Abstract
Background
Cutaneous leishmaniasis is an annoying and disfiguring disease affecting around 1,500,000 individuals globally. There are endemic pockets of this disease in Taif region. In some patients, lesion often weeps and leads to scar formation. The study was conducted to see the efficacy of fluconazole and itraconazole in the patients of cutaneous leishmaniasis and the effect of these drugs on liver enzymes and kidney markers.
Methods
Positivity of Leishmania was recorded by microscopic examinations of smears. Specific diagnosis for Leishmania major and L. tropica was made with the help of nested polymerase chain reaction. Fluconazole was given at the rate of 200mg/day while itraconazole was given at 150mg/day for six weeks. AST, ALT, creatinine and urea were estimated during medication.
Results
Leishmania major and L. tropica were the species responsible for cutaneous leishmaniasis in Taif region. 81% patients had single lesions, mostly on face followed by hands and legs. 15% of the lesions had bacterial contamination. In terms of efficacy, fluconazole gave slightly better results compared to itraconazole. After 6 weeks of medications, slightly elevated values were recorded for liver enzymes and creatinine.
Conclusion
Transmission of leishmaniasis in Taif region is probably because of poor coverage of residual insecticides spraying at hiding places in pile-ups of rocks and abandoned houses from where sand flies visit nearby houses and cattle sheds during night. Fluconazole and itraconazole may be used for the treatment of cutaneous leishmaniasis with good recovery rate and fewer side effects.
Keywords: Leishmania, skin lesions, chemotherapy, nested PCR, Taif, Saudi Arabia
Introduction
Leishmaniasis is a potential threat to human population in 88 countries affecting around 12 million people globally (1). Around l,500,000 cases of cutaneous leishmaniasis (CL) has been reported from tropical, subtropical and temperate regions of many countries each year. It is more widely distributed in three epidemiological regions, the Americas, the Mediterranean basin, and western Asia from the Middle East to Central Asia. Globally, 70 to 75% of estimated CL incidence is from Afghanistan, Algeria, Colombia, Brazil, Iran, Syria, Ethiopia, North Sudan, Costa Rica and Peru (2, 3). Patients with cutaneous leishmaniasis have one or more lesions on the skin, usually without fever/symptoms. L. major infection generally have superficial satellite papules in the periphery of lesion which heals within 4-6 months in about 50-70% cases whereas in L. tropica the ulcers on the skin are usually dry type which heal within a year or longer.
Saudi Arabia which is a tropical country has endemic pockets of cutaneous leishmaniasis in Al-Hassa, Al-Gaseem, Madina, Hail, Riyadh, Asir, Tabooq, Taif, Al-Baha, Jazan, Najran and Bisha. Lots of cases were detected in Asir, Al-Baha, Arar, El-Quassim and Riyadh provinces (4-9). L. tropica is in abundance in foothills of the Asir range in the south-west of Saudi Arabia (6). Both L. major and L. tropica were found in Al-Baha and Al-Qasim in the western region of the kingdom (10, 11). Recently, incidence of these species has increased in other countries like Egypt, Iran and Israel (12-15). Similar increases have also been reported in the density of Phlebotomus papatasi and P. sergenti which increased the risk of CL transmission in Asir and Madinah Munawwara (16, 17).
As far as the treatment is concerned, it includes thermotherapy, cryotherapy, surgical excision and chemotherapy. Most commonly used agents are antimonial compounds, amphotericin-B, ketoconazole and itraconazole (18, 19). However, due to high toxicity associated with pentavalent antimonial compounds and emergence of drug resistance; combination therapy using liposomal Amphotericin-B with pentamidines or miltefosine has also been tried as a better alternative (20). Fluconazole has also been tried in different dosage in recent past with success for the treatment of cutaneous leishmaniasis caused by Leishmania major (21, 22). Cutaneous leishmaniasis when not treated promptly causes disfiguring, resulting into social stigma particularly among women and, therefore, must be eradicated.
The aim of the study was therefore to see the efficacy of fluconazole and itraconazole in the patients of cutaneous leishmaniasis and the effect of these drugs on liver enzymes and kidney markers.
Materials and Methods
The present study on cutaneous leishmaniasis was conducted in Taif region where a few endemic pockets are available such as Turbah, Al-Garya, Missan and Hadad. These places were selected as they had varied ecological conditions, from sandy and rocky to bushy and hilly terrain. Main focus of the study was epidemiological and pathological aspects of the disease along with vector control. The study was conducted on the patients attending the hospitals located in proposed areas with the help of paramedical staff deputed there and Communicable Diseases Control Centre, MOH, Taif.
Catches of sand flies were made from few house dwellings and sheds of domesticated animals such as sheep and goats near the villages which had cases of cutaneous leishmaniasis. Sand flies captured from the abandoned houses near the animal sheds with the help of torch and WHO suction tube were used for vector incrimination. Thick sticky paper flag traps (soaked in castor oil) were placed in front of the big rocks having space above the ground where sand flies hide during day time. Sand flies when come out for feeding at dusk and dawn get stick to these paper traps. These collected flies were then cleaned with chloral dehydrate. The collected live sand flies were brought to the laboratory and anaesthetized by putting in refrigerator and dissected under Nikon binocular dissecting microscope (Nikon, SMZ1500, Japan) and then examined under the research microscope (Nikon, Eclipse E-600, Japan) to see the positivity for promastigote stage. Ongoing fumigation and spray operations were observed in the study area.
For pathological studies slides were prepared from the material obtained by scraping of the indurate end in the periphery of lesion. These smears after drying in air were fixed and stained with Wright’s eosin-methylene blue, and then checked for pathological conditions under the research microscope (Nikon, Eclipse E-600, Japan) at 1000x. Polymerase chain reaction (PCR) was performed for species identification. A nested polymerase chain reaction-based schizodeme method as described earlier (11) was used as a diagnostic tool for identifying Leishmania-kinetoplast minicircle classes for L. major and L. tropica. Positive slides were photographed with attached digital camera mounted on the microscope.
Out of 27 patients, 10 were given fluconazole at the rate of 200 mg/day while another 10 were given itraconazole at 150 mg/day for 6 weeks. Rest of the patients did not give their consent and, therefore, were not included in this study. All these patients were followed up for 8 months. After medications, patents were examined for their positivity for amastigote stage and bacterial contaminations, if any. First test was conducted after one month, there after every 10 days during the second month and then on a monthly basis. Liver and Kidney function tests were also performed in ten individuals who gave their consent. The ALT and AST enzymes were estimated as per the method described by Wilkinson et al. (23) whereas creatinine and urea were measured by the methods as describes earlier by Thomas (24) and Kalpan (25).
Statistical Analysis
The statistical analysis was conducted with the help of the Microsoft Excel Sheet (Office 2007). Results were expressed as mean ± standard deviation (SD).
Results
As for vector incrimination is concerned, we could collect only 80 sandflies from human dwellings and 700 from animal sheds during 2013. These sandflies were identified as Phlebotomus bergeroti, P. arabicus, P. papatasi and P. sergenti. Out of these, 350 female sandflies were dissected in the laboratory to see gut and gland positivity and only one specimen of P. papatasi was found positive. Both weeping and dry type of lesions were encountered on face, hands and feet in patients from Taif region. Out of 47 suspected ones, 27 individuals were found positive for cutaneous leishmaniasis from the proposed area of study. Seventeen isolates were identified as L. major and 10 as L. tropica. Nineteen patients were male and 8 were female. Elderly males suffered most as 10 patients ranged between 40-60 years, while 5 were between 20-30 years and rest 4 were children of 5-12 years. Among females, out of 8 patients; 2 were of 45 and 52 years, 4 were between 15-35 years and rest 2 were children of 7 and 11 years age. As for lesion distribution is concerned, face, arms and feet had 63, 56 and 50% lesions, respectively (Table 1). We observed single lesion in 81% patients while remaining 19% patients had two lesions involving face and lips or face and hands. Fifteen percent of the lesions were having secondary infection with Staphylococcus aureus, especially in weeping lesions where healings were delayed. The pathological changes in cutaneous leishmaniasis caused by L. major and L. tropica in Taif region showed predominantly macrophages and lymphocytes in the inflammatory infiltrate (Fig. 1), along with formation of compact epithelioid granulomas and necrosis of parasitized macrophages.
Table 1.
Distribution of lesions on different body parts of patients from Taif region during 2013
| Site | Positive smears | Negative smears | Total number | Positivity (%) |
|---|---|---|---|---|
| Face | 13 | 8 | 21 | 62 |
| Arms | 9 | 7 | 16 | 56 |
| Legs | 5 | 5 | 10 | 50 |
| Total | 27 | 20 | 47 | 56 |
Fig. 1.
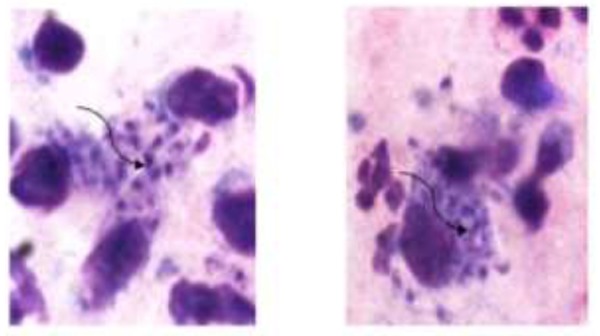
Showing amastigotes in macrophages in the smear prepared from exudates of lesion
The recovery rate in first group of patients treated with fluconazole at 200 mg/day for 6 weeks was around 70% while it was 65% in second group who were treated with itraconazole at the rate of 150 mg/day for the same duration. However, healing was little early around 7.5 to 8 weeks in second group compared with those who were treated with fluconazole at 200mg/day wherein healing took 8-9 weeks. Two patients complained mild headache and nausea in first group while one had nausea in second group especially at later stage of treatment.
As for changes in liver enzymes are concerned, AST and ALT showed slightly raised values of 63.83±3.71, 57.42±2.84 and 77.61±3.35, 68.8±1.72 IU/L, respectively in both group of patients after treatment with fluconazole and itraconazole for 45 days (Table 2 & 3).
Table 2.
Liver enzymes (AST & ALT), creatinine and urea in six cutaneous leishmaniasis patients treated with fluconazole
| Fluconazole (200 mg/d) | After 15 days | After 30 days | After 45 days | After 60 days |
|---|---|---|---|---|
| AST (IU/L) (0-45 IU/L) | 28.7±2.74 | 49.41±4.69 | 63.83±3.71 | 44.83±1.56 |
| ALT (IU/L) (3-60 IU/L) | 20.88±1.75 | 46.36±2.85 | 77.61±3.35 | 60.25±2.42 |
| Creatinine (72.0-126 μmoles/L) | 83.36±2.03 | 96±2.97 | 133.4±2.51 | 80.8±2.11 |
| Urea (3.0-6.0 μmoles/L) | 2.03±0.29 | 3.13±0.44 | 2.88±0.27 | 3.03±0.31 |
Table 3.
Liver enzymes (AST & ALT), creatinine and urea in six cutaneous leishmaniasis patients treated with itraconazole
| Itraconazole (150mg/d) | After 15 days | After 30 days | After 45 days | After 60 days |
|---|---|---|---|---|
| AST (IU/L) (0-45IU/L) | 20.85±1.73 | 42.75±2.39 | 57.42±2.84 | 44.5±1.77 |
| ALT (IU/L) (3-60IU/L) | 16.12±1.31 | 42.05±2.59 | 68.8±1.72 | 56.85±2.93 |
| Creatinine (72.0-126 μmoles/L) | 83.92±1.24 | 93.45±4.3 | 130.37±4.01 | 112.32±2.93 |
| Urea (3.0-6.0 m moles/L) | 2.05±0.46 | 2.32±0.18 | 2.92±0.27 | 3.05±0.26 |
Values of these enzymes remained at higher side but within the normal range on 15th and 30th days of medications (Fig. 2 & 3). Creatinine showed slightly elevated figure of 133.4±2.51and 130.37±4.01 μmoles/L after 45 days of treatment with fluconazole and itraconazole, while urea was within the normal range from 2.88±0.27 to 3.05±0.26 μmoles/L throughout medication (Fig. 2 & 3). Normal values of AST, ALT and creatinine were regained within two weeks after the treatment was over.
Fig. 2.
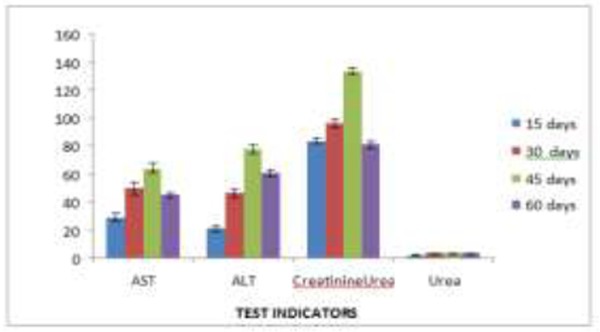
Effect of fluconazole on liver enzymes and creatinin
Fig. 3.
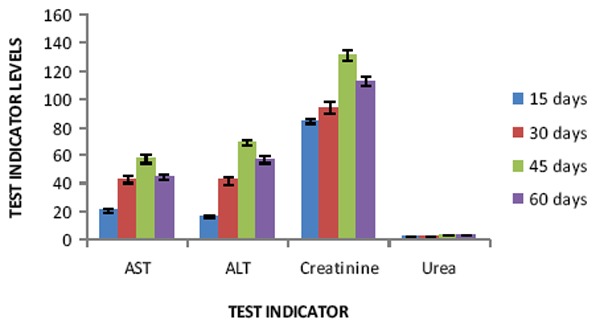
Effect of itraconazole on liver enzymes, creatinine and urea
Discussion
We incriminated Phelebotomus papatasi as vector in Taif region and recorded Leishmania major and L. tropica in 63 and 37% cases. Slightly raised levels of AST, ALT, and creatinine were observed in patients treated with fluconazole and itraconazole. In earlier studies, Phlebotomus papatasi was incriminated as vector for L. major in Al-Hassa oasis and Riyadh provinces of Saudi Arabia (9, 26). We reported positivity rate as 0.29% which is much less than that reported as 5.5% in Southern Jordan valley (27) and 10% in Shahrood district, Central Iran (28). The low positivity rate recorded in present study might be due to less number of dissections which were carried out in a short period of time. Data from 1999 to till date showed that in 1999 and 2000 there were 159 and 128 cases of Leishmaniasis, respectively. It ranged from 51-64 during 2001-2004 (29). Health statistical year books of MOH, Saudi Arabia for 2007-2009 showed 41, 45 and 31 cases of cutaneous leishmaniasis in Taif region (30-32). During 2013, 27 cases of cutaneous leishmaniasis were recorded from this region which is little less than what was reported in 2009. These data clearly indicates the establishment of sand flies in Taif region. It is probably because of varied type of breeding and hiding places which are really difficult to approach. Though control program is taking care of destroying Leishmania vector specially by fogging of infested areas, it seems that it is not helping much as most of the sand flies during fogging remain in their hiding places like spaces under the big rocks which are numerous and could not easily be approached. Besides, there are many abandoned houses and garbage pits near the animal sheds which falls in the flight range of sandflies. Ideally in these and similar difficult areas, synthetic pyrethroides should be used as residual insecticides in the hiding places along with fogging where density of sandflies remains high. Synthetic pyrethroids such as deltamethrin, cyfluthrin and lambdacyhalothrin as residual insecticides had already been used to control sand fly population with great success in many Leishmania infested countries including Moracco and Iran (33, 34).
In our study conducted in Taif region, we observed L. major and L. tropica in 63 and 37% patients, respectively, In Fars Province of Southern Iran L. major and L. tropica were found in 93 and 7% (35), while in other part of Iran L. major infection was reported only in 4% patients (36) we recorded single lesion in 81% of the cutaneous leishmaniasis patients. Almost similar observations were made earlier in Saudi Arabia in which single lesions were observed in 70-80% cases (37). Single lesions were also reported in 53 and 58% patients in Al-Hassa, Saudi Arabia and Iran (4, 38). Contrary to this, multiple lesions were reported in 78% patients from Eastern Saudi Arabia and Kwait (39, 40). L. major lesion starts as raised spot at the site of the bite which lasts often weeks or sometimes years. We observed severely inflamed nodular lesions on the exposed parts of the body which often ulcerate. In our study, pathological changes in both Leishmania major and L. tropica showed predominantly macrophages and lymphocytes in the inflammatory infiltrate along with formation of compact epithelioid granulomas as earlier observed in cutaneous leishmaniasis patients in Qatar and Sudan (41, 42). About 15% of the ulcerating lesions were secondarily infected with Staphylococcus aureus. Almost similar ulcerating lesions were noticed in endemic pockets of Saudi Arabia, Iraq and Pakistan where Staphylococcus aureus infection was observed (43-45). Weeping lesions provided easy access to the bacteria which aggravate and delay the healing process.
Keeping in view the widespread resistance against antimony compounds used for the treatment of leishmania sp., new drugs are now being tried in many countries. In Iran cDNA-AFLP approach was employed for identification of target genes involved in antimony resistance in L. tropica clinical isolates (46). Vaccine preparation protocol for cutaneous leishmaniasis has also been designed recently in Iran (47). We tried non-antimony compound and observed cure rate of 70% in Taif region with six week course of fluconazole at a dose of 200 mg/day. Slightly better results with 79% cure rate was recorded after treating the cutaneous leishmaniasis patients with 200 mg fluconazole/day for the similar duration in other parts of Saudi Arabia (20). This drug at a higher dose of 400 mg/day for 6 weeks in Iran proved more efficacious compared to the dose of 200 mg/day as time of healing get reduced to 4 weeks. However, it had pronounced side effects such as cheilitis and nausea (21). Further better results of 75-100% cure rate with fuconazole was recorded when it was administered at the rate of 5-8 mg/kg body weight per day in Brazilian patients suffering from L. braziliensis (48). It has already been established that fluconazole has a long half-life, and the concentration is 10 times higher in the skin than in plasma (49). It has also been observed that fluconazole retards the growth of Leishmania in culture by inhibiting cytochrome P-450-mediated 14-α-demethylation of lanesterol, blocking ergosterol synthesis and causing accumulation of 14-α-4methyl sterols (50). These qualities of fluconazole probably made it more effective compared to the conventional treatment. We observed 65% cure rate by giving itraconazole in patients at 150mg/day for six weeks. Almost similar result of 66.6% cure rate was obtained from India by giving itraconazole at 4mg/kg/day for the same duration without any noticeable side effect (51). Slightly low cure rate (59%) was recorded at a higher dose of 7mg/kg/day for shorter period of medication of three weeks in Iran (52). Two patients were successfully treated by administering itraconazole at 100 mg/day for two months without any side effect in Saudi Arabia (18). Cure rate of both the medicines were 65-75% and, therefore, a six-week course of 200mg fluconazole/day or 150 mg itraconazole/day may be recommended for the treatment for cutaneous leishmaniasis caused by both L. major and L. tropica. Healing time is little more with fluconazole but side effects are much less against both the drugs in aforesaid dosage, and therefore, may be preferred.
We observed slightly raised figures for AST and ALT activities, 63.83±3.71, 57.42±2.84 and 77.61±3.35, 68.8±1.72 IU/L after 45 days of treatment with fluconazole and itraconazole, respectively, which regained their normal physiological values after 15 days of termination of medications. Almost similar efficacy was observed with fluconazole and itraconazole in Kuwait, India and Saudi Arabia (19, 20, 51). Similar reversible rise with these drugs was also indicated in a review of Blum et al. (53). Since liver is major site of drug metabolism, increased level of AST and ALT in blood might be due to the effect of fluconazole and itraconazole which were administered for a prolonged duration to treat skin lesions and hence might have damaged the liver and leakage of enzyme had occurred which quickly recovered after the termination of treatment. Creatinine was also slightly increased in patients during the terminal phase of treatment with both fluconazole and itraconazole.
We have noticed lack of awareness among people regarding transmission and prevention of disease. Similar lack of awareness had also been reported among patients from Al-Hassa (54). It is expected that adequate health awareness programmes will make the people aware of breeding and hiding places which will enable them to take preventive measures along with a check on breeding of the sandflies by taking appropriate actions.
Conclusion
The sand fly vector for leishmaniasis has established itself in Taif region of Saudi Arabia, especially in rocky mountainous region which have many pile-ups of big rocks on the ground and many abandoned houses and garbage pits near the animal sheds. For control measure, fumigation is not effective as it kills only fraction of sandflies, while majority of them hide safely under the rocks, crevices of abandoned houses and cracks on garbage storage pits. Therefore, intensified spray of residual insecticides in the breeding and hiding places, and human and animal dwellings is recommended. L. major is a zoonotic infection as it operates among human as well as rodents which are available in the vicinity of human habitations. L. tropica which is considered to be anthropophilic species too is transmitted from human to human in endemic pockets especially among the inhabitants of sandy plain areas.
Acknowledgments
This study was funded by The Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah (grant No. 142-002-D1434), which is also acknowledged for technical support. The authors also wish to thank Dr Shabi Fatma Abidi for critical review and help in preparation of this manuscript. The authors declare that there is no conflict of interests.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Report of the consultative meeting on cutaneous leishmaniasis; Geneva: WHO; 2008. p. 36. [Google Scholar]
- 3.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Gindan Y, Abdulaziz O, Kubba R. Cutaneous leishmaniases in Al-Hassa Saudi Arabia. Int J Dermatol. 1984;23:194–197. doi: 10.1111/j.1365-4362.1984.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Biharis AH, Cheema AH, El-Hassan AM. Leishmania infecting man and animals in Saudi Arabia. Canine cutaneous leishmaniasis in the Eastern Province. Roy Soc Trop Med Hyg. 1987;81:925–927. doi: 10.1016/0035-9203(87)90353-1. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zahrani MA, Peters W, Evans DA, Smith V, Ching Chin I. Leishmania infecting man and wild animals in Saudi Arabia. 6. Cutaneous leishmaniasis of man in the south-west. Trans R Soc Trop Med Hyg. 1989;83:621–628. doi: 10.1016/0035-9203(89)90376-3. [DOI] [PubMed] [Google Scholar]
- 7.Kubeyinje EP, Belagavi CS, Jamil YA. Cutaneous leishmaniasis in expatriates in northern Saudi Arabia. East Afr Med J. 1997;74:249–251. [PubMed] [Google Scholar]
- 8.Magzoub M, Subaih M. Prevalence of human cutaneous Leishmaniasis in El-Quassim region of Saudi Arabia. Zagazig Vet J. 1998;26:106–110. [Google Scholar]
- 9.Mustafa MB, Hussein SM, Ibrahim EA, et al. Phlebotomus papatasi (Scopoli), vector of zoonotic cutaneous leishmaniasis in Riyadh province, Saudi Arabia. Trans R Soc Trop Med Hyg. 1994;88:40. doi: 10.1016/0035-9203(94)90489-8. [DOI] [PubMed] [Google Scholar]
- 10.Morsy TA, Al- Gahtani YM, Faris RM. Two abnormal cases of anthroponotic cutaneous leishmaniasis in Al- Baha, Saudi Arabia. J Egypt Soc Parasitol. 1991;21:675–678. [PubMed] [Google Scholar]
- 11.Shalaby I, Gherbawy Y, Jamjoom M, Banaja AE. Genotypic characterization of cutaneous leishmaniasis at Al Baha and Al Qasim Provinces (Saudi Arabia) Vector Borne Zoonotic Dis. 2011;11:807–813. doi: 10.1089/vbz.2010.0213. [DOI] [PubMed] [Google Scholar]
- 12.Svobodova M, Votypka J, Peckova J, et al. Distinct transmission cycles of Leishmania tropica in two adjacent foci Northern Israel. Emerg Infect Dis. 2006;12:1860–1868. doi: 10.3201/eid1212.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shehata MG, Samy AM, Doha SA, et al. First report of Leishmania tropica from a classical focus of L. major in North-Sinai, Egypt. Am J Trop Med Hyg. 2009;81:213–218. [PubMed] [Google Scholar]
- 14.Davami MH, Motazedian MH, Sarkari B. The changing profile of cutaneous leishmaniasis in a focus of the disease in Jahrom district, southern Iran. Ann Trop Med Parasitol. 2010;104:377–382. doi: 10.1179/136485910X12786389891083. [DOI] [PubMed] [Google Scholar]
- 15.Faiman R, Abbasi I, Jaffe C, et al. A newly emerged cutaneous leishmaniasis focus in Northern Israel and two new reservoir hosts of Leishmania major. PLoS Negl Trop Dis. 2013;7:e2058. doi: 10.1371/journal.pntd.0002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim AA, Abdoon AM. Distribution and population dynamics of Phlebotomus sand flies (Diptera: Psychodidae) in an endemic area of cutaneous leishmaniasis in Asir region Southwestern Saudi Arabia. J Entomol. 2005;2:102–108. [Google Scholar]
- 17.El-Badry AA, Al-Juhani AM, Ibrahim EKD, Al-Zubiany SF. Distribution of sand flies in El-Nekheil province, in Al-Madinah Al-Munawwarah region, western of Saudi Arabia. Parasitol Res. 2008;103:151–156. doi: 10.1007/s00436-008-0942-3. [DOI] [PubMed] [Google Scholar]
- 18.Albanese G, Giorgetti P, Santagostino L, Crippa D, Sala G. Cutaneous leishmaniasis: treatment with itraconazole. Arch Dermatol. 1989;125:1540–1542. [PubMed] [Google Scholar]
- 19.Al-Fouzan AS, Al Saleh QA, Najem NM, Rostom AI. Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole. Int J Dermatol. 1991;30:519–521. doi: 10.1111/j.1365-4362.1991.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 20.Sahar H, Al-Natour MD. Update in the treatment of cutaneous leishmaniasis. J Family Community Med. 2009;16:41–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Alrajhi AA, Ibrahim EA, De Vol EB, et al. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med. 2002;346:891–895. doi: 10.1056/NEJMoa011882. [DOI] [PubMed] [Google Scholar]
- 22.Emad M, Hayati F, Fallahzadeh MK, Namazi MR. Superior efficacy of oral fluconazole 400 mg daily versus oral fluconazole 200 mg daily in the treatment of cutaneous Leishmania major infection: a randomized clinical trial. J Am Acad Dermatol. 2011;64:606–608. doi: 10.1016/j.jaad.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson JH, Baron DN, Moss DW, Walker PG. Standardization of clinical enzyme assays: A reference method for aspartate and alanine transaminases. J Clin Pathol. 1972;25:940. doi: 10.1136/jcp.25.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas L, editor. Clinical Laboratory diagnostics. Frankfurt: TH-Books Verlagsgesellschaft; 1998. pp. 366–374. [Google Scholar]
- 25.Kalpan A, editor. Standard Methods of Clinical Chemistry. New York: New York Academic Press; 1965. pp. 245–256. [Google Scholar]
- 26.Killick-Kendrick R, Leaney AJ, Peters W, Rioux JA, Bray RS. Zoonotic cutaneous leishmaniasis in Saudi Arabia: the incrimination of Phlebotomus papatasi as the vector in the Al-Hassa Oasis. Trans R Soc Trop Med Hyg. 1985;79:252–255. doi: 10.1016/0035-9203(85)90350-5. [DOI] [PubMed] [Google Scholar]
- 27.Janini R, Saliba E, Khoury S, Oumeish O, Adwan S, Kamhawi S. Incrimination of Phlebotomus papatasi as vector of Leishmania major in the southern Jordan Valley. Med Vet Entomol. 1995;9(4):420–422. doi: 10.1111/j.1365-2915.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 28.Rassi Y, Abai MR, Javadian E, et al. Molecular data on vectors and reservoir hosts of zoonotic cutaneous leishmaniasis in central Iran. Bull Soc Pathol Exot. 2008;101:425–428. [PubMed] [Google Scholar]
- 29.Wajihullah, Alswat A. A preliminary report on epidemiology of cutaneous leishmaniasis in Taif region, Saudi Arabia. J Vet Parasitol. 2009;23:101–102. [Google Scholar]
- 30.Ministry of Health Saudi Arabia. Health Statistical Year Book. VI: Leishmaniasis. 2007:67. [Google Scholar]
- 31.Ministry of Health Saudi Arabia. Health Statistical Year Book. VI: Leishmaniasis. 2008:73. [Google Scholar]
- 32.Ministry of Health Saudi Arabia. Health Statistical Year Book. VI: Leishmaniasis. 2009:70.
- 33.Faraj C, Ouahabi S, El Bachir-Adlaoui EB, et al. In susceptibility status of Phlebotomus(Paraphlebotomus) sergenti and phlebotomus (Phlebotomus) papatasi in endemic foci of cutaneous leishmaniasis in Morocco. Parasites& Vectors. 2012;5:51. doi: 10.1186/1756-3305-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeidi Z, Vatandoost H, Akhavan AA, et al. Baseline susceptibility of a wild strain of Phlebotomus papatasi (Diptera: Psychodidae) to DDT and pyrethroids in an endemic focus of zoonotic cutaneous leishmaniasis in Iran. Pest Manag Sci. 2012;68(5):669–675. doi: 10.1002/ps.2278. [DOI] [PubMed] [Google Scholar]
- 35.Akhoundi M, Hajjaran H, Baghaei A, Mohebali M. Geographical distribution of Leishmania species of human cutaneous leishmaniasis in Fars Province Southern Iran. Iran Parasitol. 2013;8:85–91. [PMC free article] [PubMed] [Google Scholar]
- 36.Akhoundi M, Mohebali M, Asadi M, et al. Molecular characterization of Leishmania spp. in reservoir hosts in endemic foci of zoonotic cutaneous leishmaniasis in Iran. Folia Parasitol (Praha) 2013;60:218–224. doi: 10.14411/fp.2013.024. [DOI] [PubMed] [Google Scholar]
- 37.Abu Khamsin A. Cutaneous leishmaniasis: a 46-year study of the epidemiology and clinical features in Saudi Arabia (1956-2002) Int J Infect Dis. 2004;8(4):244–250. doi: 10.1016/j.ijid.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Farahmand M, Nahrevanian H, Shirazi HA, Naeimi S, Farzanehnejad Z. An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran. Braz J Infect Dis. 2011;15:17–21. [PubMed] [Google Scholar]
- 39.Buttiker W, Lewis DJ. Ecological studiesatHofuf, Eastern Saudi Arabia, in relation to dermal leishmaniasis. Tropenmed Parasitol. 1879;30:220–229. [PubMed] [Google Scholar]
- 40.Al-Taqi M, Behbehani K. Cutaneous leishmaniasis in Kuwait. Ann Trop Med Parasitol. 1980;74:495–501. doi: 10.1080/00034983.1980.11687374. [DOI] [PubMed] [Google Scholar]; Samad A, Ardehali S. Histological spectrum of cutaneous leishmaniasis due to Leishmania tropica. Trans R Soc Trop Med Hyg. 1985;79(5):631–636. doi: 10.1016/0035-9203(85)90174-9. [DOI] [PubMed] [Google Scholar]
- 41.Azadeh B, Samad A, Ardehali S. Histological spectrum of cutaneous leishmaniasis due to Leismania tropica. Trans R Soc Trop Med Hyg. 1985;79(5):631–636. doi: 10.1016/0035-9203(85)90174-9. [DOI] [PubMed] [Google Scholar]
- 42.Gaafar A, el Kadaro AY, Theander TG, et al. The pathology of cutaneous leishmaniasis due to Leishmania major in Sudan. Am J Trop Med Hyg. 1995;52(5):438–442. doi: 10.4269/ajtmh.1995.52.438. [DOI] [PubMed] [Google Scholar]
- 43.Uthman MAE, Satir AA, Tabbara KS. Clinical and histopathological features of zoonotic cutaneous leishmaniasis in Saudi Arabia. J Eur Acad Dermatol Venereol. 2005;9:431–436. doi: 10.1111/j.1468-3083.2005.01210.x. [DOI] [PubMed] [Google Scholar]
- 44.Al-Samarai AM, Al-Obaidi HS. Cutaneous leishmaniasis in Iraq. J Infect Dev Ctries. 2009;3:123–129. doi: 10.3855/jidc.59. [DOI] [PubMed] [Google Scholar]
- 45.Bhutto AM, Soomro FR, Baloch JH, Matsumoto J, Uezato H, Hashiguchi Y, Katakura K. Cutaneous leishmaniasis caused by Leishmania (L.) major infection in Sindh province, Pakistan. Acta Trop. 2009;111:295–298. doi: 10.1016/j.actatropica.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Kazemi-Rad E, Mohebali M, Khadem-Erfan MB, et al. Identification of antimony resistance markers in Leishmania tropica field isolates through a cDNA-AFLP approach. Exp Parasitol. 2013;135:344–349. doi: 10.1016/j.exppara.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Khanjani N, Gonzalez U, Leonardi-Bee J, Mohebali M, Saffari M, Khamesipour A. Copyright C 2009. The Cochrane Collaboration. Published by John Wiley & Sons Ltd; Vaccine for preventing cutaneous leishmaniasis (protocol) [Google Scholar]
- 48.Sousa AQ, Frutuoso MS, Moraes EA, Pearson RD, Pompeu MM. High-dose oral fluconazole therapy effective for cutaneous leishmaniasis due to Leishmania (Vianna) braziliensis. Clin Infect Dis. 2011;53(7):693–695. doi: 10.1093/cid/cir496. [DOI] [PubMed] [Google Scholar]
- 49.Malik A. Origin of drugs in current use: the Diflucan story, 2001. 2010 Sep 24; Available at: http://www.world-offungi.org/Mostly_Medical-/Abrar_Malik/Abrar_Malik.htm.
- 50.Como JA, Dismukes WE. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 51.Dogra J, Aneja N, Lal BB, Mishra SN. Cutaneous leishmaniasis in India. Clinical experience with itraconazole) Int J Dermatol. 1990;29(9):661–662. doi: 10.1111/j.1365-4362.1990.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 52.Momeni AZ, Jalayer T, Emamjomeh M, et al. Treatment of cutaneous leishmaniasis with itraconazole. Randomized double-blind study. Arch Dermatol. 1996;132(7):784–786. [PubMed] [Google Scholar]
- 53.Blum J, Desjeux P, Schwartz E, Beck B, Hatz C. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53:158–166. doi: 10.1093/jac/dkh058. [DOI] [PubMed] [Google Scholar]
- 54.Amin TT, Kaliyadan F, Al-Ajyan MI, et al. Public awareness and attitudes towards cutaneous leishmaniasis in an endemic region in Saudi Arabia. J Eur Acad Dermatol Venereol. 2012;26:1544–1551. doi: 10.1111/j.1468-3083.2011.04339.x. [DOI] [PubMed] [Google Scholar]


