Abstract
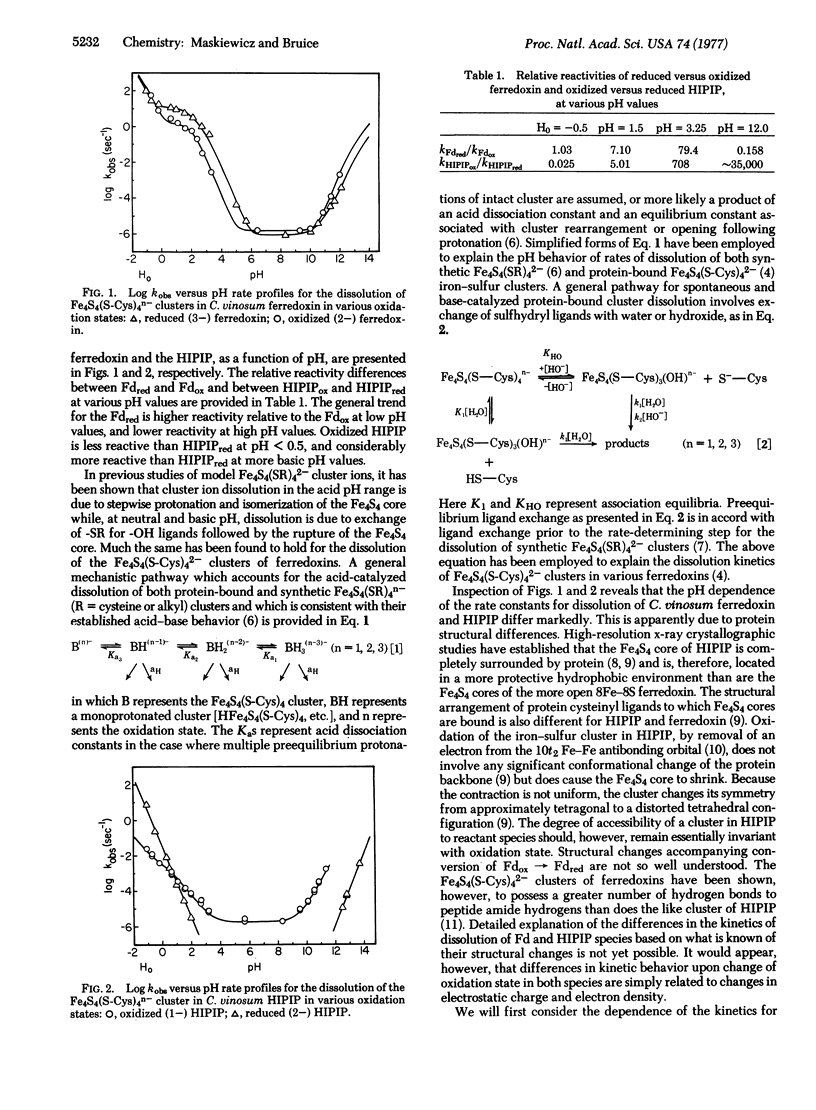
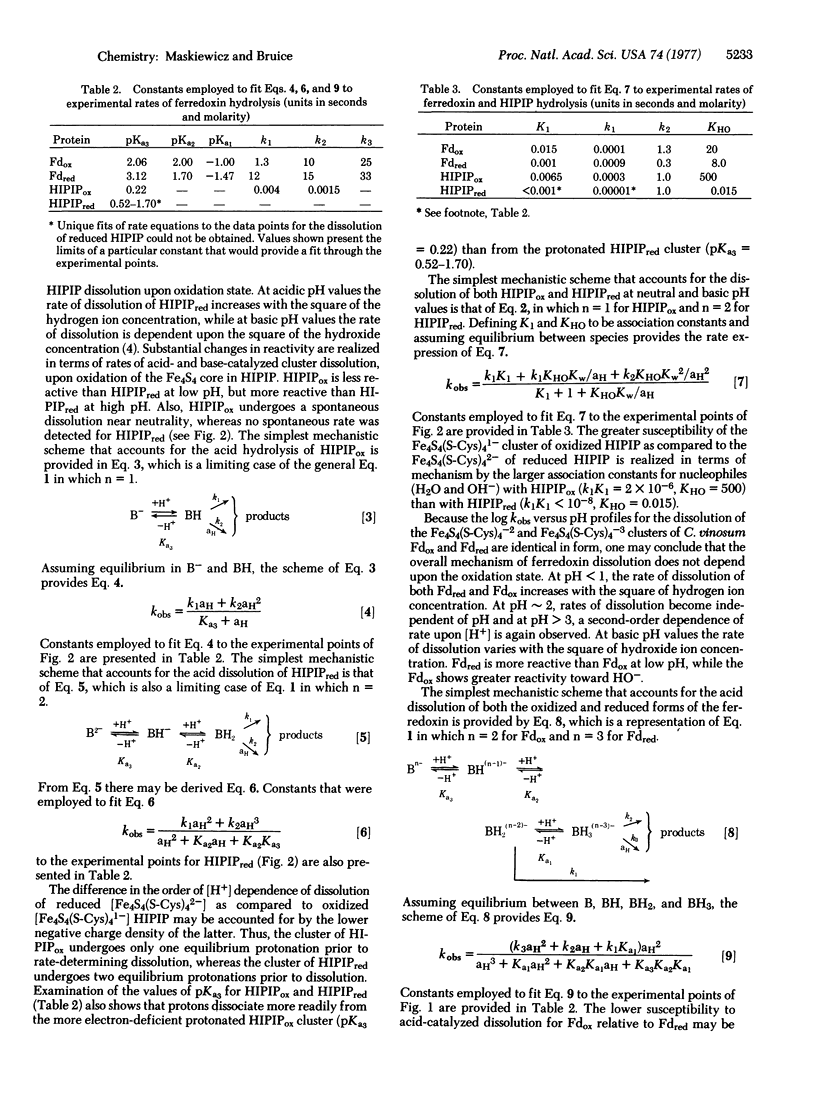
The influence of oxidation state on the pH dependence of the dissolution of the Fe4S4 clusters of Chromatium vinosum ferredoxin and high-potential iron protein (HIPIP) has been studied. The first-order rate constants (kobs) for dissolution of both the Fe4S4(S-Cys)42- and Fe4S4(S-Cys)43- clusters of the ferredoxin follow the same overall kinetic equation but with differing specific rate and equilibrium constants. The dependence of rate and equilibrium constants upon oxidation state may be rationalized on the basis of the accompanying change in electrostatic affinity of a cluster toward H+ and HO-. A more drastic change in the pH dependence of the kinetics of dissolution of the Fe4S4 cluster of the HIPIP accompanies its change in oxidation state. Whereas the values of kobs for dissolution of HIPIP containing the Fe4S4(S-Cys)42- cluster are strictly second order to [H+] and [HO-], the pH dependence for dissolution of the HIPIP Fe4S4(S-Cys)41- cluster indicates a first-order dependence upon [H+], a second-order dependence upon [HO-], and a spontaneous or water rate. These reactivity differences may be related to changes in cluster charge density. Mechanisms of dissolution involve preequilibrium protonation at acidic pH and preequilibrium ligand exchange at basic pH.
Keywords: redox state-dependent reactivities, hydrolysis kinetics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E., Watenpaugh K. D., Jensen L. H. NH---S hydrogen bonds in Peptococcus aerogenes ferredoxin, Clostridium pasteurianum rubredoxin, and Chromatium high potential iron protein. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4854–4858. doi: 10.1073/pnas.72.12.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruice T. C., Maskiewicz R., Job R. The Acid-Base Properties, Hydrolytic Mechanism, and Susceptibility to O(2) Oxidation of Fe(4)S(4)(SR)(4) Clusters. Proc Natl Acad Sci U S A. 1975 Jan;72(1):231–234. doi: 10.1073/pnas.72.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A. Comparison of oxidation-reduction site geometries in oxidized and reduced Chromatium high potential iron protein and oxidized Peptococcus aerogenes ferredoxin. J Biol Chem. 1974 Oct 10;249(19):6339–6346. [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus K., De Klerk H., Sletten K., Bartsch R. G. Chemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1967 Jun 27;140(2):291–311. doi: 10.1016/0005-2795(67)90470-9. [DOI] [PubMed] [Google Scholar]
- Job R. C., Bruice T. C. Iron-sulfur clusters II: Kinetics of ligand exchange studied on a water-soluble Fe(4)S(4)(SR)(4) cluster. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2478–2482. doi: 10.1073/pnas.72.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B., Bulen W. A., Shaw E. R., Breeze R. H. Determination of oxidation-reduction potentials by spectropolarimetric titration: application to several iron-sulfur proteins. Arch Biochem Biophys. 1974 May;162(1):301–309. doi: 10.1016/0003-9861(74)90129-5. [DOI] [PubMed] [Google Scholar]
- Maskiewicz R., Bruice T. C. Kinetic study of the dissolution of Fe4S4(2-)-cluster core ions of ferredoxins and high potential iron protein. Biochemistry. 1977 Jun 28;16(13):3024–3029. doi: 10.1021/bi00632a033. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Johnson K. H., Holm R. H., Norman J. G., Jr Letter: Theoretical model for the 4-Fe active sites in oxidized ferredoxin and reduced "high-potential" proteins. Electronic structure of the analogue [Fe4S4(SCH3)4]2-. J Am Chem Soc. 1975 Oct 29;97(22):6596–6598. doi: 10.1021/ja00855a062. [DOI] [PubMed] [Google Scholar]


