human infection results mainly from ingesting raw fleshes of frogs and snakes infected with plerocercoids, drinking raw water contaminated with cyclops harboring procercoids, or placing frog or snake flesh on open wound for treatment of skin ulcers or eye inflammations (1-6). Sparganosis poses a serious threat to human health and also cause significant economic losses (7). Phylogenetic studies to such an important parasite group is therefore of great significance to the prevention and control of human sparganosis as well as to understand the genetic structure of parasites. However, the knowledge about the phylogenetic position of the genus Spirometra, and its affiliation to other Diphyllobothriid species (such as the genera Diphyllobothrium, Digramma, Duthiersia and Schistocephalus) are still fragmentary. Therefore, there is a pressing requirement to investigate the phylogenetic relationships of Spirometra, Diphyllobothrium and other important species within the family Diphyllobothriidae.
The nuclear rDNA gene repeat unit harbors different regions that evolve at varying rates, thus adds useful and often significant resolution to molecular systematic estimates of phylogeny at a number of different taxonomic levels (8, 9). The large subunit RNA gene (lsrDNA or 28S rDNA) has been extensively utilized in estimation of the relationships existing within and among the Cestoda (9-12). In the phylogentic study, the secondary structures of the transcribed rRNA are more conserved than the primary sequences due to the compensatory or semi-compensatory mutations, and some changes of a certain helix could be specific to a taxon to help a lot in species identification (13-15).
So, the secondary structures have drawn lots of attention from phylogenetic scientists (15-17). However, until now, few researchers have been concentrated their studies on the phylogeny of Spirometra with the 28S rDNA sequences, even more considered the secondary structures.
The main aim of this study was to explore the phylogenetic location of the Spirometra sparganum isolates from China based on the primary and corresponding secondary structures of partial 28S rDNA D1 sequences. In addition, the relationships of species among Spirometra, Diphyllobothrium and other important genera within the family Diphyllobothriidae were established using the molecular data obtained.
Materials and Methods
Taxon selection and sampling
The plerocercoids (spargana) of Spirometra were collected from subcutaneous tissue and muscles of the naturally infected wild frogs (Rana nigromaculata, R. rugulosa, R. temporaria, R. limmochari) and snakes (Enhydris chinensis) at thirteen locations of China (Table 1). Spargana dissected from frogs and snakes were wrinkled, whitish, and ribbon-shaped worms, which continuously crept in normal saline.
Table 1.
Geographical origins (different locations in China) of Spirometra sparganum isolates and related taxa of the family Diphyllobothriidae used in this study, as well as their GenBank accession numbers for sequences of 28S rDNA D1 region.
| Genus | Species | Sample codes | Location | Host | GenBank No. |
|---|---|---|---|---|---|
| Spirometra | S. erinaceieuropaei | GX-NN1 | Nanning, Guangxi | Rana rugulosa | KF874629* |
| S. erinaceieuropaei | GX-NN2 | Nanning, Guangxi | R. rugulosa | KF874630* | |
| S. erinaceieuropaei | GX-NN-Yn1 | Yongning, Guangxi | Enhydris chinensis | KF874631* | |
| S. erinaceieuropaei | GX-NN-Yn2 | Yongning, Guangxi | E. chinensis | KF874632* | |
| S. erinaceieuropaei | GX-NN-Xxt1 | Xixiangtang, Guangxi | E. chinensis | KF874633* | |
| S. erinaceieuropaei | GX-NN-Xxt2 | Xixiangtang, Guangxi | E. chinensis | KF874634* | |
| S. erinaceieuropaei | GX-NN-Xxt3 | Xixiangtang, Guangxi | R. temporaria | KF874635* | |
| S. erinaceieuropaei | GX-NN-Xxt4 | Xixiangtang, Guangxi | R. temporaria | KF874636* | |
| S. erinaceieuropaei | GX-YL-Lc1 | Luchuan, Guangxi | R. rugulosa | KF874637* | |
| S. erinaceieuropaei | GX-YL-Lc2 | Luchuan, Guangxi | R. rugulosa | KF874638* | |
| S. erinaceieuropaei | GX-GL1 | Guilin, Guangxi | R. rugulosa | KF874639* | |
| S. erinaceieuropaei | GX-GL2 | Guilin, Guangxi | R. rugulosa | KF874640* | |
| S. erinaceieuropaei | GX-LG-Lt1 | Lingui, Guangxi | R. rugulosa | KF874641* | |
| S. erinaceieuropaei | GX-LG-Lt2 | Lingui, Guangxi | R. rugulosa | KF874642* | |
| S. erinaceieuropaei | GX-LG-Wt1 | Lingui, Guangxi | R. temporaria | KF874643* | |
| S. erinaceieuropaei | GX-LG-Wt2 | Lingui, Guangxi | R. temporaria | KF874644* | |
| S. erinaceieuropaei | HeN-KF1 | Kaifeng, Henan | R. temporaria | KF874645* | |
| S. erinaceieuropaei | HeN-KF2 | Kaifeng, Henan | R. temporaria | KF874646* | |
| S. erinaceieuropaei | HeN-KF-Tx1 | Tongxu, Henan | R. limmochari | KF874647* | |
| S. erinaceieuropaei | HeN-KF-Tx2 | Tongxu, Henan | R. limmochari | KF874648* | |
| S. erinaceieuropaei | HeN-LH1 | Luohe, Henan | R. limmochari | KF874649* | |
| S. erinaceieuropaei | HeN-LH2 | Luohe, Henan | R. limmochari | KF874650* | |
| S. erinaceieuropaei | HeN-XX1 | Xinxiang, Henan | R. limmochari | KF874651* | |
| S. erinaceieuropaei | HeN-XX2 | Xinxiang, Henan | R. limmochari | KF874652* | |
| S. erinaceieuropaei | HeN-ZK-Fg1 | Fugou, Henan | R. limmochari | KF874653* | |
| S. erinaceieuropaei | HeN-ZK-Fg2 | Fugou, Henan | R. limmochari | KF874654* | |
| S. erinaceieuropaei | HeN-ZZ1 | Zhengzhou, Henan | R. nigromaculata | KF874655* | |
| S. erinaceieuropaei | HeN-ZZ2 | Zhengzhou, Henan | R. nigromaculata | KF874656* | |
| S. erinaceieuropaei | N/a | N/a | N/a | AF004717 | |
| S. mansonoides | N/a | N/a | N/a | AF004718 | |
| Diphyllobothrium | D. latum | N/a | N/a | N/a | AF004719 |
| D. latum | N/a | Russia | Gymnocephalus cernuus | DQ925326 | |
| D. latum | N/a | Finland | N/a | AB302387 | |
| D. nihonkaiense | N/a | Japan | N/a | AB302388 | |
| D. pacificum | N/a | Peru | Homo sapiens | DQ925327 | |
| D. stemmacephalum | N/a | USA | Lagenorhynchus acutus | AF286943 | |
| Digramma | D. interrupta | N/a | Russia | Hemiculter lucidus | DQ925325 |
| Duthiersia | D. fimbriata | N/a | Ghana | Varanus exanthematicus | DQ925328 |
| Schistocephalus | S. solidus | N/a | USA | Gasterosteus aculatus | AF286944 |
Asterisks indicate sequences newly reported in this study (N/a = Not available)
These spargana were 1-13 cm long and 1-2.5 mm wide. To study the phylogenetic relationships among diphyllobothroid cestodes, other members of the genera Spirometra, Digramma, Diphyllobothrium, Duthiersia and Schistocephalus within the family Diphyllobothriidae were considered in the present study (Table 1), with two species of the family Taeniidae (Taenia saginata AF096224 and T. taeniaeformis AF004721) as out-group to root the resulting trees.
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from individual plerocercoid sample using the Tiangen DNeasy Blood and Tissue Kit (Tiangen, China) following the manufacturer's protocol. The 28S rDNA D1 region was amplified by PCR using the primer combination of Lee et al. 2007 (9): forward primer (JB10, 5′-GATTACCCGCTGAACTTAAGCATA-3′) and reverse primer (JB9, 5′-GCTGCATTCAC-AAACACCCCGACTC-3′).
Polymerase chain reactions (PCRs) (25μl) were performed in 2mM MgCl2, 2.5μM of each primer, 2.5μl 10 × rTaq buffer, 0.5mM of each deoxyribonucleoside triphosphate (dNTP), 1.25U of rTaq DNA polymerase (Takara, China), and 1μl of DNA (5-10 ng) sample. The amplification profile for 28S rDNA D1 region consisted of an initial denaturation for 2 min at 94 °C, 40 cycles of: 20 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C; followed by a 6 min final extension at 72 °C. PCR products were purified using the High Pure PCR Product Purification Kit (Takara, China) and sequenced in both directions using an automated sequencer (ABI Prism 3730 XL DNA Analyzer; ABI Prism, Foster City, CA) at the Genwiz Company (Beijing, China).
Sequence analysis
The 28S rDNA D1 sequences were aligned using the computer program Clustal X 2.0 (18). Molecular Evolutionary Genetics Analysis (MEGA) software version 5.0 (19) was employed to analyze the nucleotide composition, conserved sites, variable sites, parsim-info sites and singleton sites, as well as to compute uncorrected pairwise divergence. The program DAMBE 5.0 (20) was used to measure the nucleotide substitution saturation using the method of Xia et al., 2003 (21) as the substitution saturation masked the phylogenetic signal.
Phylogenetic analysis
The aligned 28S rDNA D1 sequences were analyzed by using three methods of Neighbor-Joining (NJ), maximum parsimony (MP) and Bayesian inference (BI), respectively. NJ analysis was performed in PAUP*4b10 (22) using the Kimura two-parameter distance selected by Modeltest 3.7 (23) under the Akaike information criterion and the “heuristics” search option with the “simple” addition sequence and TBR (tree bisection-reconnection) swapping. MP analysis was also performed in PAUP*4b10 (22) using heuristic searches with TBR branch swapping and 2,000 random addition sequences. Confidence in each node was assessed by boot-strapping (2000 pseudo-replicates, heuristic search of 20 random addition replicates with TBR option). BI analyses were performed in MrBayes v3.1 (24) with 5,000,000 generations, sampling trees every 100 generations. Stationarity was assessed using a convergence diagnostic. An average standard deviation of the split frequencies (ASDSF) < 0.03 were used as criteria of convergence between both runs.
Predication of secondary structure
The thermodynamic secondary structure of 28S rDNA D1 region was calculated using the program RNA structure 5.6 (25). We recognized standard Watson-Crick base pairs and noncanonical G: U interactions in all putative stem positions, because G: U can replace a Watson-Crick pair to maintain a helical region and may even have a special function in some instances (26). Information on compensatory and/or semi-compensatory base substitutions was also used to confirm local secondary structures. The RNAviz 2.0 (27) was used to produce the figures of the structures.
Results
The 28S rDNA D1 fragments of the Spirometra sparganum isolates from thirteen locations of China were successfully amplified, and sequenced to be 298 bp in eleven isolates (isolates from Luchuan, Yongning, Xixiangtang, Nanning, Guilin, Liutang and Wutong of Guangxi Zhuang Autonomous Region; and Kaifeng, Tongxu, Xinxiang and Zhengzhou of Henan province), 297 bp in two Henan isolates from Luohe and Fugou. The alignment of the fourteen 28S rDNA D1sequences obtained in the present study and the eleven sequences of the family Diphyllobothriidae obtained from the GenBank resulted in a total of 302 characters including gaps. The MEGA analysis presented 97.32% (290/298) sequence identity, 8 variable sites, and 2 singletons in the fourteen sparganum isolates from China. The average contents of A, C, G and T in these sparganum isolates were 24.8, 22.8, 31.6, and 20.8%, respectively. The nucleotide frequencies were a little biased toward G + C, averaging 54.4 %. The genetic divergence of 28S rDNA D1 sequences of species within the family Diphyllobothriidae ranged from 0 to 6.1%. However, when only took account of Chinese isolates, the upper limits would decline to 0.4%.
The test of substitution saturation showed that the observed index of substitution saturation (Iss) for 28S rDNA D1-alignments was 0.0461, and the corresponding critical index substitution saturation (Iss.c) was 0.6149 for a symmetrical tree and 0.3805 for an extreme asymmetrical tree. The index of Iss was significantly lower than the corresponding Iss.c (p = 0.0000), indicating that there was little saturation in our sequences. The most suitable substitution model was K2 for 28S rDNA D1 region following model selection by Modeltest.
In order to explore the phylogenetic location of the Spirometra sparganum isolates from China and the relationships of important species within the family Diphyllobothriidae, phylogenetic trees were reconstructed based on partial 28S rDNA D1 sequences under NJ, MP, BI three inference methods, respectively, (Fig. 1). As shown in Fig. 1, the monophyly of the family Diphyllobothriidae was supported by all three methods with high support values (100/100/1). Within Diphyllobothriidae, the genus Duthiersia was in the basal of the family, the genera Schistocephalus, Digramma, Diphyllobothrium and Spirometra made up a monophyletic group (60/69/0.72). The clade including all isolates from China and two species (Spirometra erinaceieuropaei AF004717 and Spirometra mansonoides AF004718) obtained from the GenBank was supported by MP and BI methods (69/0.99). The genera Schistocephalus, Digramma and Diphyllobothrium were recovered as a single clade but with very weak support.
Fig 1.
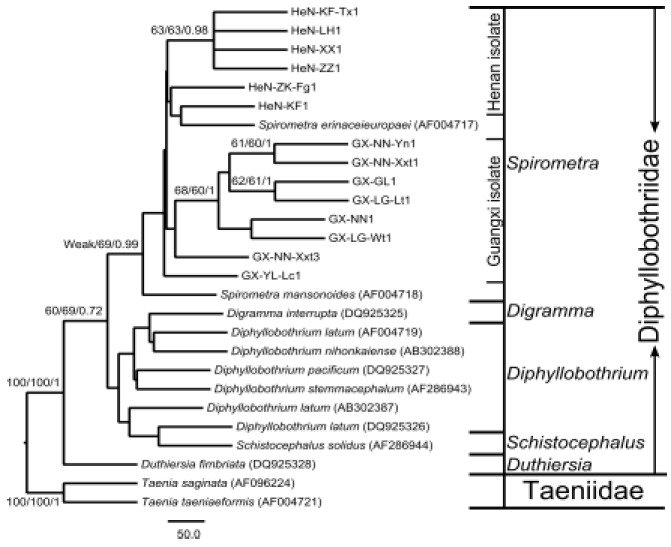
Phylogenetic relationship among the examined Spirometra erinaceieuropaei sparganum isolates from China and other Diphyllobothriid species inferred by Neighbor-Joining (NJ), maximum parsimony (MP) and Bayesian inference (BI) analyses based on 28S rRNA D1 sequences. The numbers along branches indicate bootstrap values and posterior probabilities resulting from different analyses in the order: NJ/MP/BI. The bootstrap values lower than 60 and the posterior probabilities lower than 0.6 are given as ‘Weak’
Our core secondary structure model of 28S rRNA D1 region based on the Spirometra isolate from Naning of China is shown in Fig. 2. We recognized totally fifteen stems, which were numbered 1-15. Positions within stems were indicated by numbers after dashes: 1-1 indicates the first [5’-side] base in stem 1, paired with its complement. Two of the fifteen stems were supported by positional covariance among the 25 Diphyllobothriid sequences included in this analysis. One was position 9-3 in stem 9 of Diphyllobothrium nihonkaiense, D. pacificum, D. stemmacephalum and Digramma interrupta, respectively; the other was position 10-4 in stem 10 of Duthiersia fimbriata and Spirometra mansonoides (Fig. 3). All sequences within the genus Spirometra had a bulge of a cytosine residue (Bulge C in Fig. 2) in the stem 13, but the bulge structure was absent in the genera Diphyllobothrium, Digramma, Duthiersia and Schistocephalus (Fig. 3). Total and varietal sites, and nucleotide percentages for Diphyllobothriid 28S rRNA D1 stems and loops are given in Table 2. Varietal sites in sequences from all Chinese isolates were appeared in loops. However, these sites were more likely to reveal in stems of Diphyllobothrium, Digramma, Duthiersia and Schistocephalus. In loops, adenine is the most abundant base (averagely 41.9%) followed by guanine (averagely 30.0%), and cytosine (averagely 15.1%). In stems, the average percentage of G + C (58.3%) was higher than the percentage of A + T (41.7%).
Fig 2.
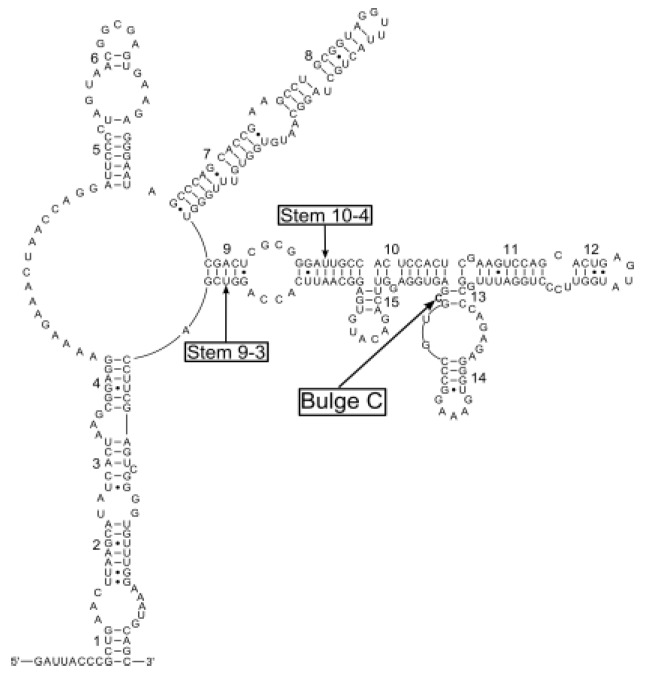
A Core secondary structure model for the Diphyllobothriid 28S rRNA D1 region illustrated using a Spirometra erinaceieuropaei sparganum isolate from Nanning of China (GenBank Accession No. KF874629). Base pairing is indicated as follows: standard canonical pairs by lines (G-C, A-U), wobble G:U pairs by dots (G•U)
Fig 3.
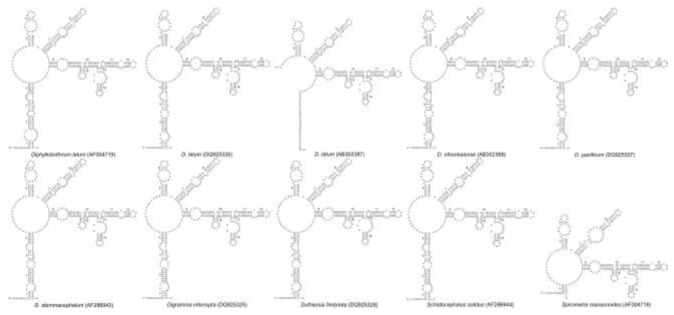
Secondary structure models for the 28S rRNA D1 region of species within genera Diphyllobothrium, Digramma, Duthiersia, Schistocephalus and Spiromtra mansonoides. Base pairing is indicated as follows: standard canonical pairs by lines (G-C, A-U), wobble G:U pairs by dots (G•U)
Table 2.
Molecular properties of Diphyllobothriid 28S rRNA D1 stems and loops
| Sequences | Length (bp) | Total sites | Varietal sites | Loops | Stems | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loop | Stem | Loop | Stem | %A | %C | %G | %T | %A | %C | %G | %T | ||
| Spirometra | |||||||||||||
| GX-NN1 | 298 | 91 | 190 | 0 | 0 | 42.9 | 15.4 | 29.7 | 12.0 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-NN-Yn1 | 298 | 91 | 190 | 1 | 0 | 41.8 | 15.4 | 30.8 | 12.0 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-NN-Xxt1 | 298 | 91 | 190 | 1 | 0 | 41.8 | 15.4 | 30.8 | 12.0 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-NN-Xxt3 | 298 | 91 | 190 | 1 | 0 | 41.8 | 16.5 | 29.7 | 12.0 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-YL-Lc1 | 298 | 91 | 190 | 2 | 0 | 42.9 | 15.4 | 30.8 | 10.9 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-GL1 | 298 | 91 | 190 | 2 | 0 | 41.8 | 14.3 | 30.8 | 13.1 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-LG-Lt1 | 298 | 91 | 190 | 3 | 0 | 40.7 | 14.3 | 30.8 | 14.2 | 16.8 | 24.7 | 33.2 | 25.3 |
| GX-LG-Wt1 | 298 | 91 | 190 | 1 | 0 | 42.9 | 14.3 | 29.7 | 13.1 | 16.8 | 24.7 | 33.2 | 25.3 |
| HeN-KF1 | 298 | 90 | 190 | 1 | 0 | 42.2 | 15.6 | 30.0 | 12.2 | 16.8 | 24.7 | 33.2 | 25.3 |
| HeN-KF-Tx1 | 298 | 91 | 190 | 2 | 0 | 40.7 | 15.4 | 31.9 | 12.0 | 16.8 | 24.7 | 33.2 | 25.3 |
| HeN-LH1 | 297 | 90 | 190 | 2 | 0 | 41.1 | 15.6 | 31.1 | 12.2 | 16.8 | 24.7 | 33.2 | 25.3 |
| HeN-XX1 | 298 | 91 | 190 | 2 | 0 | 40.7 | 15.4 | 31.9 | 12.0 | 16.8 | 24.7 | 33.2 | 25.3 |
| HeN-ZK-Fg1 | 297 | 90 | 190 | 1 | 0 | 42.2 | 15.6 | 30.0 | 12.2 | 16.8 | 24.7 | 33.2 | 25.3 |
| HeN-ZZ1 | 298 | 91 | 190 | 3 | 0 | 40.7 | 15.4 | 30.8 | 13.1 | 16.8 | 24.7 | 33.2 | 25.3 |
| S. erinaceieuropaei | 302 | 92 | 192 | 1 | 2 | 42.4 | 15.2 | 29.3 | 13.1 | 17.2 | 24.5 | 32.8 | 25.5 |
| S. mansonoides | 251 | 84 | 152 | 3 | 4 | 39.3 | 14.3 | 34.5 | 11.9 | 17.1 | 25.7 | 32.9 | 24.3 |
| Diphyllobothrium | |||||||||||||
| D. latum (AF004719) | 299 | 93 | 188 | 3 | 0 | 40.9 | 15.1 | 30.1 | 13.9 | 17.0 | 25.0 | 33.0 | 25.0 |
| D. latum (DQ925326) | 297 | 89 | 192 | 2 | 3 | 42.7 | 14.6 | 28.1 | 14.6 | 16.1 | 25.0 | 33.9 | 25.0 |
| D. latum (AB302387) | 249 | 69 | 146 | 1 | 1 | 37.7 | 17.4 | 31.9 | 13.0 | 16.4 | 26.0 | 33.6 | 24.0 |
| D. nihonkaiense | 297 | 89 | 192 | 2 | 4 | 43.8 | 14.6 | 28.1 | 13.5 | 16.1 | 25.0 | 33.9 | 25.0 |
| D. pacificum | 297 | 89 | 192 | 2 | 4 | 43.8 | 14.6 | 27.0 | 14.6 | 16.1 | 25.5 | 33.9 | 24.5 |
| D. stemmacephalum | 297 | 89 | 192 | 2 | 4 | 42.7 | 14.6 | 28.1 | 14.6 | 16.1 | 25.5 | 33.9 | 24.5 |
| Digramma interrupta | 297 | 89 | 192 | 2 | 4 | 42.7 | 14.6 | 28.1 | 14.6 | 16.1 | 25.0 | 33.9 | 25.0 |
| Duthiersia fimbriata | 297 | 93 | 188 | 4 | 8 | 45.2 | 15.1 | 25.8 | 13.9 | 14.4 | 24.5 | 35.6 | 25.5 |
| Schistocephalus solidus | 296 | 89 | 192 | 1 | 3 | 42.7 | 14.6 | 29.2 | 13.5 | 16.1 | 25.0 | 33.4 | 25.5 |
| Average | 294.0 | 89.4 | 187.1 | 1.8 | 1.5 | 41.9 | 15.1 | 30.0 | 13.0 | 16.6 | 24.9 | 33.4 | 25.1 |
Discussion
In this study, the phylogenetic position of the Spirometra sparganum isolates from China was explored based on the 28S rDNA D1 sequence, and the secondary structure of 28S rDNA D1 region was inferred. The phylogeny reveals that all thirteen Chinese sparganum isolates combined with Spirometra erinaceieuropaei and S. mansonoides species make up a monophyletic group. The secondary structure of 28S rDNA D1 sequences within the genus Spirometra (S. erinaceieuropaei and S. mansonoides) has a bulge of a cytosine residue (Bulge C) in the stem 13.
Unlike the nucleotide frequencies of Diphyllobothriid mitochondrial sequences which are more biased toward A + T (28, 29), our analysis of the 28S rDNA D1 region shows that the G + C nucleotide frequencies are higher than A + T, which is consistent with the study of Lee et al. 2007 (9). This may indicates that the Diphyllobothriid nuclear ribosomal DNA sequence is generally GC rich. In the phylogenetic trees, a high posterior probability (0.99) supports the monophyly of the genus Spirometra. Inside the clade of Spirometra, the species Spirometra mansonoides was in the basal, all isolates from China and the species Spirometra erinaceieuropaei made up a sub-clade. As the species Spirometra erinaceieuropaei is mainly distributed in Asia, and S. mansonoides is the most common in North America (30, 31), the sparganum isolates in China should be represent S. erinaceieuropaei. The genera Schistocephalus, Digramma and Diphyllobothrium were revealed as a monophyletic group, which is consistent with the conclusion of Brabec et al. 2006 (11) based on both small and large subunit nuclear ribosomal RNA genes. And the close relationship between Schistocephalus and Diphyllobothrium is also supported by the analysis of Olson et al. 2001 (10). In our analysis, the sister-group relationship between Spirometra and Schistocephalus + Digramma + Diphyllobothrium was moderate supported by NJ, MP and BI inference methods, this tree topology indicated that the two important species groups (Spirometra and Diphyllobothrium) of the family Diphyllobothriidae might be sibling relationship. Certainly, the confirmed relationship between Spirometra and Diphyllobothrium should wait for an even more comprehensive phylogenetic study with broader sampling of the more terminal representative taxa and more other effective molecular markers as well as morphological data.
In the secondary structure of 28S rDNA D1 region, a higher percentage of adenine in unpaired regions and higher nucleotide frequencies of G + C are consistent with results of previous studies (32, 33). As insertion or deletion, the multiple sequence alignments sometimes seem less reliable during the phylogenetic research, but the secondary structures could help to make a more reliable assignment of nucleotide homology in this situation (14). What's more, some changes, such as expansions and deletions, of a certain helix could be specific to a taxon to help a lot in species identification (15).
Conclusion
According to the results of phylogenetic analyses, the thirteen sparganum isolates from China represent S. erinaceieuropaei. The ‘Bulge C’ in the stem 13 of the 28S rDNA D1 secondary structure could be used as a suitable mark to identify the Spirometra species.
Acknowledgments
We thank Mr JC Lu and ZW Zhang, Ms. J Jiang, LY Li, and H Wen for collecting valuable specimens used in this study. This study was supported by the National Natural Science Foundation of China (No. 81172612). The authors declare that there is no conflict of interests.
References
- 1.Fukushima T, Yamane Y. How does the sparganosis occur? Parasitol Today. 1999;15:124. doi: 10.1016/s0169-4758(99)01405-2. [DOI] [PubMed] [Google Scholar]
- 2.Nithiuthai S, Anantaphruti MT, Waikagul J, Gajadhar A. Waterborne zoonotic helminthiases. Vet Parasitol. 2004;126:167–193. doi: 10.1016/j.vetpar.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Magnino S, Colin P, Dei-Cas E, Madsen M, McLauchlin J, Nöckler K, Maradona MP, Tsigarida E, Vanopdenbosch E, Van Peteghem C. Biological risks associated with consumption of reptile products. Int J Food Microbiol. 2009;134:163–175. doi: 10.1016/j.ijfoodmicro.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Shirakawa K, Yamasaki H, Ito A, Miyajima H. Cerebral sparganosis: the wandering lesion. Neurology. 2010;74:180. doi: 10.1212/WNL.0b013e3181c91a15. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Lin XM, Zhang HW, Xu BL, Wang ZQ. Sparganosis, Henan Province, central China. Emerg Infect Dis. 2011;17:146–147. doi: 10.3201/eid1701.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu DD, Cui J, Wang L, Liu LN, Wei T, Z Q. Immunoproteomic analysis of the excretory-secretory proteins from Spirometra mansoni sparganum. Iran J Parasitol. 2013;8:408–416. [PMC free article] [PubMed] [Google Scholar]
- 7.Wiwanitkit V. A review of human sparganosis in Thailand. Int J Infect Dis. 2005;9:312–316. doi: 10.1016/j.ijid.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Lee SU, Huh S, Sohn WM. Molecular phylogenic location of the Plagiorchis muris (Digenea, Plagiorchiidae) based on sequences of partial 28S rDNA and mitochondrial cytochrome c oxidase subunit I. Korean J Parasitol. 2004;42:71–75. doi: 10.3347/kjp.2004.42.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SU, Chun HC, Huh S. Molecular phylogeny of parasitic Platyhelminthes based on sequences of partial 28S rDNA D1 and mitochondrial cytochrome c oxidase subunit I. Korean J Parasitol. 2007;45:181–189. doi: 10.3347/kjp.2007.45.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson PD, Littlewood DTJ, Bray RA, Mariaux J. Interrelationships and Evolution of the Tapeworms (Platyhelminthes: Cestoda) Mol Phylogenet Evol. 2001;19:443–467. doi: 10.1006/mpev.2001.0930. [DOI] [PubMed] [Google Scholar]
- 11.Brabec J, Kuchta R, Scholz T. Paraphyly of the Pseudophyllidea (Platyhelminthes: Cestoda): Circumscription of monophyletic clades based on phylogenetic analysis of ribosomal RNA. Int J Parasitol. 2006;36:1535–1541. doi: 10.1016/j.ijpara.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Waeschenbach A, Webster BL, Bray RA, Littlewood DTJ. Added resolution among ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with complete small and large subunit nuclear ribosomal RNA genes. Mol Phylogenet Evol. 2007;45:311–325. doi: 10.1016/j.ympev.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Crease TJ, Colbourne JK. The unusually long small-subunit ribosomal RNA of the Crustacean, Daphnia pulex: sequence and predicted secondary structure. J Mol Evol. 1998;46:307–313. doi: 10.1007/pl00006307. [DOI] [PubMed] [Google Scholar]
- 14.Hwang UW, Ree HI, Kim W. Evolution of hypervariable regions, V4 and V7, of insect 18S rRNA and their phylogenetic implications. Zoolog Sci. 2000;17:111–121. doi: 10.2108/zsj.17.111. [DOI] [PubMed] [Google Scholar]
- 15.Zhao YE, Wang ZH, Xu Y, Wu LP, Hu L. Secondary structure prediction for complete rDNA sequences (18S, 5.8S, and 28S rDNA) of Demodex folliculorum, and comparison of divergent domains structures across Acari. Exp Parasitol. 2013;135:370–381. doi: 10.1016/j.exppara.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie JJ, Yoder MJ, Wharton RA. Predicted secondary structure for 28S and 18S rRNA from Ichneumonoidea (Insecta: Hymenoptera: Apocrita): impact on sequence alignment and phylogeny estimation. J Mol Evo. 2005;61:114–137. doi: 10.1007/s00239-004-0246-x. [DOI] [PubMed] [Google Scholar]
- 17.Pepato AR, da Rocha CE, Dunlop JA. Phylogenetic position of the acariform mites: sensitivity to homology assessment under total evidence. BMC Evol Biol. 2010;10:235. doi: 10.1186/1471-2148-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins DG, Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia XH, Xie ZH. DAMBE: Data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- 21.Xia XH, Xie ZH, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evol. 2003;26:1–7. doi: 10.1016/s1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 22.Swofford DL. Phylogenetic Analysis Using Parsimony (PAUP) and Other Methods. Version 4.0b10. Sinauer Associates; Sunderland, MA: 2003. [Google Scholar]
- 23.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 24.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 25.Mathews DH, Disney MD, Childs JL, Schroeder SJ, Zuker M, Turner DH. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc Natl Acad Sci USA. 2004;101:7287–7292. doi: 10.1073/pnas.0401799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Knippenberg PH, Formenoy LJ, Heus HA. Is there a special function for U: G basepairs in ribosomal RNA. Biochimica et Biophysica Acta. 1990;1050:14–17. doi: 10.1016/0167-4781(90)90134-n. [DOI] [PubMed] [Google Scholar]
- 27.De Rijk P, Wuyts J, De Wachter R. RnaViz 2: an improved representation of RNA secondary structure. Bioinformatics. 2003;19:299–300. doi: 10.1093/bioinformatics/19.2.299. [DOI] [PubMed] [Google Scholar]
- 28.Dai RS, Liu GH, Song HQ, Lin RQ, Yuan ZG, Li MW, Huang SY, Liu W, Zhu XQ. Sequence variability in two mitochondrial DNA regions and internal transcribed spacer among three cestodes infecting animals and humans from China. J Helminthol. 2012;86:245–251. doi: 10.1017/S0022149X11000319. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Liu GH, Li F, He DS, Wang T, Sheng XF, Zeng DL, Yang FF, Liu Y. Sequence variability in three mitochondrial DNA regions of Spirometra erinaceieuropaei spargana of human and animal health significance. J Helminthol. 2012;86:271–275. doi: 10.1017/S0022149X1100037X. [DOI] [PubMed] [Google Scholar]
- 30.Bogitsch BJ, Carter CE, Oeltmann TN. Human parasitology. 3. Academic; New York, NY: 2005. [Google Scholar]
- 31.Roberts LS, Janovy J, Jr, Gerald D. Foundations of Parasitology. 8. McGraw-Hill; New York: 2009. [Google Scholar]
- 32.Springer MS, Douzery E. Secondary structure and patterns of evolution among mammalian mitochondrial 16S rRNA molecules. J Mol Evol. 1996;43:357–373. doi: 10.1007/BF02339010. [DOI] [PubMed] [Google Scholar]
- 33.Burk A, Douzery E, Springer MS. The Secondary Structure of Mammalian Mitochondrial 16S rRNA Molecules: Refinements Based on a Comparative Phylogenetic Approach. J Mamm Evol. 2000;9:225–252. [Google Scholar]


