Abstract
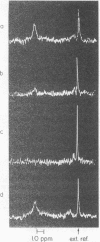
Purified rhodopsin was incorporated into phospholipid bilayers of egg phosphatidylcholine to give recombinant membrane vesicles, which were examined by proton and phosphorus nuclear magnetic resonance spectroscopy. Increased rhodopsin content in the membranes appears to progressively inhibit the molecular motions of the methyl, methylene, and phosphate groups of the phospholipid molecules. This indicates that regions of the rhodopsin molecule interact in a manner that affects the phospholipids from the aqueous interface to the bilayer midline. In the dark, the recombinant vesicles were sealed to europium, manganese, or cobalt ions. Light exposure allowed rapid equilibration of Mn2+ and Co2+, and somewhat slower equilibration of Eu3+ across the membrane. Light changed the membrane permeability, and the gradient in chemical potential resulted in a net ion movement across the rhodopsin:phospholipid recombinant membrane. The results suggest rhodopsin is a transmembrane protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berden J. A., Barker R. W., Radda G. K. NMR studies on phospholipid bilayers. Some factors affecting lipid distribution. Biochim Biophys Acta. 1975 Jan 28;375(2):186–208. doi: 10.1016/0005-2736(75)90188-1. [DOI] [PubMed] [Google Scholar]
- Blasie J. K. The location of photopigment molecules in the cross-section of frog retinal receptor disk membranes. Biophys J. 1972 Feb;12(2):191–204. doi: 10.1016/S0006-3495(72)86079-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Wilkins M. H. Structure of retinal photoreceptor membranes. Nature. 1972 Apr 7;236(5345):313–314. doi: 10.1038/236313a0. [DOI] [PubMed] [Google Scholar]
- Chabre M. X-ray diffraction studies of retinal rods. I. Structure of the disc membrane, effect of illumination. Biochim Biophys Acta. 1975 Mar 25;382(3):322–335. doi: 10.1016/0005-2736(75)90274-6. [DOI] [PubMed] [Google Scholar]
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Hubbell W. L. Temperature- and light-dependent structural changes in rhodopsin-lipid membranes. Exp Eye Res. 1973 Dec 24;17(6):517–532. doi: 10.1016/0014-4835(73)90082-1. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruyff B., Richards R. E. Factors affecting the motion of the polar headgroup in phospholipid bilayers. A 31P NMR study of unsonicated phosphatidylcholine liposomes. Biochim Biophys Acta. 1976 Mar 19;426(3):433–446. doi: 10.1016/0005-2736(76)90388-6. [DOI] [PubMed] [Google Scholar]
- Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. IV. Aldehydes and cation permeability of artificial phospholipid miclles. Biochim Biophys Acta. 1969 Jun 3;183(1):90–97. doi: 10.1016/0005-2736(69)90132-1. [DOI] [PubMed] [Google Scholar]
- Daemen F. J. Vertebrate rod outer segment membranes. Biochim Biophys Acta. 1973 Nov 28;300(3):255–288. doi: 10.1016/0304-4157(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J Mol Biol. 1972 Jan 28;63(2):265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Englander S. W. Molecular structure of membrane-bound rhodopsin. Nature. 1975 Apr 17;254(5501):625–627. doi: 10.1038/254625a0. [DOI] [PubMed] [Google Scholar]
- Dutton A., Rees E. D., Singer S. J. An experiment eliminating the rotating carrier mechanism for the active transport of Ca ion in sarcoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1532–1536. doi: 10.1073/pnas.73.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G. Initiation of impulses in visual cells of Limulus. J Physiol. 1959 Oct;148:14–28. doi: 10.1113/jphysiol.1959.sp006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson G. W., Chan S. I. Nuclear magnetic relaxation behavior of lecithin multilayers. J Am Chem Soc. 1974 Mar 6;96(5):1312–1319. doi: 10.1021/ja00812a009. [DOI] [PubMed] [Google Scholar]
- Gent M. P., Prestegard J. H. Cholesterol-phosphatidylcholine interactions in vesicle systems. Implication of vesicle size and proton magnetic resonance line-width changes. Biochemistry. 1974 Sep 10;13(19):4027–4033. doi: 10.1021/bi00716a033. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Lipid requirements for Rhodopsin regenerability. Biochemistry. 1973 Oct 23;12(22):4517–4523. doi: 10.1021/bi00746a033. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. L., Chan S. I. Voltage-induced formation of alamethicin pores in lecithin bilayer vesicles. Biochemistry. 1976 Jun 15;15(12):2551–2555. doi: 10.1021/bi00657a010. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Levine Y. K., Metcalfe J. C. High resolution proton relaxation studies of lecithins. Biochim Biophys Acta. 1972 Jan 17;255(1):43–56. doi: 10.1016/0005-2736(72)90006-5. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Horwitz A. F., Klein M. P. Transbilayer asymmetry and surface homogeneity of mixed phospholipids in cosonicated vesicles. Biochemistry. 1973 Jul 3;12(14):2637–2645. doi: 10.1021/bi00738a014. [DOI] [PubMed] [Google Scholar]
- Montal M., Darszon A., Trissl H. W. Transmembrane channel formation in rhodopsin-containing bilayer membranes. Nature. 1977 May 19;267(5608):221–225. doi: 10.1038/267221a0. [DOI] [PubMed] [Google Scholar]
- O'Brien D. F., Costa L. F., Ott R. A. Photochemical functionality of rhodopsin-phospholipid recombinant membranes. Biochemistry. 1977 Apr 5;16(7):1295–1303. doi: 10.1021/bi00626a009. [DOI] [PubMed] [Google Scholar]
- Poo M. M., Cone R. A. Lateral diffusion of phodopsin in Necturus rods. Exp Eye Res. 1973 Dec 24;17(6):503–510. doi: 10.1016/0014-4835(73)90079-1. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Smith H. G., Jr, Fager R. S., Litman R. J. Light-activated calcium release from sonicated bovine retinal rod outer segment disks. Biochemistry. 1977 Apr 5;16(7):1399–1405. doi: 10.1021/bi00626a025. [DOI] [PubMed] [Google Scholar]
- Stubbs G. W., Litman B. J. Microviscosity of the hydrocarbon region of the bovine retinal rod outer segment disk membrane determined by fluorescent probe measurements. Biochemistry. 1976 Jun 29;15(13):2766–2772. doi: 10.1021/bi00658a009. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R., Radda G. K. Differential scanning calorimetry and 31P NMR studies on sonicated and unsonicated phosphatidylcholine liposomes. Biochim Biophys Acta. 1975 Sep 16;406(1):6–20. doi: 10.1016/0005-2736(75)90038-3. [DOI] [PubMed] [Google Scholar]